Effect of Melatonin on the Production Performance, Blood Biochemical Parameters, Nutrient Digestibility, and Gastrointestinal Microbiome of Liaoning Cashmere Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Melatonin Administration
2.3. Apparent Digestibility of Nutrients
2.4. Blood Sampling and Biochemical Analyses
2.5. Cashmere Sample Collection and Determination
2.6. Gastrointestinal Microbiota Analysis
2.7. Statistical Analyses
3. Results
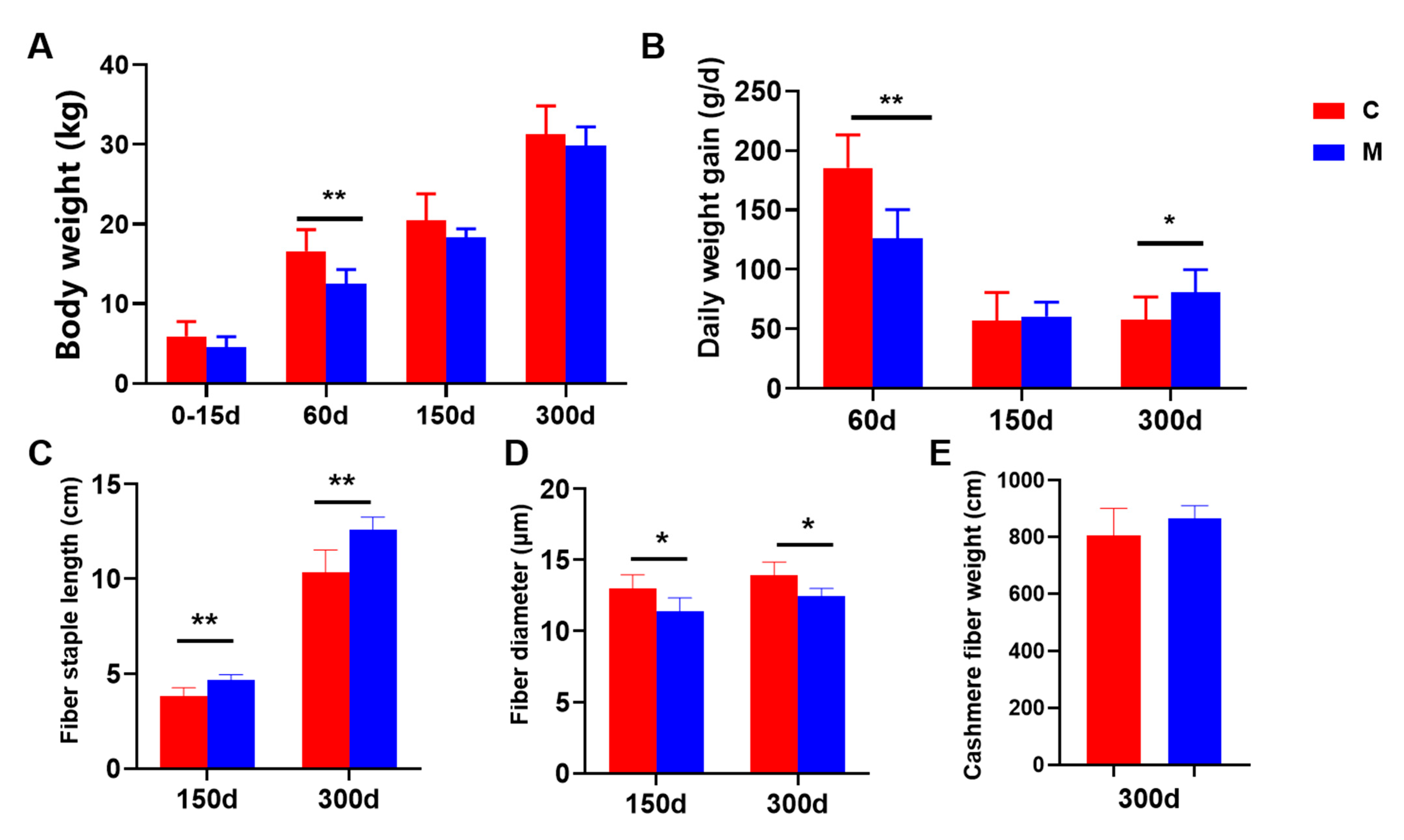
3.1. Liaoning Cashmere Goats’ Production Performance
3.2. Apparent Nutritional Digestion
3.3. Blood Biochemical Parameters
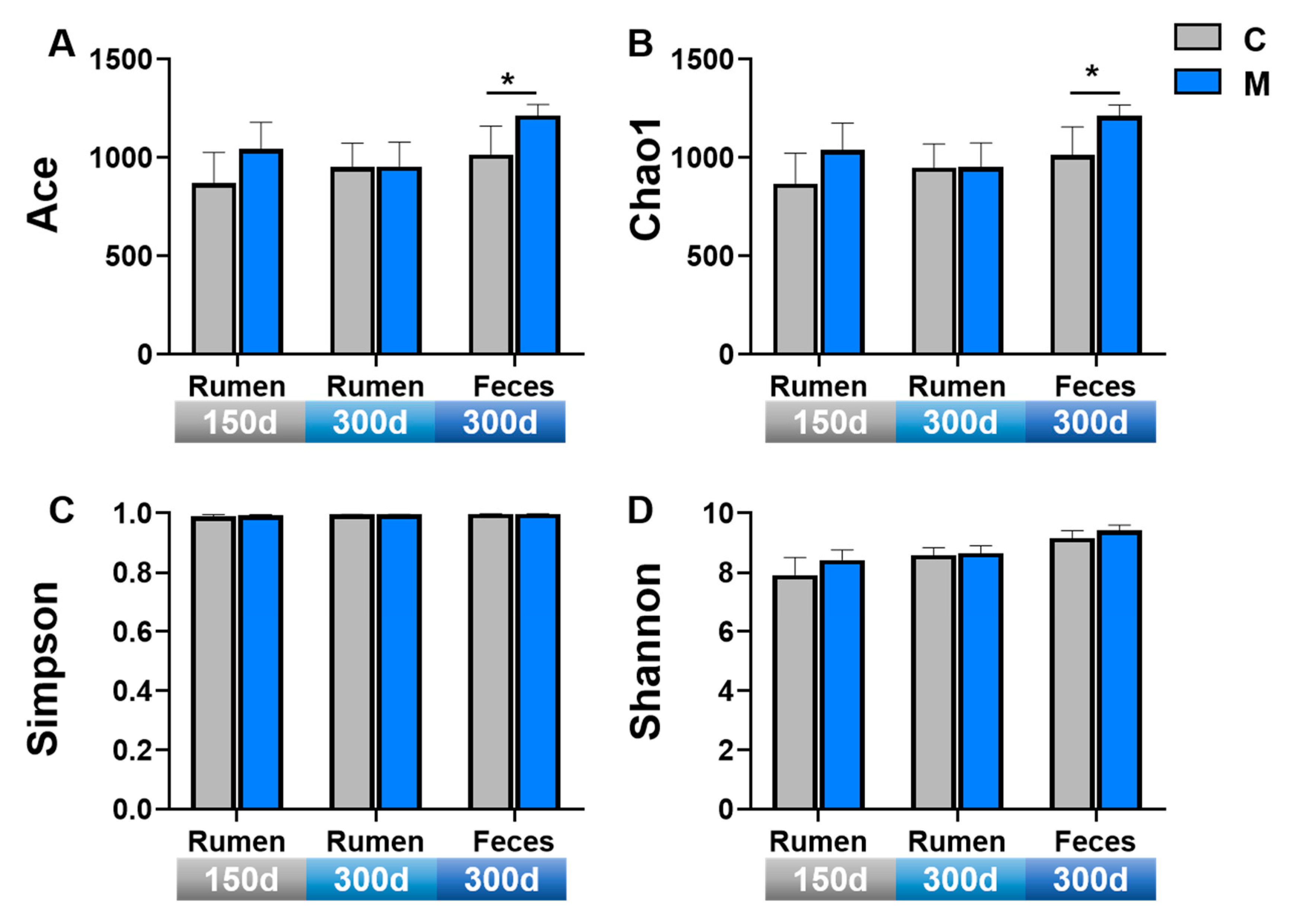
3.4. Gastrointestinal Microbiota Diversity
4. Discussion
4.1. Effect of Melatonin Implantation on Production Performance of Liaoning Cashmere Goats
4.2. Effect of Melatonin Implantation on Liaoning Cashmere Goats’ Apparent Nutritional Digestion
4.3. Effect of Melatonin Implantation on Blood Biochemical Parameters in Liaoning Cashmere Goats
4.4. Effect of Melatonin Implantation on Gastrointestinal Microbiome Diversity in Liaoning Cashmere Goats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, C.; Xu, J.; Sun, C.; Jia, Z.; Zhang, W. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2015, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Xu, J.H.; Ren, Q.C.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef]
- Wang, W.; Ye, L.; Dou, X.; Liu, H.; Han, D. Effects of Rumen-Protected Methionine Supplementation on Growth Performance, Nutrient Digestion, Nitrogen Utilisation and Plasma Amino Acid Profiles of Liaoning Cashmere Goats. Animals 2023, 13, 2995. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.J.; Lemley, C.O.; Vonnahme, K.A.; Swanson, K.C. Effects of nutrient restriction and melatonin supplementation from mid-to-late gestation on maternal and fetal small intestinal carbohydrase activities in sheep. Domest. Anim. Endocrinol. 2021, 74, 106555. [Google Scholar] [CrossRef] [PubMed]
- Lemley, C.O.; Camacho, L.E.; Meyer, A.M.; Kapphahn, M.; Caton, J.S.; Vonnahme, K.A. Dietary melatonin supplementation alters uteroplacental amino acid flux during intrauterine growth restriction in ewes. Anim. Int. J. Anim. Biosci. 2013, 7, 1500–1507. