Detection of Viruses and Elimination of Sweet Potato Feathery Mottle Virus in High-Yielding Varieties of Sweet Potato (Ipomoea batatas) from Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Plant Materials, and Greenhouse Conditions
2.2. Virus Testing
2.2.1. ELISA
2.2.2. RT–PCR
2.2.3. Graft Indexing to I. setosa
2.3. Heat Therapy, Meristem Culture, and Plant Regeneration
3. Results and Discussion
3.1. Graft Inoculation Drastically Improves the Detection of SPFMV by DAS–ELISA and RT–PCR
3.2. Report of the SPVC in Ethiopia
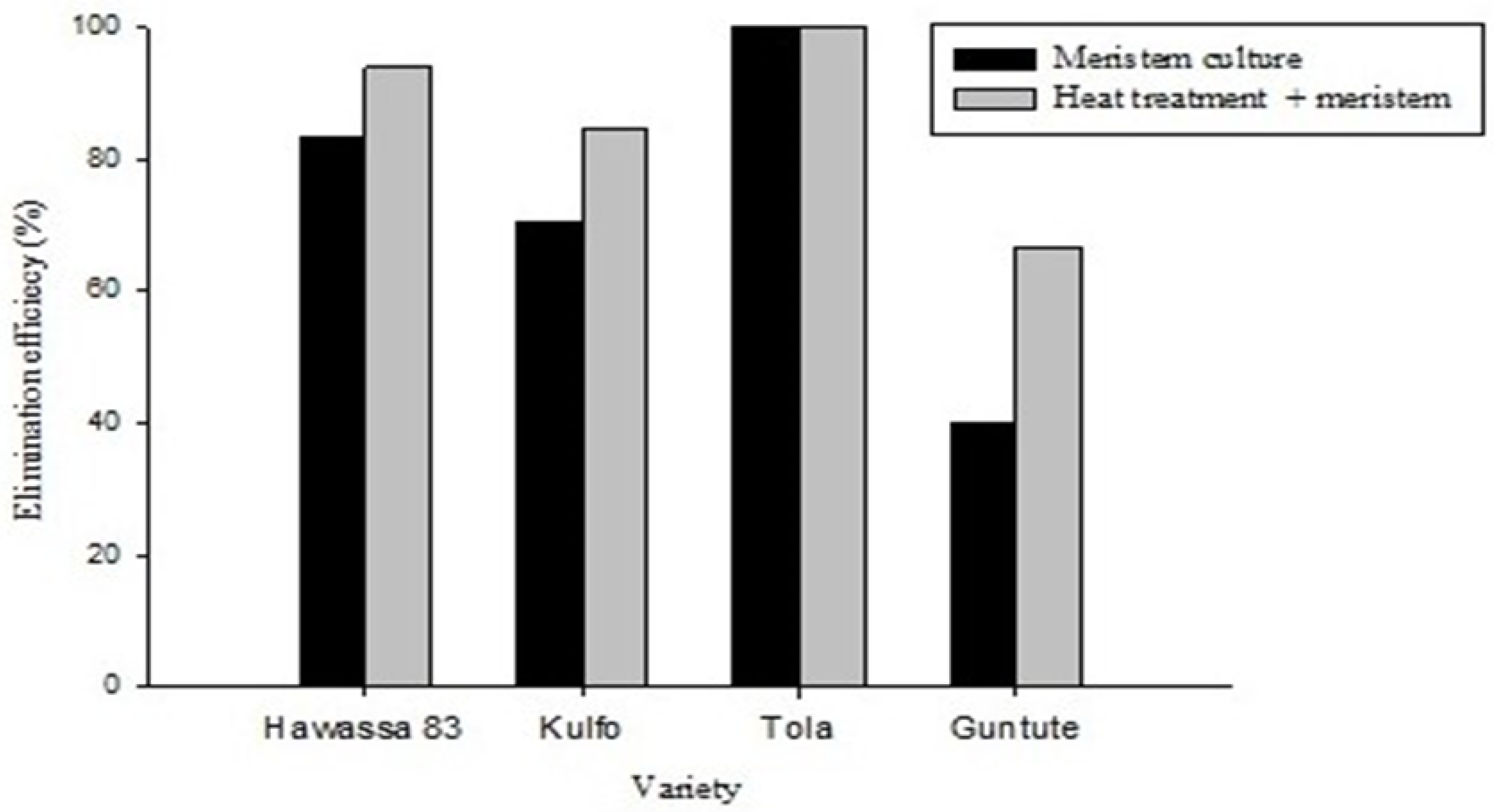
3.3. Heat Therapy Followed by Meristem Tip Culture Increased the Efficiency of SPFMV Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, J.; Lynam, J.; Lemaga, B.; Crissman, C.; Barker, I.; Thiele, G.; Namanda, S.; Wheatley, C.; Andrade, M. Sweet potato in Sub-Saharan Africa. In The Sweetpotato; Springer: Berlin/Heidelberg, Germany, 2009; pp. 359–390. [Google Scholar]
- Belehu, T. Agronomical and Physiological Factors Affecting Growth, Development and Yield of Sweet Potato in Ethiopia. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2003. [Google Scholar]
- Truong, V.D.; Avula, R.Y.; Pecota, K.V.; Yencho, G.C. Sweetpotato production, processing, and nutritional quality. In Handbook of Vegetables and Vegetable Processing; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 811–838. [Google Scholar]
- Aldow, A.M. Factors Affecting Sweet Potato Production in Crop-Livestock Farming Systems in Ethiopia. Master’s Thesis, Norwegian University of Life Sciences (NMBU), As, Norway, 2017. [Google Scholar]
