The Effect of Organic Materials with Different Degrees of Decomposition on the Content of Nickel in the Lettuce Leaves Cultivated in Mineral Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Analyses of Substrates
2.2. Analysis of Nickel Content in Lettuce Leaves
2.3. Nickel Toxicity Index
- -
- Ti < 1—yield inhibition;
- -
- Ti = 1—no effect of increased nickel content in the substrate on yields;
- -
- Ti > 1—positive effect of nickel in the substrate on yielding.
2.4. Statistical Analysis
3. Results
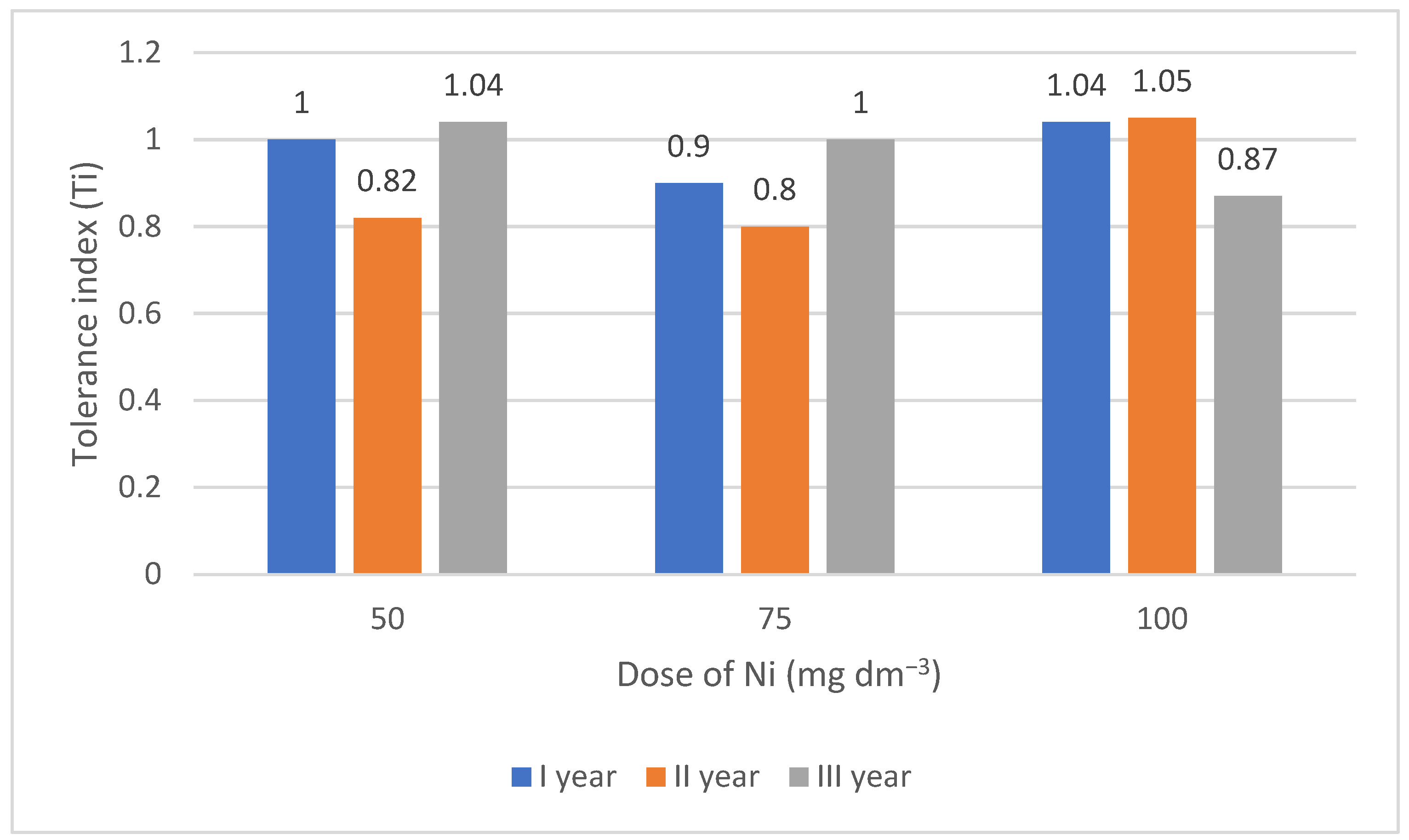