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, T.; Wu, Y.; Xu, Y.; Du, L.; Wang, T.; Luo, Y.; Wang, Y.; Li, Z.; Xuan, Z.; et al. The multi-kingdom microbiome of the goat gastrointestinal tract. Microbiome 2023, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Zhuang, Y.; Cui, K.; Bi, Y.; Zhang, N. Metagenomics reveals the temporal dynamics of the rumen resistome and microbiome in goat kids. Microbiome 2024, 12, 14. [Google Scholar] [CrossRef]
- Ma, L.; Tao, S.; Song, T.; Lyu, W.; Li, Y.; Wang, W.; Shen, Q.; Ni, Y.; Zhu, J.; Zhao, J.; et al. Clostridium butyricum and carbohydrate active enzymes contribute to the reduced fat deposition in pigs. iMeta 2024, 3, e160. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nature reviews. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar]
- Yu, Y.; Yang, J.; Zheng, L.Y.; Sheng, Q.; Li, C.Y.; Wang, M.; Zhang, X.Y.; McMinn, A.; Zhang, Y.Z.; Song, X.Y.; et al. Diversity of D-Amino Acid Utilizing Bacteria from Kongsfjorden, Arctic and the Metabolic Pathways for Seven D-Amino Acids. Front. Microbiol. 2020, 10, 2983. [Google Scholar] [CrossRef]
- Torrallardona, D.; Harris, C.I.; Fuller, M.F. Lysine synthesized by the gastrointestinal microflora of pigs is absorbed, mostly in the small intestine. American journal of physiology. Endocrinol. Metab. 2003, 284, E1177–E1180. [Google Scholar]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857s–1864s. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liang, J.; Wang, T.; Zhao, R.; Ma, Y.; Gao, Y.; Zhao, S.; Chen, G.; Liu, B. Cysteamine-supplemented diet for cashmere goats: A potential strategy to inhibit rumen biohydrogenation and enhance plasma antioxidant capacity. Front. Vet. Sci. 2022, 9, 997091. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M.; Grota, L.J. Immunohistological localization of melatonin in the rat digestive system. Experientia 1977, 33, 662–663. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Feng, J.; Wan, B.; Tu, Q.; Cai, W.; Jin, F.; Tang, G.; Rodrigues, L.R.; Zhang, X.; et al. Modulation of chronic obstructive pulmonary disease progression by antioxidant metabolites from Pediococcus pentosaceus: Enhancing gut probiotics abundance and the tryptophan-melatonin pathway. Gut Microbes 2024, 16, 2320283. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Zhang, D.; Ma, K.; Gong, Y.; Luo, X.; Liu, J.; Cui, S. Intestinal melatonin levels and gut microbiota homeostasis are independent of the pineal gland in pigs. Front. Microbiol. 