Development of Elite Mother Palms from the Best-Performing Slow-Vertical-Growth Oil Palm (Elaeis guineensis Jacq.) Genotypes
Abstract
:1. Introduction
2. Materials and Methods
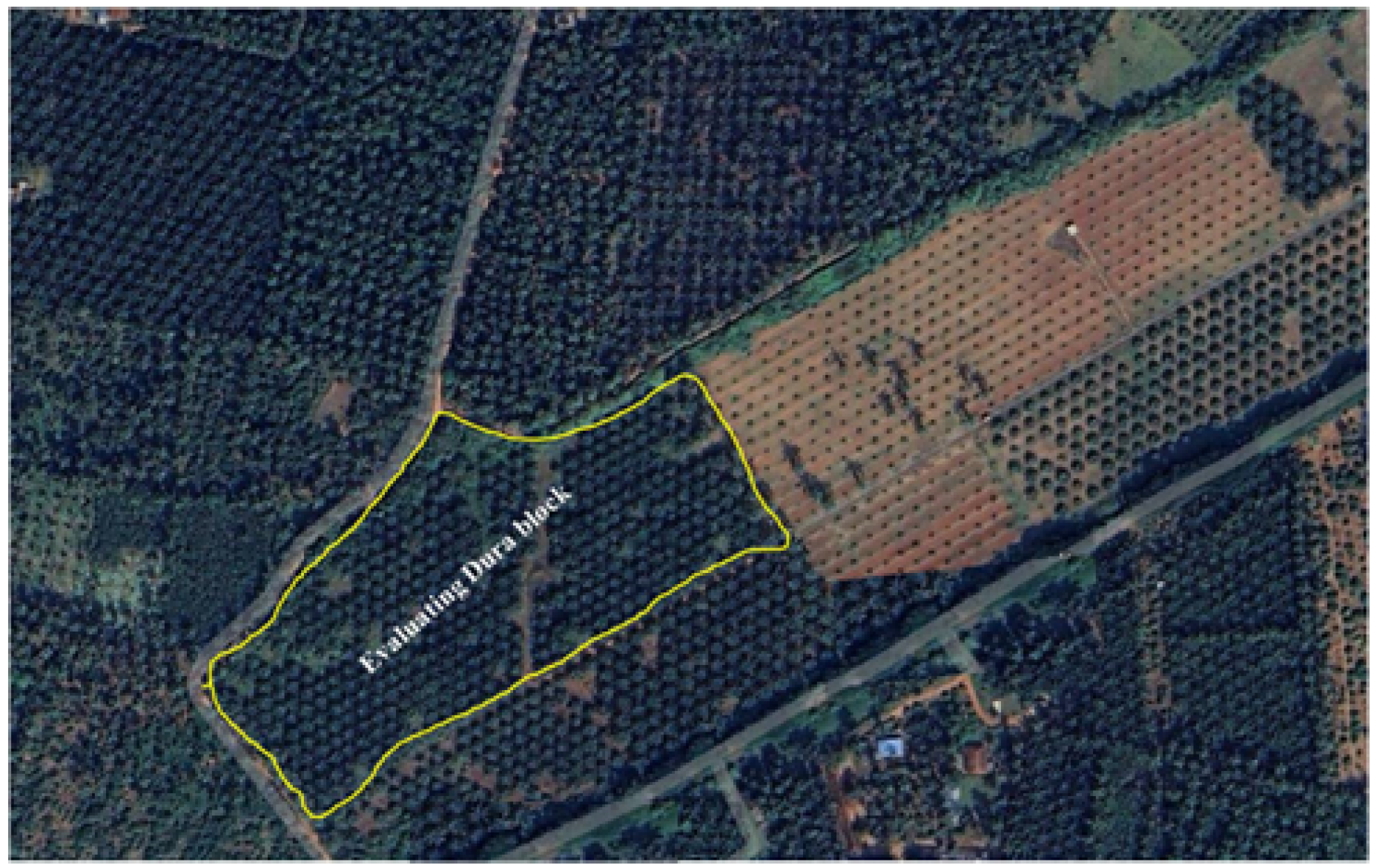
2.1. Experimental Area Description
2.2. Oil Palm Genotypes
2.3. Biometric Observations
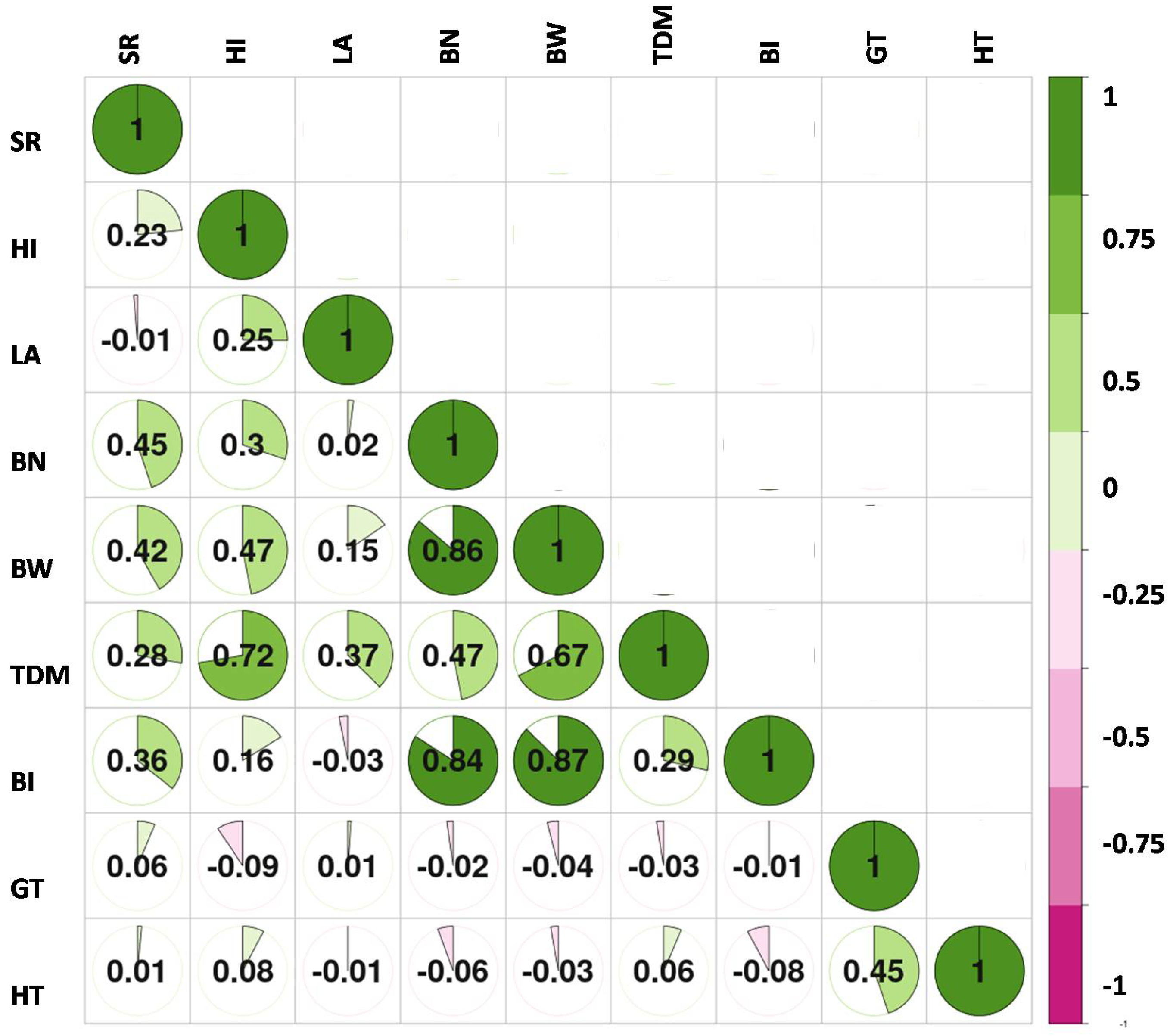
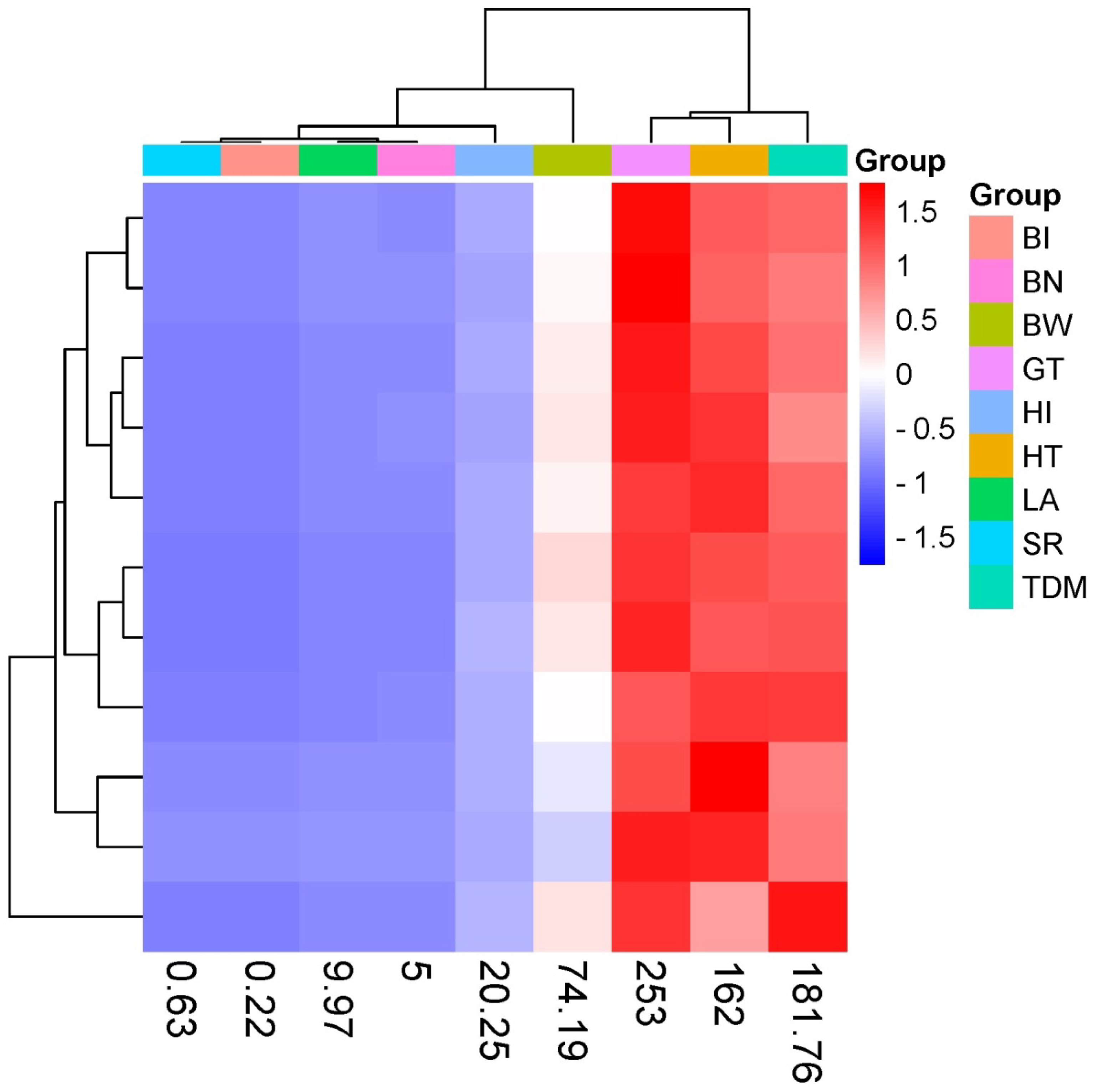
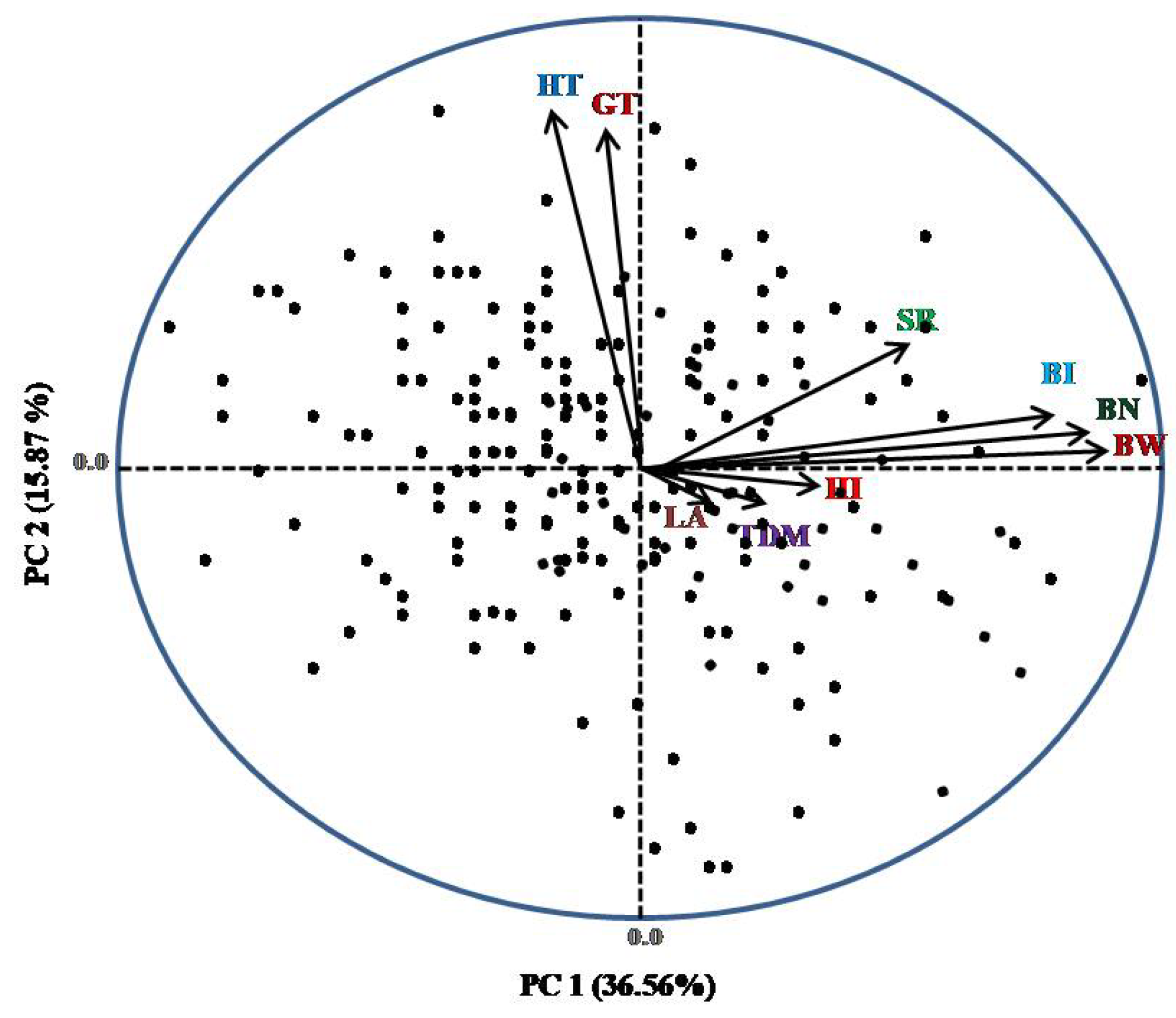
2.4. Data Analysis
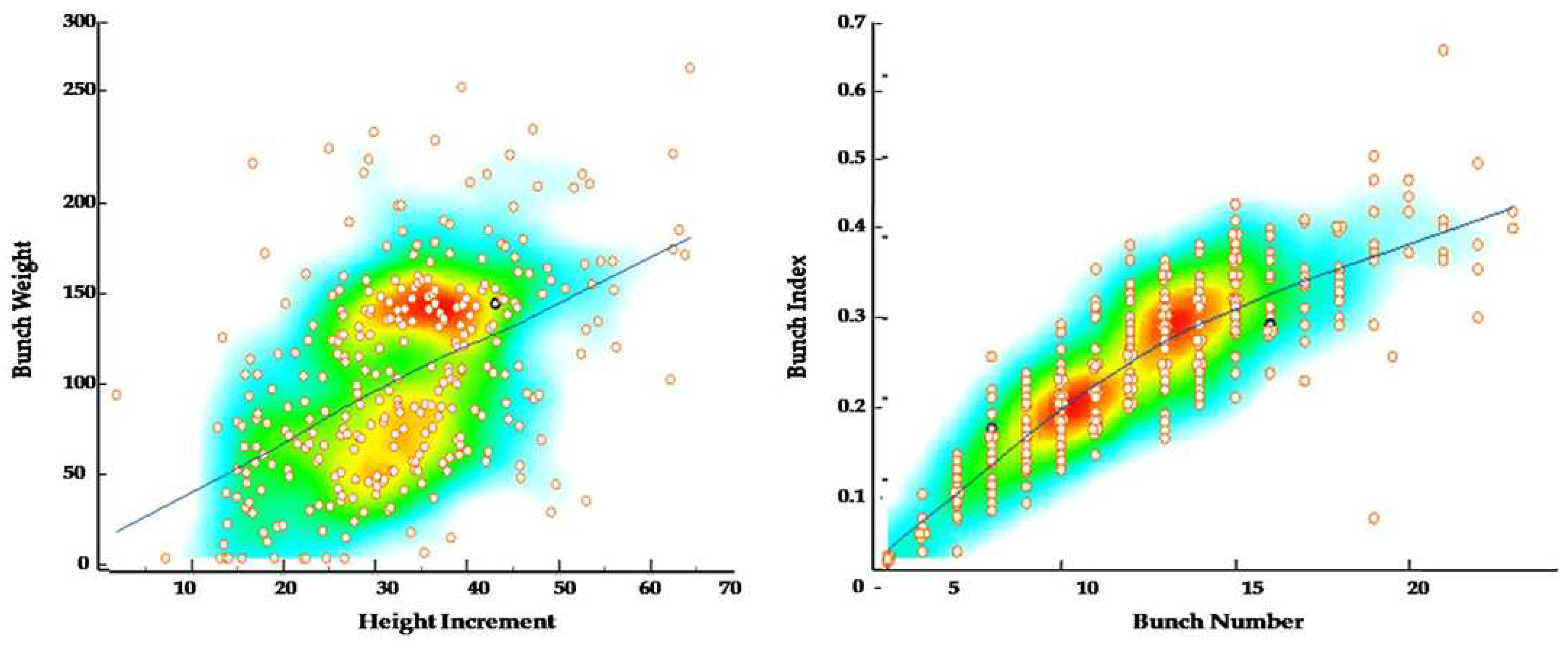
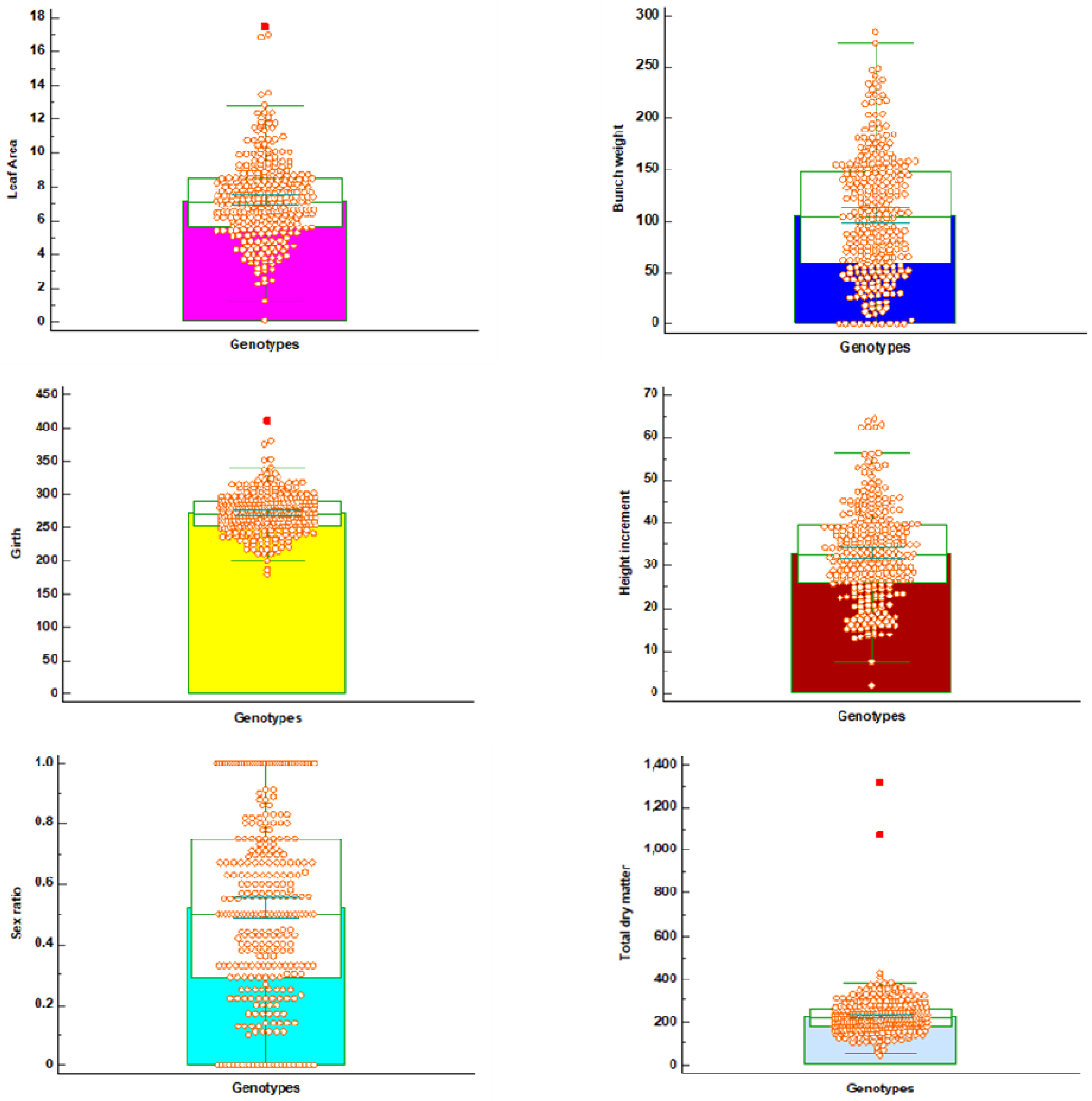
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Production, Supply and Distribution Online (PS&D); United States Department of Agriculture Production Commodity Data, USDA: Gainesville, FL, USA, 2023.
- Shi, P.; Wang, Y.; Zhang, D.; Htwe, Y.M.; Ihase, L.O. Analysis on fruit oil content and evaluation on germplasm in Oil Palm. HortScience 2019, 54, 1275–1279. [Google Scholar] [CrossRef]
- Harun, M.H.; Noor, M.R. Fruit set and Oil Palm bunch components. J. Oil Palm Res. 2002, 14, 24–33. [Google Scholar]
- Myint, K.; Amiruddin, M.; Rafii, M.Y.; Abd, M.Y.; Izan, S. Character interrelationships and path analysis for yield components in MPOB-Senegal oil palm germplasm. Sains Malaysiana 2021, 50, 699–709. [Google Scholar]
- Bakoume, C.; Louise, C. Breeding for oil yield and short oil palms in the second cycle of selection at La Dibamba (Cameroon). Euphytica 2007, 156, 195–202. [Google Scholar] [CrossRef]