- Clark, C.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Gibson, R.W.; Mukasa, S.B.; Tugume, A.K.; Tairo, F.D. Sweet potato viruses: 15 years of progress on understanding and managing complex diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T. Characterization of Viruses of Pepper (Capsicum spp.) and Sweet Potato (Ipomoea batatas) from Ethiopia; Cuvillier Verlag: Göttingen, Germany, 2004; 126p. [Google Scholar]
- Adane, A. Associated viruses threatening sweet potato improvement and production in Ethiopia. Afr. Crop Sci. J. 2010, 18, 207–213. [Google Scholar]
- Tesfaye, T.; Feyissa, T.; Abraham, A. Survey and serological detection of sweet potato (Ipomoea batatas (L.) Lam) viruses in Ethiopia. J. Appl. Biosci. 2011, 41, 2746–2756. [Google Scholar]
- Feyissa, T.; Dugassa, G. In vitro production of virus-free sweet potato [Ipomoea batatas (L.) Lam] by meristem culture and thermotherapy. Ethiop. J. Sci. 2011, 34, 17–28. [Google Scholar]
- Wondimu, T.; Feyissa, T.; Bedadav, G. Meristem culture of selected sweet potato (Ipomoea batatas L. Lam.) cultivars to produce virus-free planting material. J. Hortic. Sci. Biotechnol. 2012, 87, 255–260. [Google Scholar] [CrossRef]
- Buko, D.H.; Spetz, C.; Hvoslef-Eide, A.K. Next generation sequencing as a method to verify virus elimination using heat treatment and meristem tip culture in the five most widely used sweet potato varieties in Ethiopia. Afr. J. Biotechnol. 2020, 19, 458–463. [Google Scholar]
- Buko, D.H. Sweet Potato Virus in Ethiopia: Detection, Characterization, Elimination and Management. Ph.D. Thesis, Norwegian University of Life Sciences, Ås, Norway, 2019. Available online: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/11250/2711545/105146_Dereje%20Haile%20Buko%20PhD%20thesis.pdf?isAllowed=y&sequence=1 (accessed on 1 June 2021).