2024, 15, 1352586. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Zhang, Y.; Zhang, S.; Cui, Z.; Liu, W.; Li, L.; Xia, J.; Zou, Y.; Qi, Z. ASMT determines gut microbiota and increases neurobehavioral adaptability to exercise in female mice. Commun. Biol. 2023, 6, 1126. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, M.M.; Vargas-Bello-Pérez, E.; Zhuang, Y.; Fu, Y.; Zhang, N. Impact of dietary supplementation of β-hydroxybutyric acid on performance, nutrient digestibility, organ development and serum stress indicators in early-weaned goat kids. Anim. Nutr. 2021, 9, 16–22. [Google Scholar] [CrossRef]
- Chen, L.; Sun, J.; Pan, Z.; Lu, Y.; Wang, Z.; Yang, L.; Sun, G. Analysis of Chemical Constituents of Chrysanthemum morifolium Extract and Its Effect on Postprandial Lipid Metabolism in Healthy Adults. Molecules 2023, 28, 579. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef]
- Henry, R.J. Clinical Chemistry, Principles and Techniques, 2nd ed.; Harper and Row: Hagerstown, MD, USA, 1974. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lyu, W.; Zeng, T.; Wang, W.; Chen, Q.; Zhao, J.; Zhang, G.; Lu, L.; Yang, H.; Xiao, Y. Duck gut metagenome reveals the microbiome signatures linked to intestinal regional, temporal development, and rearing condition. Imeta 2024, 3, e198. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Luis, S.; Canto, F. Implanting melatonin at lambing enhances lamb growth and maintains high fat content in milk. Vet. Res. Commun. 2021, 45, 181–188. [Google Scholar] [CrossRef]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Liu, K.; Yu, W.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2019, 60, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Yao, L.; Duan, T.; Qin, J.; He, L.; Zhang, W. Melatonin promotes the development of the secondary hair follicles by regulating circMPP5. J. Anim. Sci. Biotechnol. 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Duan, C.; Yao, L.; Qin, J.; He, L.; Zhang, W. Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1. Int. J. Mol. Sci. 2023, 24, 3403. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, Q.; Zheng, Z.B.; Diao, X.G.; He, L.W.; Zhang, W. Supplementary Feeding of Grazing Inner Mongolian Cashmere Goats during Pregnancy-Based on “Nutrient Requirements of Cashmere Goats”. Animals 2023, 13, 473. [Google Scholar] [CrossRef]