- Junaidah, J.; Rafii, M.Y.; Chin, C.W.; Saleh, G. Performance of tenera oil palm population derived from crosses between Deli dura and pisifera from different sources on inland soils. J. Oil Palm Res. 2011, 23, 1210–1221. [Google Scholar]
- Upaz-Vera, A.; Ayala-Diaz, I.; Barrera, C.F.; Hernan, M.R. Genetic gains for obtaining improved progenies of oil palm in Colombia. Euphytica 2023, 219, 38. [Google Scholar] [CrossRef]
- Nor Azwani, A.B.; Fadila, A.M.; Mohd Din, A.; Rajanaidu, N.; Norziha, A.; Suzana, M.; Marhalil, M.; Zulkifli, Y.; Kushairi, A. Potential oil palm genetic materials derived from introgression of germplasm (MPOB-Nigeria, MPOB-Zaire and MPOB-Cameroon accessions) to advanced (AVROS) breeding population. J. Oil Palm Res. 2020, 32, 569–581. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Arolu, I.W.; Rafii, M.Y.; Marjuni, M.; Hanafi, M.M.; Sulaiman, Z.; Rahim, H.A.; Abidin, M.I.Z.; Amiruddin, M.D.; Din, A.K.; Nookiah, R. Breeding of high yielding and dwarf oil palm planting materials using Deli dura × Nigerian pisifera population. Euphytica 2017, 213, 1–15. [Google Scholar] [CrossRef]
- Sunilkumar, K.; Mathur, R.K.; Sparjanbabu, D.S.; Pillai, R.S. Evaluation of interspecific oil palm hybrids for dwarfness. J. Plant. Crops 2015, 43, 29–34. [Google Scholar]
