Stomatal Density Variation Within and Among Different Soybean Cultivars Across Various Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of the Optimal Number of Epidermal Sections to Sample from a Single Leaf
2.2. Variation in Stomatal Density Within a Leaf and Between Different Leaves Across Various Growth Stages
2.3. Evaluation of Stomatal Density Among Different Cultivars Across Various Growth Stages
2.4. Quantification of Stomatal Density
2.5. Data Analysis
3. Results
3.1. Optimal Number of Epidermal Sections for Representing the Whole Leaf
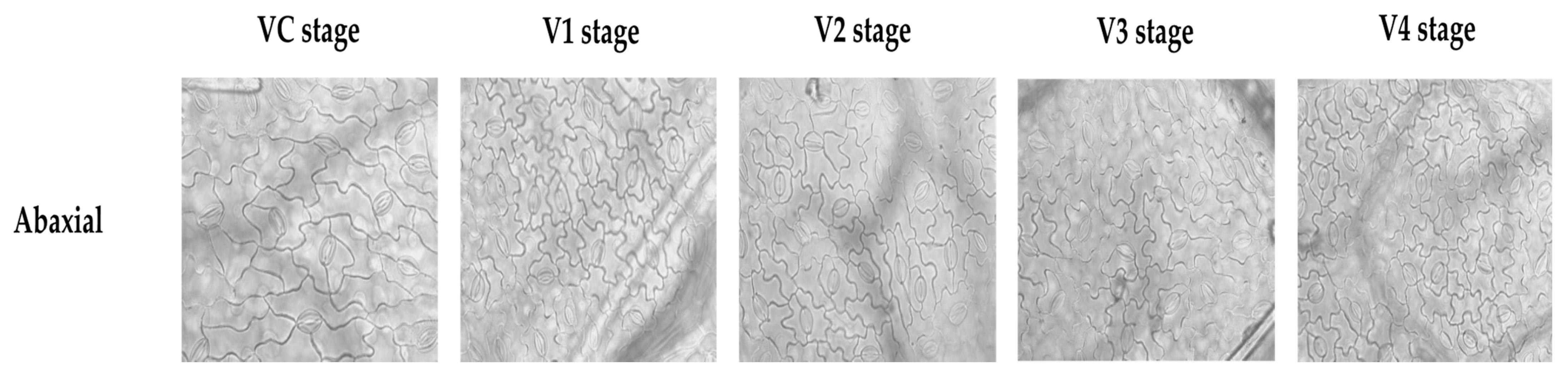
3.2. The Impact of Leaflet Location and Leaf Positions Across Growth Stages on Stomatal Density
3.3. Interaction Effects of Growth Stage, Leaflet Location and Leaf Position on Stomatal Density
3.4. Variation in Stomatal Density Among Different Cultivars Across Various Growth Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Yu, Q.; Xu, C.; Li, J.; Qin, G. Rapid estimation of stomatal density and stomatal area of plant leaves based on object-oriented classification and its ecological trade-off strategy analysis. Forests 2018, 9, 616. [Google Scholar] [CrossRef]
- Carpenter, K.J. Stomatal architecture and evolution in basal angiosperms. Am. J. Bot. 2005, 92, 1595–1615. [Google Scholar] [CrossRef]
- Baillie, A.L.; Fleming, A.J. The developmental relationship between stomata and mesophyll airspace. New Phytol. 2020, 225, 1120–1126. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Mcadam, S.A.; Murphy, M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef]
- Zhong, M.; Cerabolini, B.E.L.; Castro-Díez, P.; Puyravaud, J.; Cornelissen, J.H.C. Allometric co-variation of xylem and stomata across diverse woody seedlings. Plant Cell Environ. 2020, 43, 2301–2310. [Google Scholar] [CrossRef]
- Franks, P.J.; Casson, S. Connecting stomatal development and physiology. New Phytol. 2014, 201, 1079–1082. [Google Scholar] [CrossRef]
- Lehmann, P.; Or, D. Effects of stomata clustering on leaf gas exchange. New Phytol. 2015, 207, 1015–1025. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, L.; Li, M.; Wang, J.; Yan, P.; He, N. Stomatal arrangement pattern: A new direction to explore plant adaptation and evolution. Front. Plant Sci. 2021, 12, 655255. [Google Scholar] [CrossRef]
- Geisler, M.J.; Sack, F.D. Variable timing of developmental progression in the stomatal pathway in Arabidopsis cotyledons. New Phytol. 2002, 153, 469–476. [Google Scholar] [CrossRef]
- Wall, S.; Vialet-Chabrand, S.; Davey, P.; Van, R.J.; Galle, A.; Cockram, J.; Lawson, T. Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytol. 2022, 235, 1743–1756. [Google Scholar] [CrossRef]
- Willmer, C.; Fricker, M. The distribution of stomata. Stomata. 2022, 12–35. [Google Scholar]
- Lu, Z. The sensitivity of adaxial and abaxial stomatal resistance in wheat leaf to soil water stress. Acta Oecol. 1988, 14, 223–227. [Google Scholar]
- Wang, X.Q.; Wu, W.H.; Assmann, S.M. Differential responses of abaxial and adaxial guard cells of broad bean to abscisic acid and calcium. Plant Physiol. 1998, 118, 1421–1429. [Google Scholar] [CrossRef]
- Wang, R.; Yu, G.; He, N.; Wang, Q.; Xia, F.; Zhao, N.; Xu, Z.; Ge, J. Elevation-related variation in leaf stomatal traits as a function of plant functional type: Evidence from changbai mountain, China. PLoS ONE. 2014, 9, e115395. [Google Scholar] [CrossRef]
- Kardiman, R.; Raebild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef]
- Bergmann, D.C.; Sack, F.D. Stomatal development. Annu. Rev. Plant Biol. 2007, 58, 163–181. [Google Scholar] [CrossRef]
- Casson, S.A.; Hetherington, A.M. Environmental regulation of stomatal development. Curr. Opin. Plant Biol. 2010, 13, 90–95. [Google Scholar] [CrossRef]
- Delgado, D.; Alonso-Blanco, C.; Fenoll, C.; Mena, M. Natural variation in stomatal abundance of Arabidopsis thaliana includes cryptic diversity for different developmental processes. Ann. Bot. 2011, 107, 1247–1258. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Hou, J.; He, N. Spatial variation of stomatal morphological traits in grassland plants of the Loess Plateau. Ecol. Indic. 2021, 128, 107857. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef]