- Clark, C.A.; Moyer, J.W. Compendium of Sweet Potato Diseases; American Phytopathological Society: St. Paul, MN, USA, 1988. [Google Scholar]
- Opiyo, S.; Ateka, E.; Owuor, P.; Manguro, L.; Miano, D. Development of a multiplex PCR technique for simultaneous detection of Sweet potato feathery mottle virus and Sweet potato chlorotic stunt virus. J. Plant Pathol. 2010, 92, 363–366. [Google Scholar]
- Van der Want, J.; Dijkstra, J. A history of plant virology. Arch. Virol. 2006, 151, 1467–1498. [Google Scholar] [CrossRef] [PubMed]
- Golino, D.; Fuchs, M.; Al Rwahnih, M.; Farrar, K.; Schmidt, A.; Martelli, G. Regulatory aspects of grape viruses and virus diseases: Certification, quarantine, and harmonization. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Berlin/Heidelberg, Germany, 2017; pp. 581–598. [Google Scholar]
- Spiegel, S.; Stein, A.; Tam, Y. In vitro thermotherapy of rosaceous fruit trees. In Proceedings of the XVIth International Symposium on Fruit Tree Virus Diseases, Rome, Italy, 27 June–2 July 1994; pp. 419–420. [Google Scholar]
- Wang, Q.C.; Valkonen, J.P. Elimination of two viruses which interact synergistically from sweet potato by shoot tip culture and cryotherapy. J. Virol. Methods 2008, 154, 135–145. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Nyland, G.; Goheen, A.C. Heat therapy of virus diseases of perennial plants. Annu. Rev. Phytopathol. 1969, 7, 331. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zuo, R.; Abad, J.; Xu, D.; Bao, G.; Li, R. Simultaneous detection and differentiation of four closely related sweet potato potyviruses by a multiplex one-step RT–PCR. J. Virol. Methods 2012, 186, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kathurima, T.; Bett, B.; Miano, D.; Kim, D. Diagnostics of viruses infecting local farmer preferred sweet potato cultivars in Kenya. Afr. J. Agric. Res. 2011, 6, 3718–3724. [Google Scholar]
- Zheng, Y.; Gao, S.; Padmanabhan, C.; Li, R.; Galvez, M.; Gutierrez, D.; Fuentes, S.; Ling, K.S.; Kreuze, J.; Fei, Z. VirusDetect: An automated pipeline for efficient virus discovery using deep sequencing of small RNAs. Virology 2017, 500, 130–138. [Google Scholar] [CrossRef]
- Aritua, V.; Bua, B.; Barg, E.; Vetten, H.; Adipala, E.; Gibson, R. Incidence of five viruses infecting sweet potato es in Uganda; the first evidence of Sweet potato caulimo-like virus in Africa. Plant Pathol. 2007, 56, 324–331. [Google Scholar] [CrossRef]
- Dennien, S.; Homare, D.; Hughes, M.; Lovatt, J.; Coleman, E.; Jackson, G. Growing Healthy Sweet Potato: Best Practices for Producing Planting Material; ACIAR: Canberra, Australian, 2013. [Google Scholar]
- Buko, D.H.; Hvoslef-Eide, T.A. Optimization of plant growth regulators for meristem initiation and subsequent multiplication of five virus tested elite sweet potato varieties from Ethiopia. Afr. J. Biotechnol. 2020, 19, 332–343. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Green, S.; Kuo, Y.; Lee, D. Uneven distribution of two potyviruses (Sweet potato feathery mottle virus and sweet potato latent virus) in sweet potato plants and its implication on virus indexing of meristem derived plants. Int. J. Pest Manag. 1988, 34, 298–302. [Google Scholar] [CrossRef]