- Tupaz-Vera, A.; Ayala-Diaz, I.; Barrera, C.F.; Romero, H.M. Selection of elite dura-type parents to produce dwarf progenies of Elaeisguineensis Using Genetic Parameters. Agronomy 2021, 11, 2581. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Climate and Soils of the Oil Palm-Growing Regions. In The Oil Palm, 4th ed.; Blackwell Science Ltd.: Oxford, UK, 2003; pp. 53–88. [Google Scholar]
- Pedapati, A.; Mathur, R.K.; Ravichandran, G.; Babu, B.K.; Bhagya, H.P. Evaluation of bunch quality components in Dura x Dura progenies of Zambia and Camaroon sources of oil palm germplasm. J. Environ. Biol. 2021, 42, 1567–1577. [Google Scholar] [CrossRef]
- Okoye, M.; Okwuagwu, C.; Uguru, M. Population improvement for fresh fruit bunch yield and yield components in oil palm (Elaeis guineensis Jacq.). Am.-Eurasian J. Sci. Res. 2009, 4, 59–63. [Google Scholar]
- Tanya, P.; Hadkam, Y.; Taeprayoon, P.; Srinives, P. Estimates of repeatability and path coefficient of bunch and fruit traits in bang boet dura oil palm. J. Oil Palm Res. 2013, 25, 108–115. [Google Scholar]
- Anitha, P.; Mathur, R.K.; Ravichandran, G.; Kalyana Babu, B.; Bhagya, H.P. Assessment of genetic divergence and principal component analysis in oil palm (Elaeis guineensis Jacq.). J. Oilseeds Res. 2018, 35, 159–165. [Google Scholar]