- Wang, L.; Lu, D.; Sun, H.; Zhao, X.; Shan, D.; Yang, G.; Fang, L. Effects of photoperiod and melatonin on nitrogen partitioning and production performance of Inner Mongolia White Cashmere Goats. Front. Agric. China 2007, 1, 229–236. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Sun, H.Z.; Li, S.L.; Sang, D.; Zhang, C.H.; Jin, L.; Antonini, M.; Zhao, C.F. Effects of photoperiod on nutrient digestibility, hair follicle activity and cashmere quality in Inner Mongolia white cashmere goats. Asian-Australas. J. Anim. Sci. 2019, 32, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. TEM 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef]
- Zhao, C.; Duan, Y.; Diao, X.; He, L.; Zhang, W. Effects of Dietary Selenium Yeast Supplementation in Pregnant Cashmere Goats on the Development of Offspring Hair Follicles. Animals 2024, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, Z.; Shi, Z.; Liu, C.; Lu, Q. Melatonin Alleviates Renal Injury in Mouse Model of Sepsis. Front. Pharmacol. 2021, 12, 697643. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J. Pineal Res. 2022, 73, e12812. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Zhou, Z.; Gomes, M.S.; Peixoto, P.M.G.; Bonsaglia, E.C.R.; Canisso, I.F.; Weimer, B.C.; Lima, F.S. Rumen and lower gut microbiomes relationship with feed efficiency and production traits throughout the lactation of Holstein dairy cows. Sci. Rep. 2022, 12, 4904. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, S.; Wang, T.; Lu, Y.; Han, H.; Liu, X.; Lv, D.; Ma, X.; Guan, S.; Yao, Y.; et al. Effects of melatonin on rumen microorganisms and methane production in dairy cow: Results from in vitro and in vivo studies. Microbiome 2023, 11, 196. [Google Scholar]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, H.; Zhang, P.; Yue, S.; Zhai, B.; Zou, J.; Cheng, J.; Zhao, C.; Guo, D.; Wang, J. Paeonol Ameliorates Ulcerative Colitis in Mice by Modulating the Gut Microbiota and Metabolites. Metabolites 2022, 12, 956. [Google Scholar] [CrossRef]
- Park, S.Y.; Rao, C.; Coyte, K.Z.; Kuziel, G.A.; Zhang, Y.; Huang, W.; Franzosa, E.A.; Weng, J.K.; Huttenhower, C.; Rakoff-Nahoum, S. Strain-level fitness in the gut microbiome is an emergent property of glycans and a single metabolite. Cell 2022, 185, 513–529.e521. [Google Scholar] [CrossRef]