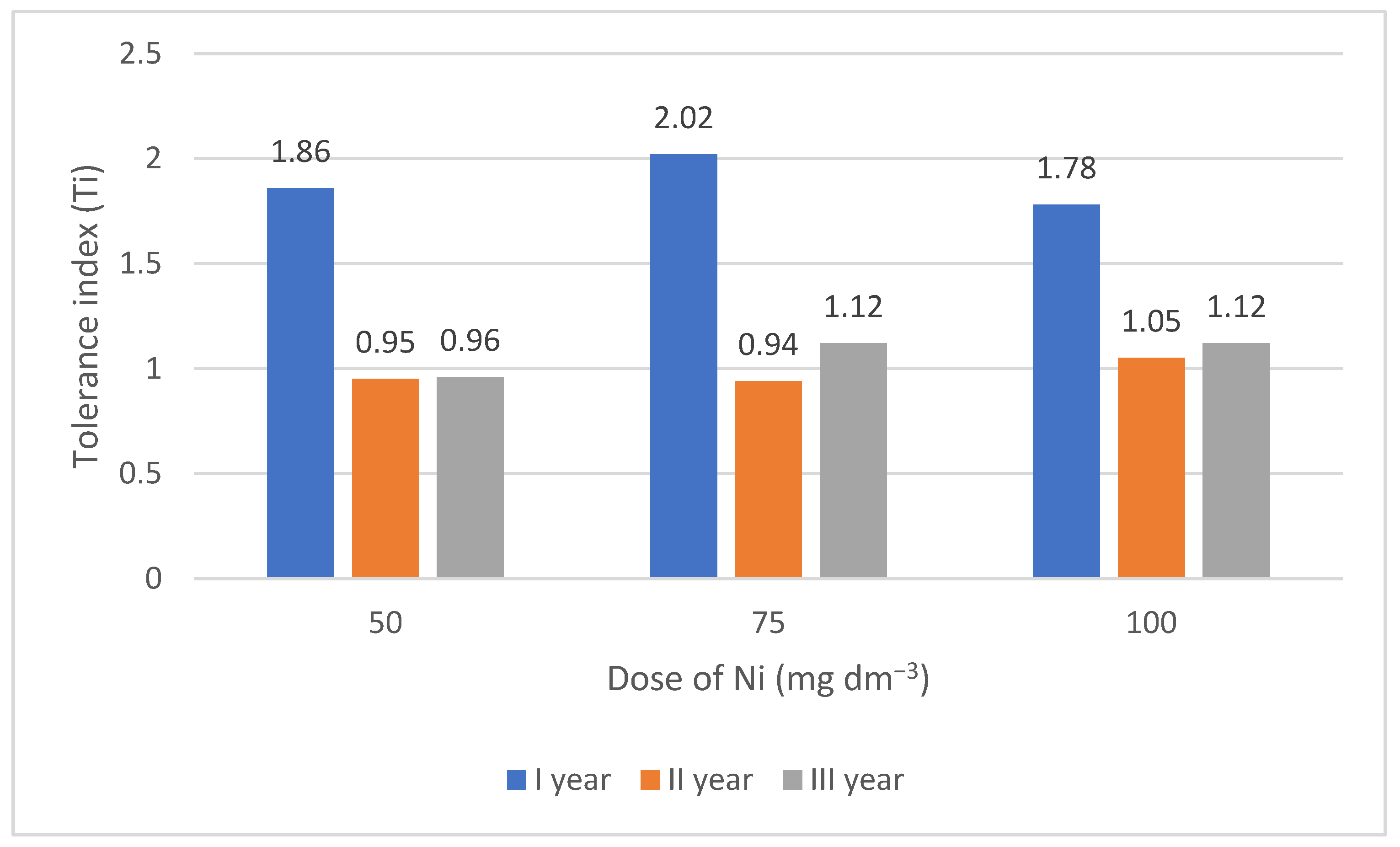
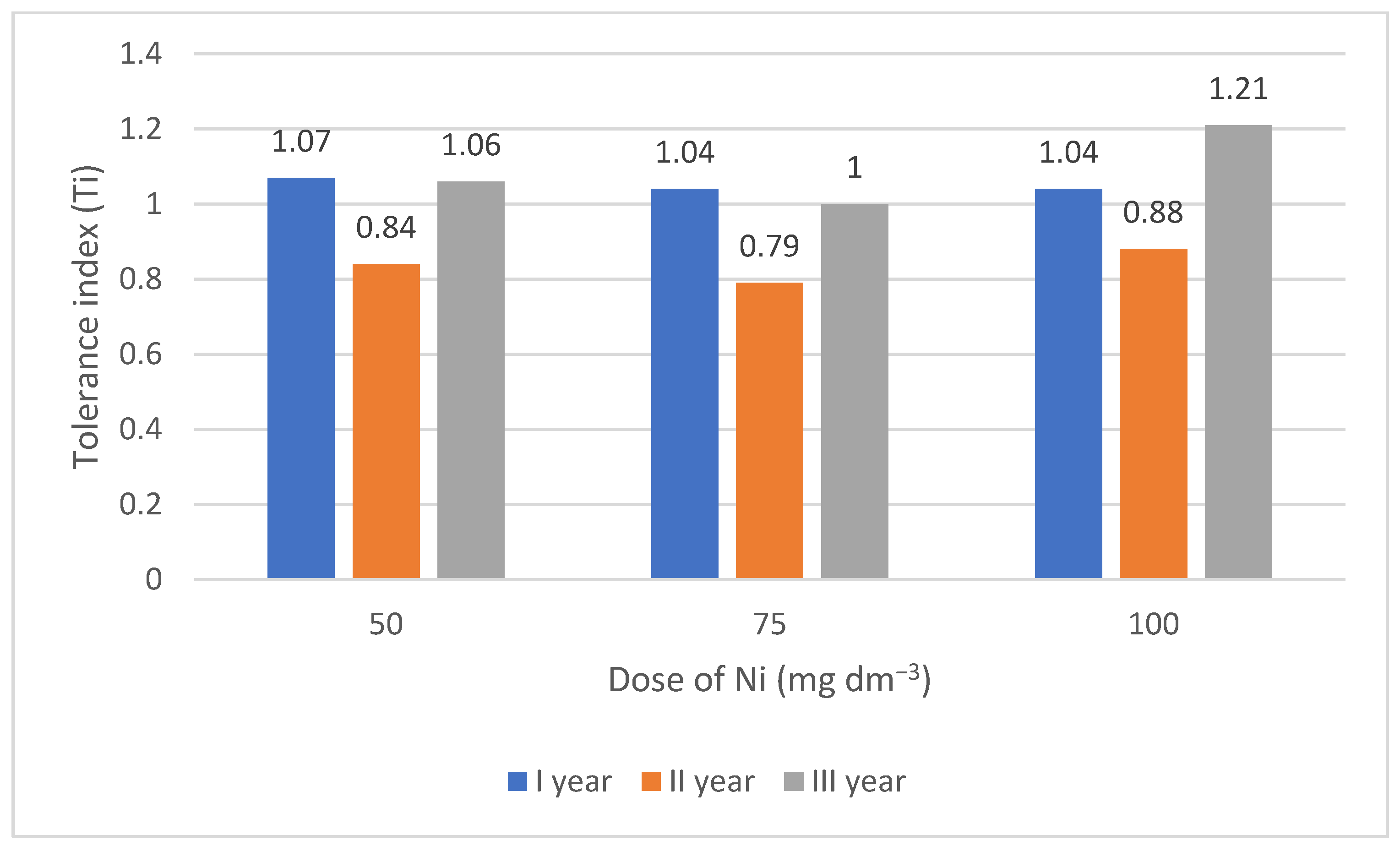
3.1. Tolerance Index (Ti) of Lettuce to Increasing Doses of Nickel
3.2. Nickel Content in Lettuce Leaves
3.3. Nickel Content (mg dm−3) in Substrates After Lettuce Cultivation
3.4. Organic Carbon Content in Substrates After Lettuce Cultivation
3.5. Sorption Capacity (T) in Substrates After Lettuce Cultivation
4. Discussion
5. Conclusions
- The type of organic material introduced into the mineral soil as a source of soil organic matter is important for the nickel content of lettuce leaves.
- The introduction of organic matter into mineral soil can reduce, as well as increase, nickel content in lettuce leaves.
- Brown coal introduced at 30% by volume into mineral soil reduces the nickel content of lettuce leaves, increases the organic carbon content of the soil, and affects the higher sorption capacity of the substrates.
- High peat introduced at 30% by volume to mineral soil increases the nickel content of lettuce leaves, increases the organic carbon content of the substrate, and affects the higher sorption capacity of the substrates.