- Suzana, M.; Zulkifli, Y.; Marhalil, M.; Rajanaidu, N.; Ong-Abdullah, M. Principal component and cluster analyses on Tanzania oil palm Elaeis guineensis Jacq. germplasm. J. Oil Palm Res. 2020, 32, 24–33. [Google Scholar]
- Billotte, N.; Risterucci, A.M.; Barcelos, E.; Noyer, J.L.; Amblard, P.; Baurens, F.C. Development, characterization, and across-taxa utility of oil palm (Elaeis guineensis Jacq.) Microsatellite markers. Genome 2001, 44, 413–425. [Google Scholar] [CrossRef]
- Swaray, S.; Amiruddin, M.D.; Rafii, M.Y.; Jamian, S.; Ismail, M.F.; Jalloh, M.; Eswa, M.; Marjuni, M.; Akos, I.S.; Yusuff, O. Oil Palm Inflorescence Sex Ratio and Fruit Set Assessment in dura × pisifera Biparental Progenies on Fibric Peat Soil. Agronomy 2021, 11, 1380. [Google Scholar] [CrossRef]
- Henson, I.E. Modelling oil palm yield based on source and sink. Oil Palm Bull. 2007, 54, 27–51. [Google Scholar]
- Noh, A.; Rafii, M.Y.; Saleh, G.; Kushairi, A. Genetic performance of 40 Deli dura × AVROS pisifera full-sib families. J. Oil Palm Res. 2010, 22, 781–795. [Google Scholar]
- Hageman, J.A.; Malosetti, M.; Eeuwijk, F.A.V. Two-mode clustering of genotype by trait and genotype by environment data. Euphytica 2012, 183, 349–359. [Google Scholar] [CrossRef]
- Krualee, S.; Sdoodee, S.; Eksomtramage, T.; Sereeprasert, V. Correlation and path analysis of palm oil yield components in oil palm (Elaeis guineensis Jacq.). Agric. Nat. Resour. 2013, 47, 528–533. [Google Scholar]