- Moyer, J.W.; Salazar, L. Viruses and virus-like diseases of sweet potato. Plant Dis. 1989, 73, 451–455. [Google Scholar] [CrossRef]
- Gibson, R.; Mwanga, R.; Kasule, S.; Mpembe, I.; Carey, E. Apparent absence of viruses in most asymptomatic field-grown sweet potato in Uganda. Ann. Appl. Biol. 1997, 130, 481–490. [Google Scholar] [CrossRef]
- Tugume, A.; Mukasa, S.; Valkonen, J. Natural wild hosts of sweet potato feathery mottle virus show spatial differences in virus incidence and virus-like diseases in Uganda. Phytopathology 2008, 98, 640–652. [Google Scholar] [CrossRef]
- Paprstein, F.; Sedlak, J.; Polak, J.; Svobodova, L.; Hassan, M.; Bryxiova, M. Results of in vitro thermotherapy of apple cultivars. Plant Cell Tissue Organ Cult. 2008, 94, 347–352. [Google Scholar] [CrossRef]
- Waswa, M.; Kakuhenzire, R.; Ochwo-Ssemakula, M. Effect of thermotherapy duration, virus type and cultivar interactions on elimination of potato viruses X and S in infected seed stocks. Afr. J. Plant Sci. 2017, 11, 61–70. [Google Scholar]
- Bilska, A.; Sowiński, P. Closure of plasmodesmata in maize (Zea mays) at low temperature: A new mechanism for inhibition of photosynthesis. Ann. Bot. 2010, 106, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Hull, R. Plant Virology; Academic Press: Norwich, UK, 2013; 1104p. [Google Scholar]
- Zhang, Z.; Lee, Y.; Spetz, C.; Clarke, J.L.; Wang, Q.; Blystad, D.-R. Invasion of shoot apical meristems by Chrysanthemum stunt viroid differs among Argyranthemum cultivars. Front. Plant Sci. 2015, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Kosmowski, F.; Aragaw, A.; Kilian, A.; Ambel, A.; Ilukor, J.; Yigezu, B.; Stevenson, J. Varietal identification in household surveys: Results from three household-based methods against the benchmark of DNA fingerprinting in southern Ethiopia. Exp. Agric. 2018, 55, 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Panis, B.; Engelmann, F.; Lambardi, M.; Valkonen, J. Cryotherapy of shoot tips: A technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann. Appl. Biol. 2009, 154, 351–363. [Google Scholar] [CrossRef]
- Dodds, J.; Ng, S.Y.C. In vitro methods for pathogen elimination and international distribution of sweet potato germplasm exploration, maintenance and utilization of sweet potato genetic resources. In Proceedings of the Report 1st Planning Conference of Exploration, Maintenance and Utilization of Sweet Potato Resources, International Potato Centre, Lima, Perú, 23–27 February 1987. [Google Scholar]
- El Far, M.M.; Ashoub, A. Utility of thermotherapy and meristem tip for freeing sweet potato from viral infection. Aust. J. Basic Appl. Sci. 2009, 3, 153–159. [Google Scholar]