- Wheat straw introduced at 30% by volume into the mineral soil increases the nickel content of lettuce leaves and does not increase the organic carbon content or sorption capacity of the substrates.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A micronutrient essential for higher plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, UK, 1995; pp. 1–889. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments, 2nd ed.; Springer: New York, NY, USA, 2001; pp. 1–867. [Google Scholar]
- Gillette, B. Nickel named “Allergen of the Year”. ACDS adds to list of substances warranting more attention. Dermatol. Times 2008, 4, 15–16. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679–700. [Google Scholar] [CrossRef] [PubMed]
- Bosiacki, M.; Kleiber, T.; Markiewicz, B. Continuous and Induced Phytoextraction-Plant-Based Methods of Remove Heavy Metals from Contaminated Soil. In Environmental Risk Assessment of Soil Contamination; Hernandez-Soriano, M.C., Ed.; InTech: Rijeka, Croatia, 2014; Volume 20, pp. 575–612. ISBN 978-953-51-1235-8. [Google Scholar]
- Murray, H.; Pinchin, T.A.; Macfie, S.M. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J. Soils Sediments 2011, 11, 815–829. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, S.G.; Zhu, L.N.; Liu, H.T.; Yang, J.K.; Hua, D.L. Effects of different amendments on fractions and uptake by winter wheat in slightly alkaline soil contaminated by cadmium and nickel. Huan Jing Ke Xue Huanjing Kexue 2020, 41, 460–468. [Google Scholar]
- Naveed, M.; Ditta, A.; Ahmad, M.; Mustafa, A.; Ahmad, Z.; Conde-Cid, M.; Tahir, S.; Shah, S.A.A.; Abrar, M.M.; Fahad, S. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ. Sci. Pollut. Res. 2021, 28, 45629–45645. [Google Scholar] [CrossRef]
- Bosiacki, M.; Tyksinski, W. Effect of organic substance with diversified decomposition degree on cadmium and lead uptake by lettuce (Lactuva sativa L.). Rocz. Akad. Rol. W Pozn. CCCLVI Ogrod. 2004, 37, 19–28. [Google Scholar]
- Bosiacki, M.; Tyksiński, W. Dependence between the content of organic carbon and the content of cadmium and lead in horticultural substrates. Acta Agrophysica 2006, 7, 517–526. [Google Scholar]
- Janssen, B.H. A simple model for calculating decomposition and accumulation of ‘young’ organic matter. Plant Soil 1984, 76, 297–304. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; van Riemsdijk, W.H.; Koopal, L.K.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.; Naidu, R. Speciation and phytoavailability of cadmium in selected surface soils of South Australia. Soil Res. 2000, 38, 991–1004. [Google Scholar] [CrossRef]
- Mocek, A.; Drzymała, S. The Genesis, Analysis, Classification of Soils; Publishing Company by Poznan University of Life Sciences: Poznan, Poland, 2010. [Google Scholar]
- Kozik, E.; Golcz, A. Plant nutrient. In Research Methods in Plant Sciences vol. 3. Soil Sickness; Narwal, S.S., Politycka, B., Wu, Z., Sampietro, D.A., Eds.; Studium Press LLC.: Huston, TX, USA, 2011; pp. 21–41. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1987, 42, 421–428. [Google Scholar] [CrossRef]
- Golcz, A. Soil salinity and acidity. In Research Methods in Plant Sciences vol. 3. Soil Sickness; Narwal, S.S., Politycka, B., Wu, Z., Sampietro, D.A., Eds.; Studium Press LLC.: Huston, TX, USA, 2011; pp. 43–53. [Google Scholar]