- Popet, P.; Eksomtramage, T.; Anothai, J.; Khomphet, T. Correlation and path analysis in commercial tenera oil palms collected from Southern Thailand. Indian J. Agric. Res. 2022, 56, 485–488. [Google Scholar] [CrossRef]
- Salim, W.N.S.T.M.; Yaakub, Z.; Bakar, N.A.A.; Nasir, F.M.; Marjuni, M.; Amiruddin, M.D.; Ong-Abdullah, M. Genetic variability of MPOB-Cameroon oil palm germplasm based on morphological traits using multivariate analysis. J. Oil Palm Res. 2023, 35, 476–490. [Google Scholar]
- Gomes Junior, R.A.; de Freitas, A.F.; da Cunha, R.N.V.; Pina, A.J.d.A.; Campos, H.O.B.; Lopes, R. Selection gains for the palm oil production from progenies of American oil palm with oil palm. Pesqui. Agropecu. Bras. 2021, 56, e02321. [Google Scholar] [CrossRef]
- Murugesan, P.; Mary Rani, K.L.; Naveen Kumar, P.; Ramajayam, D.; Sunil Kumar, K.; Mathur, R.K.; Ravichandran, G.; Arunachalam, V. Genetic diversity of vegetative and bunch traits of African oil palm (Elaeis guineensis) germplasm in India. Indian J. Agric. Sci. 2015, 85, 892–895. [Google Scholar] [CrossRef]
- Zhou, L.X.; Xiao, Y.; Xia, W.; Yang, Y.D. Analysis of genetic diversity and population structure of oil palm (Elaeis guineensis) from China and Malaysia based on species-specific simple sequence repeat markers. Genet. Mol. Res. 2015, 14, 16247–16254. [Google Scholar] [CrossRef]