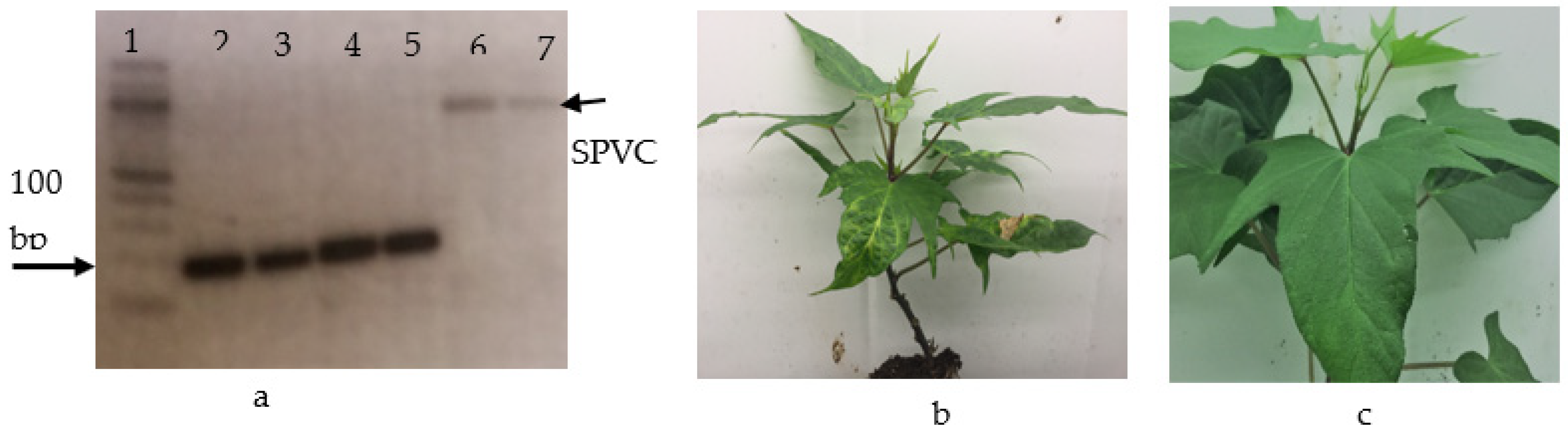
| Varieties | Number of Plants Collected | Source | Yield (qt/ha) | Year Released | Root Flesh Color | Maturity Period |
|---|---|---|---|---|---|---|
| ‘Berkume’ | 3 | Haramaya University | 322 | 2007 | White | 3–4 months |
| ‘Guntute’ | 3 | HARC * | 354 | 1996 | Orange | 4–5 months |
| ‘Hawassa-83’ | 3 | Farmers field in Wolayta zone | 366 | 1990 | White | 5–6 months |
| ‘Kulfo’ | 3 | HARC | 270 | 2005 | Orange | 4–5 months |
| ‘Tola’ | 3 | BARC ** | 322 | 2012 | White | 3–4 months |
| Variety | Original Source Plant | I. setosa upon Graft-Inoculation with the Source Plant at 30 Days Post-Inoculation | ||||
|---|---|---|---|---|---|---|
| Symptoms | Viruses Detected by ELISA | Viruses Detected by RT–PCR | Symptoms | Viruses Detected by ELISA | Viruses Detected by RT–PCR | |
| ‘Hawassa-83’ A | SL | ND | SPFMV | CRS, LD, FM, LC, VC | SPFMV | SPFMV |
| ‘Hawassa-83’ B | SL | ND | SPFMV | CRS, LD, F, LC, VC | SPFMV | SPFMV |
| ‘Hawassa-83’ C | SL | ND | ND | CRS, LD, FM, LC, VC | SPFMV | SPFMV |
| ‘Berkume’ A | SL | ND | ND | SL | - | - |
| ‘Berkume’ B | SL | ND | ND | SL | - | - |
| ‘Berkume’ C | SL | ND | ND | SL | - | - |
| ‘Tola’ A | SL | ND | ND | CRS | SPFMV | SPFMV |
| ‘Tola’ B | SL | ND | SPFMV | CRS | SPFMV | SPFMV |
| ‘Tola’ C | SL | ND | ND | CRS | SPFMV | SPFMV |
| ‘Kulfo’ A | SL | ND | ND | CRS | SPFMV | SPFMV |
| ‘Kulfo’ B | SL | ND | SPFMV | CRS | SPFMV | SPFMV |
| ‘Kulfo’ C | SL | ND | SPFMV | CRS | SPFMV | SPFMV |
| ‘Guntute’ A | SL | ND | ND | RCS, VC, LC, FM | SPFMV | SPFMV |
| ‘Guntute’ B | CRS, S, VC | SPFMV & SPCSV | SPFMV & SPCSV | CRP, CY, LD, VC | SPFMV, SPCSV | SPFMV, SPCSV and SPVC |
| ‘Guntute’ C | SL | ND | ND | CRP, VC, LC, FM | SPFMV | SPFMV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buko, D.H.; Spetz, C.; Hvoslef-Eide, T. Detection of Viruses and Elimination of Sweet Potato Feathery Mottle Virus in High-Yielding Varieties of Sweet Potato (Ipomoea batatas) from Ethiopia. Agriculture 2024, 14, 1929. https://doi.org/10.3390/agriculture14111929
Buko DH, Spetz C, Hvoslef-Eide T. Detection of Viruses and Elimination of Sweet Potato Feathery Mottle Virus in High-Yielding Varieties of Sweet Potato (Ipomoea batatas) from Ethiopia. Agriculture. 2024; 14(11):1929. https://doi.org/10.3390/agriculture14111929
Chicago/Turabian StyleBuko, Dereje Haile, Carl Spetz, and Trine (A.K.) Hvoslef-Eide. 2024. "Detection of Viruses and Elimination of Sweet Potato Feathery Mottle Virus in High-Yielding Varieties of Sweet Potato (Ipomoea batatas) from Ethiopia" Agriculture 14, no. 11: 1929. https://doi.org/10.3390/agriculture14111929
APA StyleBuko, D. H., Spetz, C., & Hvoslef-Eide, T. (2024). Detection of Viruses and Elimination of Sweet Potato Feathery Mottle Virus in High-Yielding Varieties of Sweet Potato (Ipomoea batatas) from Ethiopia. Agriculture, 14(11), 1929. https://doi.org/10.3390/agriculture14111929