- Lityński, T.; Jurkowska, H.; Gorlach, E. Analiza Chemiczno-Rolnicza; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1976. [Google Scholar]
- Bosiacki, M.; Roszyk, J. The compering methods of mineralization of plant material on the content of heavy metals. Apar. Badaw. I Dydakt. 2010, 4, 37–41. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Bichemistry Trace Elements II; PWN: Warszawa, Poland, 1999. [Google Scholar]
- Senesi, N. Sustainable use of organic wastes in agricultural soils. In Proceedings of the II Simpósio internacional sobre Gerenciamento de Resíduos Agropecuários Agroindustriais—II SiGERA, Parana, Brazil, 15–17 March 2011. Volume I—Palestras. 29. [Google Scholar]
- Jabłonska-Ceglarek, R.; Zaniewicz-Bajkowska, A.; Franczuk, J. Reduction of cadmium and lead uptake from soil by vegetables (White headed cabbage, Red beet) due to organic fertilization with second crops, farmyard manure and winter rye straw. Zesz. Probl. PostępÓW Nauk. Rol. 1999, 466, 365–371. [Google Scholar]
- Zaniewicz-Bajkowska, A.; Rosa, R.; Franczuk, J.; Kosterna, E. Direct and secondary effect of liming and organic fertilization on cadmium content in soil and in vegetables. Plant Soil Environ. 2007, 53, 473. [Google Scholar] [CrossRef]
- Mercik, S.; Kubik, I. Chelating of heavy metals by the humus acids and influence of peat on the uptake of Zn, Pb and Cd by plants. Zesz. Probl. PostępÓW Nauk. Rol. 1995, 422, 19–30. [Google Scholar]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Esposito, E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef]
- Rupa, T.R.; Sinivas, R.C.; Subha, R.A.; Singh, M. Effect of farmyard manure and phosphorus on Zn transformation and phytoavailability in two altisol of India. Bioresour. Technol. 2003, 87, 279–288. [Google Scholar] [CrossRef]
- Alashty, S.R.; Bahmanyar, M.A.; Sepanlou, M.G. Change of pH, organic carbon (OC), electrical conductivity (EC), nickel (Ni) and chrome (Cr) in soil and concentration of Ni and Cr in radish and lettuce plants as influenced by three year application of municipal compost. Afr. J. Agric. Res. 2011, 6, 3740–3746. [Google Scholar]
- Tejada, M.; Moreno, J.L.; Hernández, M.T.; García, C. Soil amendments with organic wastes reduce the toxicity of nickel to soil enzyme activities. Eur. J. Soil Biol. 2008, 44, 129–140. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1994; p. 496. [Google Scholar]
- Plaza, C.; Hernandez, D.; Fernandez, J.M.; Polo, A. Long-term effects of amendment with liquid swine manure on proton binding behavior of soil humic substances. Chemosphere 2006, 65, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Lobartini, J.C.; Tan, K.H.; Pape, C. The nature of humic acid-apatite interaction products and their availability to plant growth. Commun. Soil Sci. Plant Anal. 1994, 25, 2355–2369. [Google Scholar] [CrossRef]
- Ociepa, E. The Effect of Fertilization for Changing of Solubility of Zinc and Nickel in the Soil and Assimilating this Metals for Maize and Virginia Fanpetals (Sida Hermaphrodita). Inżynieria I Ochr. Sr. 2011, 14, 41–48. [Google Scholar]
- Kuziemska, B.; Kalembasa, D.; Kalembasa, S. Effect of liming and organic materials on content of selected metals in of cocksfoot grown in soil contaminated with nickel. Acta Agrophysica 2014, 21, 293–304. [Google Scholar]