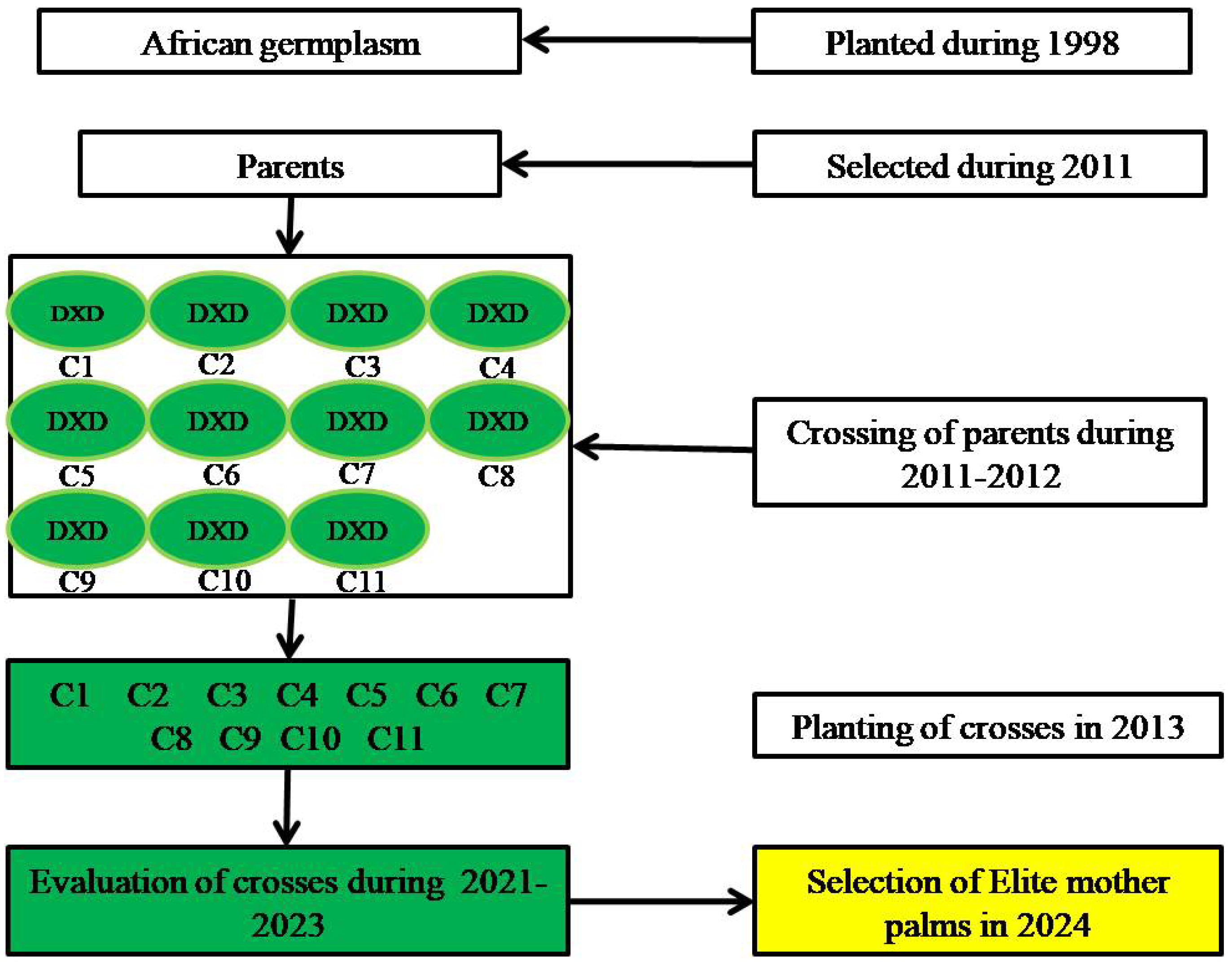

| SN | Entry | Genotype | Population | Parent Source |
|---|---|---|---|---|
| 1. | C1 | 42 CD × 43 CD | 28 | Zambia |
| 2. | C2 | 497 CD × 43 CD | 28 | Zambia |
| 3. | C3 | 497 CD × 465 CD | 28 | Zambia |
| 4. | C4 | 465 CD × 42 CD | 28 | Zambia |
| 5. | C5 | 40 CD × 282 CD | 28 | Zambia |
| 6. | C6 | 410 CD × 42 CD | 28 | Zambia |
| 7. | C7 | 42 CD × 93 CD | 28 | Zambia × Tanzania |
| 8. | C8 | 42 CD × 42 CD | 28 | Zambia |
| 9. | C9 | 40 CD × 42 CD | 28 | Zambia |
| 10. | C10 | 42 CD × 257 CD | 28 | Zambia |
| 11. | C11 | 206 CD × 4 D | 28 | Zambia |
| Variable | Mean | Std Dev | Min | Max | cv | GCV (%) | PCV (%) |
|---|---|---|---|---|---|---|---|
| BI | 0.24 | 0.11 | 0.00 | 0.63 | 0.46 | 62.5 | 45.83 |
| BN | 7.49 | 3.93 | 0.00 | 21.33 | 0.52 | 66.9 | 52.47 |
| BW | 105.95 | 59.35 | 0.00 | 220.52 | 0.56 | 83.5 | 56.01 |
| GT | 271.96 | 30.35 | 180.00 | 410.00 | 0.11 | 13.8 | 11.16 |
| HI | 32.99 | 11.08 | 1.88 | 62.17 | 0.34 | 40.9 | 33.59 |
| HT | 252.37 | 83.27 | 33.00 | 434.00 | 0.33 | 43.4 | 33.00 |
| LA | 7.22 | 2.46 | 0.11 | 17.43 | 0.34 | 40. | 34.07 |
| SR | 0.52 | 0.30 | 0.00 | 1.00 | 0.58 | 88.5 | 57.69 |
| TDM | 226.08 | 101.89 | 42.16 | 1312.02 | 0.45 | 37.9 | 45.07 |
| Crosses | SR | HI | LA | BN | BW | TDM | BI | GT | HT |