- Fakih, I.; Got, J.; Robles-Rodriguez, C.E.; Siegel, A.; Forano, E.; Muñoz-Tamayo, R. Dynamic genome-based metabolic modeling of the predominant cellulolytic rumen bacterium Fibrobacter succinogenes S85. mSystems 2023, 8, e0102722. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cao, Q.; Sun, Y.; Huang, S.; Liu, X.; Li, D. 16S rRNA genes- and metagenome-based confirmation of syntrophic butyrate-oxidizing methanogenesis enriched in high butyrate loading. Bioresour. Technol. 2022, 345, 126483. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Qiu, M.; Bao, L.; Wu, K.; Meng, X.; Zhao, Y.; Feng, L.; Duan, S.; He, Y.; et al. Sialic acid exacerbates gut dysbiosis-associated mastitis through the microbiota-gut-mammary axis by fueling gut microbiota disruption. Microbiome 2023, 11, 78. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, S.Y.; Ru, F.X.; Feng, J.J.; Tao, J.; Wei, Z.Y.; Li, X.; Qian, C.; Lin, Q.; Chen, J.H. Gastric juice microbiota in pediatric chronic gastritis that clinically tested positive and negative for Helicobacter pylori. Front. Microbiol. 2023, 14, 1112709. [Google Scholar] [CrossRef]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, 14. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Wang, Y.; Yu, J.; Wang, B.; Wen, Q.; Zheng, X. Donor selection for fecal bacterial transplantation and its combined effects with inulin on early growth and ileal development in chicks. J. Appl. Microbiol. 2023, 134, 99. [Google Scholar] [CrossRef]




| Ingredient | (%) | Nutrient Levels 2 | |
|---|---|---|---|
| Alfalfa | 35.00 | ME, (MJ/Kg) | 9.69 |
| Peanut straw | 35.00 | DM, (%) | 86.99 |
| Corn | 16.00 | EE, (%) | 3.62 |
| Soybean meal | 9.50 | CP, (%) | 14.80 |
| Fermented soybean meal | 3.50 | Ca, (%) | 1.00 |
| Dicalcium phosphate | 0.10 | P, (%) | 0.32 |
| Salt | 0.50 | NDF, (%) | 24.66 |
| Premix 1 | 0.30 | ADF, (%) | 47.38 |
| Total | 100.00 | ASH, (%) | 6.70 |
| Ingredient | (%) | Nutrient levels |
| Item | 150 d | 300 d | ||||
|---|---|---|---|---|---|---|
| Control | Melatonin | p-Value | Control | Melatonin | p-Value | |
| Intake N (g/d) | 16.50 ± 0.77 | 16.19 ± 0.36 | 0.4054 | 26.46 ± 2.08 | 24.62 ± 1.19 | 0.1264 |
| Feces N (g/d) | 4.38 ± 0.39 | 3.89 ± 0.60 | 0.1417 | 6.05 ± 0.62 | 5.73 ± 0.82 | 0.5461 |