- Rehman, M.Z.U.; Rizwan, M.; Ali, S.; Fatima, N.; Yousaf, B.; Naeem, A.; Sabir, M.; Ahmad, H.R.; Ok, Y.S. Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol. Environ. Saf. 2016, 133, 218–225. [Google Scholar] [CrossRef]
- Balaguer, J.; Almendro, M.B.; Gomez, I.; Navarro Pedreño, J.; Mataix, J. Tomato growth and yield affected by nickel presented in the nutrient solution. Acta Hortic. 1993, 458, 269–272. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Effect of nickel and grafting combination on yield, fruit quality, antioxidative enzyme activities, lipid peroxidation and mineral composition of tomato. J. Plant Nutr. Soil Sci. 2015, 178, 848–860. [Google Scholar] [CrossRef]
- Palacios, G.; Carbonell-Barrachina, A.; Gomez, I.; Mataix, J. The influence of organic amendment and nickel pollution on tomato fruit yield and quality. J. Environ. Sci. Health Part B 1999, 34, 133–150. [Google Scholar] [CrossRef]
- Poulik, Z. Influence of nickel contaminated soils on lettuce and tomatoes. Sci. Hortic. 1999, 81, 243–250. [Google Scholar] [CrossRef]
- Matraszek, R.; Szymanska, M.; Wróblewska, M. Effect of nickel on yielding and mineral composition of the selected vegetables. Acta Sci. Polonorum. Tech. Agrar. 2002, 1, 3–22. [Google Scholar]

| mg dm−3 | Mineral Soil (Loamy Sand) | ls + High Peat | ls + Brown Coal | ls + Wheat Straw |
|---|---|---|---|---|
| N-NH4 | 7 | 11 | traces | 2 |
| N-NO3 | 14 | 4 | 5 | 9 |
| P | 16 | 34 | 28 | 33 |
| K | 52 | 82 | 78 | 89 |
| Ca | 6255 | 6188 | 6311 | 6198 |
| Mg | 98 | 52 | 178 | 87 |
| Na | 14 | 10 | 28 | 16 |
| S-SO4 | 12 | 8 | 17 | 15 |
| Cl | 7 | 14 | 9 | 8 |
| Fe | 34.9 | 50.7 | 48.6 | 38.7 |
| Mn | 8.2 | 11.5 | 9.1 | 8.8 |
| Cu | 2.5 | 3.4 | 2.8 | 2.6 |
| Zn | 11.1 | 6.7 | 12.1 | 10.1 |
| Ni | 1.32 | 1.47 | 1.37 | 1.28 |
| EC (mS cm−1) | 0.193 | 0.190 | 0.211 | 0.197 |
| pH (H2O) | 7.15 | 7.03 | 7.20 | 7.09 |
| Metal | Year of Research | Reference Material | |||
|---|---|---|---|---|---|
| Certified Content | +/− | Content Obtained | Difference | ||
| Ni | I year | 2.47 | 0.07 | 2.34 | −0.13 |
| II year | 2.21 | −0.26 | |||
| III year | 2.28 | −0.19 | |||
| Substrate | Dose of Ni (mg dm−3) | Year of Research | Mean B·C | Mean B | |||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| mineral soil (ls) | 0 | 3.28 *a | 5.69 b | 5.15 b | 4.71 a | 11.04 b | |||
| 50 | 8.89 cd | 13.18 jk | 10.26 efg | 10.78 c | |||||
| 75 | 11.21 f–i | 15.85 m | 12.04 hij | 13.03 d | |||||
| 100 | 17.66 op | 15.80 m | 13.43 jk | 15.63 g | |||||
| Mean B·A | 10.26 bc | 12.63 f | 10.22 b | ||||||
| ls + high peat | 0 | 2.97 a | 5.58 b | 4.95 b | 4.50 a | 11.61 c | |||
| 50 | 9.90 def | 13.24 jk | 10.42 efg | 11.19 c | |||||
| 75 | 12.17 hij | 16.65 mno | 12.52 ij | 13.78 e | |||||
| 100 | 16.81 mno | 18.28 pq | 15.83 m | 16.97 h | |||||
| Mean B·A | 10.46 bc | 13.44 g | 10.93 dc | ||||||
| ls + brown coal | 0 | 2.80 a | 5.68 b | 4.93 b | 4.47 a | 10.41 a | |||
| 50 | 9.53 cde | 10.52 efg | 9.54 cde | 9.86 b | |||||
| 75 | 14.39 kl | 13.03 jk | 10.97 fgh | 12.79 d | |||||
| 100 | 15.73 m | 15.48 lm | 12.36 ij | 14.52 f | |||||
| Mean B·A | 10.61 bcd | 11.18 de | 9.45 a | ||||||
| ls + wheat straw | 0 | 2.70 a | 5.50 b | 4.82 b | 4.34 a | 12.04 d | |||
| 50 | 8.53 c | 14.04 k | 11.40 ghi | 11.32 c | |||||
| 75 | 13.93 k | 17.60 nop | 14.14 k | 15.23 fg | |||||
| 100 | 16.27 mn | 19.24 q | 16.31 mno | 17.27 h | |||||
| Mean B·A | 10.36 bc | 14.09 h | 11.67 e | ||||||