|---|---|---|---|---|---|---|---|---|---|
| 47 CD × 43 CD | 0.391 a | 24.879 e | 7.54 abcd | 6.321 de | 86.91 f | 191.897 f | 0.221 a | 260.146 f | 199.036 g |
| 497 CD × 43 CD | 0.459 a | 24.743 e | 6.531 d | 7.605 cd | 94.734 e | 182.062 g | 0.265 a | 273.393 d | 202.839 g |
| 497 CD × 465 CD | 0.481 a | 28.455 d | 8.393 a | 7.5 cd | 106.389 d | 194.127 f | 0.265 a | 260.232 f | 225.179 f |
| 465 CD × 42 CD | 0.677 a | 37.594 bc | 7.742 abc | 8.25 bc | 145.229 a | 251.459 c | 0.296 a | 288.743 b | 267.464 d |
| 40 CD × 282 CD | 0.58 a | 29.107 d | 6.494 d | 9.893 a | 115.469 c | 191.957 f | 0.307 a | 278.471 c | 259.679 e |
| 410 CD × 42 CD | 0.559 a | 43.504 a | 6.645 d | 9.107 ab | 116.988 c | 231.898 d | 0.261 a | 267.821 e | 228.464 f |
| 42 CD × 93 CD | 0.401 a | 35.509 c | 6.908 cd | 5.357 ef | 85.438 f | 225.836 d | 0.197 a | 269.464 e | 344.393 a |
| 42 CD × 42 CD | 0.421 a | 21.625 f | 6.757 cd | 4.464 f | 54.952 g | 215.528 e | 0.158 a | 294.964 a | 289.393 c |
| 40 CD × 42 CD | 0.56 a | 38.75 b | 8.173 ab | 7.5 cd | 118.9 c | 271.891 b | 0.245 a | 250.9 h | 168.857 h |
| 42 CD × 257 CD | 0.587 a | 35.621 c | 7.246 bcd | 8.306 bc | 125.6 b | 250.456 c | 0.251 a | 291 b | 306.929 b |