| Urine N (g/d) | 4.48 ± 0.83 a | 3.54 ± 0.48 b | 0.0403 | 7.85 ± 0.64 a | 6.13 ± 0.99 b | 0.0199 |
| Digestible N (g/d) | 12.12 ± 0.88 | 12.30 ± 0.64 | 0.6960 | 20.40 ± 1.87 | 18.89 ± 1.30 | 0.6960 |
| Retention N (g/d) | 7.64 ± 0.22 b | 8.77 ± 1.01 a | 0.0316 | 12.54 ± 1.75 | 12.76 ± 1.18 | 0.8296 |
| FN/NI (%) | 26.60 ± 2.78 | 23.00 ± 3.66 | 0.2167 | 20.30 ± 1.91 | 23.30 ± 3.40 | 0.2167 |
| UN/NI (%) | 26.98 ± 4.21 a | 21.90 ± 3.30 b | 0.0464 | 29.84 ± 3.03 a | 24.84 ± 3.59 b | 0.0425 |
| RN/NI (%) | 46.42 ± 2.17 b | 54.10 ± 5.74 a | 0.0159 | 47.21 ± 3.84 | 51.86 ± 4.43 | 0.8296 |
| NAD (%) | 73.40 ± 2.78 | 76.00 ± 3.60 | 0.2167 | 77.04 ± 2.13 | 76.70 ± 3.40 | 0.8713 |
| Item | 150 d | 300 d | ||||
|---|---|---|---|---|---|---|
| Control | Melatonin | p-Value | Control | Melatonin | p-Value | |
| GE intake (MJ/d) | 11.92 ± 0.51 | 11.84 ± 0.37 | 0.8929 | 19.19 ± 0.46 | 18.53 ± 0.10 | 0.5431 |
| FE (MJ/d) | 3.85 ± 0.35 | 3.28 ± 0.53 | 0.3126 | 5.24 ± 0.10 | 4.84 ± 0.16 | 0.3743 |
| UE (MJ/d) | 0.19 ± 0.06 a | 0.12 ± 0.03 b | 0.0444 | 0.42 ± 0.02 | 0.36 ± 0.09 | 0.3113 |
| DE (MJ/d) | 8.34 ± 0.47 | 8.55 ± 0.56 | 0.7096 | 13.95 ± 0.42 | 13.68 ± 0.19 | 0.7891 |
| ME (MJ/d) | 8.15 ± 0.45 | 8.43 ± 0.58 | 0.6359 | 13.53 ± 0.41 | 13.32 ± 0.18 | 0.8107 |
| Item | 150 d | 300 d | ||||
|---|---|---|---|---|---|---|
| Control | Melatonin | p-Value | Control | Melatonin | p-Value | |
| Dry matter | ||||||
| Intake (g/d) | 699.14 ± 38.76 | 676.88 ± 21.38 | 0.2550 | 1155.02 ± 27.33 | 1114.82 ± 6.21 | 0.5351 |
| Digestive bulk (g/d) | 481.92 ± 38.91 | 473.60 ± 33.30 | 0.6978 | 849.77 ± 25.76 | 830.83 ± 11.25 | 0.7787 |
| Digestibility (%) | 68.89 ± 2.70 | 69.97 ± 4.51 | 0.6466 | 73.36 ± 0.69 | 74.51 ± 0.85 | 0.6566 |
| Organic matter | ||||||
| Intake (g/d) | 651.95 ± 36.20 | 631.06 ± 20.01 | 0.2532 | 1074.68 ± 25.51 | 1037.55 ± 5.76 | 0.5423 |
| Digestive bulk (g/d) | 464.57 ± 36.80 | 451.10 ± 26.03 | 0.4642 | 808.22 ± 24.09 | 789.80 ± 9.91 | 0.7532 |
| Digestibility (%) | 71.23 ± 2.56 | 71.50 ± 3.81 | 0.8961 | 75.00 ± 0.67 | 76.11 ± 0.80 | 0.6461 |
| Ether extract | ||||||
| Intake (g/d) | 28.06 ± 1.32 | 27.02 ± 0.85 | 0.1430 | 28.98 ± 0.64 | 27.82 ± 0.17 | 0.4503 |
| Digestive bulk (g/d) | 24.02 ± 1.40 | 22.55 ± 1.13 | 0.0804 | 22.90 ± 0.66 | 21.90 ± 0.36 | 0.5651 |