| Mean A | 10.42 a | 12.83 b | 10.56 a | ||||||
| Mean C | dose 0 Ni (I–III) | dose 50 Ni (I–III) | dose 75 Ni (I–III) | dose 100 Ni (I–III) | |||||
| 4.50 a | 10.79 b | 13.71 c | 16.62 d | ||||||
| Mean A·C | year | dose 0 Ni | dose 50 Ni | dose 75 Ni | dose 100 Ni | ||||
| I | 2.94 a | 9.21 d | 12.93 f | 16.62 i | |||||
| II | 5.61 c | 12.75 f | 15.78 h | 17.20 i | |||||
| III | 4.96 b | 10.40 e | 12.42 f | 14.48 g | |||||
| Substrate | Dose of Ni (mg dm−3) | Year of Research | Mean B·C | Mean B | |||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| mineral soil (ls) | 0 | 0.55 *a | 0.63 a | 0.61 a | 0.60 a | 8.23 a | |||
| 50 | 6.45 bc | 6.42 bc | 7.56 c | 6.81 b | |||||
| 75 | 10.16 de | 10.77 de | 11.34 ef | 10.76 d | |||||
| 100 | 14.84 hi | 15.06 hi | 14.36 h | 14.75 e | |||||
| Mean B·A | 8.00 a | 8.22 a | 8.47 a | ||||||
| ls + high peat | 0 | 0.56 a | 0.89 a | 0.52 a | 0.65 a | 12.80 b | |||
| 50 | 10.14 de | 12.52 fg | 9.36 d | 10.67 d | |||||
| 75 | 19.59 lm | 18.43 kl | 12.47 fg | 16.83 f | |||||
| 100 | 25.08 p | 22.27 o | 21.79 no | 23.05 h | |||||
| Mean B·A | 13.84 cd | 13.53 c | 11.04 b | ||||||
| ls + brown coal | 0 | 0.65 a | 0.91 a | 0.57 a | 0.71 a | 12.65 b | |||
| 50 | 9.39 d | 9.47 d | 6.04 b | 8.30 c | |||||
| 75 | 17.40 k | 15.92 ij | 11.09 e | 14.80 e | |||||
| 100 | 35.52 s | 28.94 q | 15.89 ij | 26.78 j | |||||
| Mean B·A | 15.74 e | 13.81 cd | 8.40 a | ||||||
| ls + wheat straw | 0 | 0.49 a | 0.99 a | 0.64 a | 0.71 a | 13.95 c | |||
| 50 | 6.91 bc | 13.71 gh | 12.80 g | 11.14 d | |||||
| 75 | 17.24 jk | 20.69 mn | 19.41 lm | 19.11 g | |||||
| 100 | 21.31 no | 22.42 o | 30.83 r | 24.85 i | |||||
| Mean B·A | 11.49 b | 14.45 d | 15.92 e | ||||||
| Mean A | 12.27 b | 12.50 b | 10.96 a | ||||||
| Mean C | dose 0 Ni (I–III) | dose 50 Ni (I–III) | dose 75 Ni (I–III) | dose 100 Ni (I–III) | |||||
| 0.67 a | 9.23 b | 15.37 c | 22.36 d | ||||||
| Mean A·C | year | dose 0 Ni | dose 50 Ni | dose 75 Ni | dose 100 Ni | ||||
| I | 0.56 a | 8.22 b | 16.10 f | 24.19 i | |||||
| II | 0.85 a | 10.53 d | 16.45 f | 22.17 h | |||||
| III | 0.59 a | 8.94 c | 13.58 e | 20.72 g | |||||
| Substrate | Dose of Ni (mg dm−3) | Year of Research | Mean B·C | Mean B | |||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| mineral soil (ls) | 0 | 1.01 *ab | 1.14 b | 1.02 ab | 1.06 a | 1.01 a | |||
| 50 | 0.96 a | 1.05 ab | 0.95 a | 0.99 a | |||||
| 75 | 1.02 ab | 1.07 ab | 0.93 a | 1.01 a | |||||
| 100 | 1.02 ab | 1.04 ab | 0.93 a | 1.00 a | |||||
| Mean B·A | 1.00 ab | 1.07 b | 0.96 a | ||||||
| ls + high peat | 0 | 2.06 cde | 2.15 de | 2.06 cde | 2.09 bc | 2.07 c | |||
| 50 | 2.04 cde | 2.04 cde | 2.04 cde | 2.04 bc | |||||
| 75 | 2.07 cde | 2.16 e | 2.06 cde | 2.10 c | |||||
| 100 | 2.03 cde | 2.15 de | 2.00 cde | 2.06 bc | |||||
| Mean B·A | 2.05 c | 2.12 d | 2.04 c | ||||||
| ls + brown coal | 0 | 2.01 cde | 2.04 cde | 2.04 cde | 2.03 bc | 2.01 b | |||
| 50 | 2.01 cde | 2.01 cde | 2.04 cde | 2.02 bc | |||||
| 75 | 1.95 c | 1.98 cd | 2.06 cde | 2.00 b | |||||
| 100 | 2.03 cde | 1.97 c | 2.01 cde | 2.00 bc | |||||
| Mean B·A | 2.00 c | 2.00 c | 2.04 c | ||||||
| ls + wheat straw | 0 | 0.99 ab | 1.08 ab | 1.02 ab | 1.03 a | 1.03 a | |||
| 50 | 0.99 ab | 1.05 ab | 1.02 ab | 1.02 a | |||||
| 75 | 1.05 ab | 1.05 ab | 1.04 ab | 1.05 a | |||||
| 100 | 1.05 ab | 1.08 ab | 0.98 ab | 1.04 a | |||||