| 206 CD × 4 CD | 0.615 a | 43.08 a | 6.999 cd | 8.071 bc | 114.838 c | 279.758 a | 0.218 a | 256.375 g | 283.786 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedapati, A.; Suresh, K.; Mathur, R.K.; Ravichandran, G.; Kumar, P.N.; Bhagya, H.P.; Babu, B.K.; Narayana, K.S. Development of Elite Mother Palms from the Best-Performing Slow-Vertical-Growth Oil Palm (Elaeis guineensis Jacq.) Genotypes. Agriculture 2024, 14, 2007. https://doi.org/10.3390/agriculture14112007
Pedapati A, Suresh K, Mathur RK, Ravichandran G, Kumar PN, Bhagya HP, Babu BK, Narayana KS. Development of Elite Mother Palms from the Best-Performing Slow-Vertical-Growth Oil Palm (Elaeis guineensis Jacq.) Genotypes. Agriculture. 2024; 14(11):2007. https://doi.org/10.3390/agriculture14112007
Chicago/Turabian StylePedapati, Anitha, Kancherla Suresh, Ravi Kumar Mathur, Govindan Ravichandran, Prathapani Naveen Kumar, Hosahalli Parvathappa Bhagya, Banisetti Kalyana Babu, and Kariyappa Sankar Narayana. 2024. "Development of Elite Mother Palms from the Best-Performing Slow-Vertical-Growth Oil Palm (Elaeis guineensis Jacq.) Genotypes" Agriculture 14, no. 11: 2007. https://doi.org/10.3390/agriculture14112007
APA StylePedapati, A., Suresh, K., Mathur, R. K., Ravichandran, G., Kumar, P. N., Bhagya, H. P., Babu, B. K., & Narayana, K. S. (2024). Development of Elite Mother Palms from the Best-Performing Slow-Vertical-Growth Oil Palm (Elaeis guineensis Jacq.) Genotypes. Agriculture, 14(11), 2007. https://doi.org/10.3390/agriculture14112007