| Digestibility (%) | 85.57 ± 2.21 | 83.45 ± 3.31 | 0.2455 | 78.81 ± 0.80 | 78.76 ± 1.30 | 0.9834 |
| Crude fiber | ||||||
| Intake (g/d) | 177.19 ± 14.02 | 169.39 ± 7.62 | 0.2673 | 296.71 ± 7.99 | 289.61 ± 1.49 | 0.7036 |
| Digestive bulk (g/d) | 102.3 ± 10.99 | 95.48 ± 12.66 | 0.3668 | 173.75 ± 7.10 | 176.59 ± 4.78 | 0.8856 |
| Digestibility (%) | 57.63 ± 3.01 | 56.36 ± 7.07 | 0.7146 | 58.19 ± 1.21 | 60.98 ± 1.63 | 0.5524 |
| Neutral detergent fiber | ||||||
| Intake (g/d) | 355.69 ± 23.80 | 342.11 ± 13.08 | 0.2576 | 533.84 ± 13.62 | 518.56 ± 2.70 | 0.6315 |
| Digestive bulk (g/d) | 217.06 ± 6.03 | 211.03 ± 13.52 | 0.6641 | 338.22 ± 12.56 | 338.26 ± 6.43 | 0.9936 |
| Digestibility (%) | 61.01 ± 2.63 | 61.65 ± 6.93 | 0.8468 | 63.03 ± 1.04 | 65.24 ± 1.23 | 0.5538 |
| Acid detergent fiber | ||||||
| Intake (g/d) | 245.08 ± 18.72 | 234.34 ± 10.32 | 0.2554 | 407.89 ± 10.83 | 397.68 ± 2.04 | 0.6828 |
| Digestive bulk (g/d) | 143.39 ± 12.65 | 133.69 ± 18.24 | 0.3350 | 254.87 ± 10.83 | 397.68 ± 2.04 | 0.9035 |
| Digestibility (%) | 58.58 ± 3.86 | 57.00 ± 7.10 | 0.6626 | 62.04 ± 1.13 | 64.90 ± 1.21 | 0.4614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Han, D.; Su, Z.; He, L.; Zhang, W. Effect of Melatonin on the Production Performance, Blood Biochemical Parameters, Nutrient Digestibility, and Gastrointestinal Microbiome of Liaoning Cashmere Goats. Agriculture 2024, 14, 1983. https://doi.org/10.3390/agriculture14111983
Zheng Z, Han D, Su Z, He L, Zhang W. Effect of Melatonin on the Production Performance, Blood Biochemical Parameters, Nutrient Digestibility, and Gastrointestinal Microbiome of Liaoning Cashmere Goats. Agriculture. 2024; 14(11):1983. https://doi.org/10.3390/agriculture14111983
Chicago/Turabian StyleZheng, Zibin, Di Han, Zhenyu Su, Liwen He, and Wei Zhang. 2024. "Effect of Melatonin on the Production Performance, Blood Biochemical Parameters, Nutrient Digestibility, and Gastrointestinal Microbiome of Liaoning Cashmere Goats" Agriculture 14, no. 11: 1983. https://doi.org/10.3390/agriculture14111983
APA StyleZheng, Z., Han, D., Su, Z., He, L., & Zhang, W. (2024). Effect of Melatonin on the Production Performance, Blood Biochemical Parameters, Nutrient Digestibility, and Gastrointestinal Microbiome of Liaoning Cashmere Goats. Agriculture, 14(11), 1983. https://doi.org/10.3390/agriculture14111983