| Mean B·A | 1.02 ab | 1.07 b | 1.01 ab | ||||||
| Mean A | 1.52 a | 1.56 b | 1.51 a | ||||||
| Mean C | dose 0 Ni (I–III) | dose 50 Ni (I–III) | dose 75 Ni (I–III) | dose 100 Ni (I–III) | |||||
| 1.55 a | 1.52 a | 1.54 a | 1.52 a | ||||||
| Mean A·C | year | dose 0 Ni | dose 50 Ni | dose 75 Ni | dose 100 Ni | ||||
| I | 1.52 ab | 1.50 a | 1.52 ab | 1.53 ab | |||||
| II | 1.60 b | 1.54 ab | 1.56 ab | 1.56 ab | |||||
| III | 1.53 ab | 1.51 a | 1.52 ab | 1.48 a | |||||
| Substrate | Dose of Ni (mg dm−3) | Year of Research | Mean B·C | Mean B | |||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| mineral soil (ls) | 0 | 49.79 *b–i | 49.71 a–g | 50.00 d–j | 49.83 ab | 49.74 a | |||
| 50 | 49.75 b–h | 49.46 ab | 49.85 b–i | 49.69 a | |||||
| 75 | 49.75 b–h | 49.64 a–e | 49.90 c–i | 49.76 a | |||||
| 100 | 49.75 b–h | 49.35 a | 49.90 c–i | 49.67 a | |||||
| Mean B·A | 49.76 bc | 49.54 a | 49.91 cd | ||||||
| ls + high peat | 0 | 50.18 i–l | 49.95 c–i | 50.05 f–j | 50.06 c | 50.02 b | |||
| 50 | 50.08 g–k | 49.99 d–j | 49.90 c–i | 49.99 bc | |||||
| 75 | 50.35 j–m | 49.65 a–f | 50.14 h–k | 50.05 bc | |||||
| 100 | 50.03 e–j | 49.98 c–j | 49.98 c–j | 49.99 bc | |||||
| Mean B·A | 50.16 e | 49.89 cd | 50.02 de | ||||||
| ls + brown coal | 0 | 51.65 p | 50.65 m | 50.53 lm | 50.94 e | 50.83 c | |||
| 50 | 51.55 op | 50.65 m | 50.55 lm | 50.92 e | |||||
| 75 | 51.25 no | 50.55 lm | 50.45 klm | 50.75 de | |||||
| 100 | 51.18 n | 50.58 m | 50.35 j–m | 50.70 d | |||||
| Mean B·A | 51.41 g | 50.61 f | 50.47 f | ||||||
| ls + wheat straw | 0 | 49.94 c–i | 49.76 b–h | 49.91 c–i | 49.87 abc | 49.77 a | |||
| 50 | 49.85 b–i | 49.58 abc | 49.85 b–i | 49.76 a | |||||
| 75 | 49.83 b–i | 49.60 a–d | 49.75 b–h | 49.73 a | |||||
| 100 | 49.80 b–i | 49.65 a–f | 49.75 b–h | 49.73 a | |||||
| Mean B·A | 49.85 cd | 49.65 ab | 49.82 bc | ||||||
| Mean A | 50.29 c | 49.92 a | 50.05 b | ||||||
| Mean C | dose 0 Ni (I–III) | dose 50 Ni (I–III) | dose 75 Ni (I–III) | dose 100 Ni (I–III) | |||||
| 50.18 b | 50.09 ab | 50.07 a | 50.02 a | ||||||
| Mean A·C | year | dose 0 Ni | dose 50 Ni | dose 75 Ni | dose 100 Ni | ||||
| I | 50.39 g | 50.31 fg | 50.29 efg | 50.19 def | |||||
| II | 50.02 a–d | 49.92 ab | 49.86 a | 49.89 ab | |||||
| III | 50.12 cde | 50.04 a–d | 50.06 bcd | 49.99 abc | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiak, K.; Bosiacki, M. The Effect of Organic Materials with Different Degrees of Decomposition on the Content of Nickel in the Lettuce Leaves Cultivated in Mineral Soil. Agriculture 2024, 14, 1970. https://doi.org/10.3390/agriculture14111970
Misiak K, Bosiacki M. The Effect of Organic Materials with Different Degrees of Decomposition on the Content of Nickel in the Lettuce Leaves Cultivated in Mineral Soil. Agriculture. 2024; 14(11):1970. https://doi.org/10.3390/agriculture14111970
Chicago/Turabian StyleMisiak, Kamil, and Maciej Bosiacki. 2024. "The Effect of Organic Materials with Different Degrees of Decomposition on the Content of Nickel in the Lettuce Leaves Cultivated in Mineral Soil" Agriculture 14, no. 11: 1970. https://doi.org/10.3390/agriculture14111970
APA StyleMisiak, K., & Bosiacki, M. (2024). The Effect of Organic Materials with Different Degrees of Decomposition on the Content of Nickel in the Lettuce Leaves Cultivated in Mineral Soil. Agriculture, 14(11), 1970. https://doi.org/10.3390/agriculture14111970