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S.; et al. Induced genetic variations in stomatal density and size of rice strongly affect water-use efficiency, drought tolerance, and responses to abiotic stresses. Front. Plant Sci. 2021, 13, 801706. [Google Scholar]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef]
- Engineer, C.B.; Ghassemian, M.; Anderson, J.C.; Peck, S.C.; Hu, H.; Schroeder, J.I. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 2014, 513, 246–250. [Google Scholar] [CrossRef]
- Hamanishi, E.T.; Thomas, B.R.; Campbell, M.M. Drought induces alterations in the stomatal development program in Populus. J. Exp. Bot. 2012, 63, 4959–4971. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Crawford, A.J.; Mclachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018, 221, 371–384. [Google Scholar] [CrossRef]
- Bresson, C.C.; Vitasse, Y.; Kremer, A.; Delzon, S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011, 31, 1164–1174. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujii, K.; Shiraiwa, T. Variability of leaf morphology and stomatal conductance in soybean [Glycine max (L.) Merr.] cultivars. Crop Sci. 2010, 50, 2525–2532. [Google Scholar] [CrossRef]
- Amaliah, N.; Zubaidah, S.; Kuswantoro, H. Trichomes and stomata diversity in Soybean (Glycine max L. Merr) Lines. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012025. [Google Scholar] [CrossRef]
- Sakoda, K.; Watanabe, T.; Sukemura, S.; Kobayashi, S.; Nagasaki, Y.; Tanaka, Y.; Shiraiwa, T. Genetic diversity in stomatal density among soybeans elucidated using high-throughput technique based on an algorithm for object detection. Sci. Rep. 2019, 9, 7610. [Google Scholar] [CrossRef]
- Hultine, K.R.; Marshall, J.D. A comparison of three methods for determining the stomatal density of pine needles. J. Exp. Bot. 2001, 52, 369–373. [Google Scholar] [CrossRef]
- Sultana, S.N.; Park, H.; Choi, S.H.; Jo, H.; Song, J.T.; Lee, J.-D.; Kang, Y.J. Optimizing the experimental method for stomata-profiling automation of soybean leaves based on deep learning. Plants 2021, 10, 2714. [Google Scholar] [CrossRef]
- Poole, I.; Weyers, J.D.B.; Lawson, T.; Raven, J.A. Variations in stomatal density and index: Implications for palaeoclimatic reconstructions. Plant Cell Environ. 1996, 19, 705–712. [Google Scholar] [CrossRef]
- Bernard, R.L.; Cremeens, C.R. Registration of ‘Williams 82’ Soybean. Crop Sci. 1998, 28, 1027–1028. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill1. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Lee, C.; Choi, M.S.; Kim, H.T.; Yun, H.T.; Lee, B.; Chung, Y.-S.; Kim, R.W.; Choi, H.-K. Soybean [Glycine max (L.) Merrill]: Importance as a crop and pedigree reconstruction of korean varieties. Plant Breed. Biotech. 2015, 3, 179–196. [Google Scholar] [CrossRef]
- Kim, H.T.; Ko, J.M.; Lee, B.W.; Yun, H.T.; Lee, Y.H.; Shin, S.O.; Seo, M.J.; Choi, M.S.; Jeon, M.G.; Kang, B.K.; et al. Large seed, lodging resistant and high yield soybean cultivar ‘Seonpung’ for Soy-paste and Tofu. Korean J. Breed. Sci. 2017, 49, 96–102. [Google Scholar] [CrossRef]
- Lee, J.-D.; Kim, M.; Kulkarni, K.P.; Song, J.T. Agronomic traits and fatty acid composition of high-oleic acid cultivar Hosim. Plant Breed. Biotech. 2018, 6, 44–50. [Google Scholar] [CrossRef]
- Park, K.Y.; Moon, J.K.; Yun, H.T.; Lee, Y.H.; Kim, S.L.; Ryu, Y.H.; Kim, Y.H.; Ku, J.H.; Lee, E.S.; Ha, K.S.; et al. A new soybean cultivar for fermented soyfood and tofu with high yield,” Daepung”. Korean J. Breed. Sci. 2005, 37, 111–112. [Google Scholar]
- Liu, C.; He, N.; Zhang, J.; Li, Y.; Wang, Q.; Sack, L.; Yu, G. Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency. Funct. Ecol. 2018, 32, 20–28. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Lee, Y.-E. Bioactivity changes of non-Germinated Pungsannamul-kong according to Roasting conditions. Food Eng. Prog. 2021, 25, 61–69. [Google Scholar] [CrossRef]
- Bhaiswar, N.; Dixit, D. A review: Methods of automatic stomata detection and counting through microscopic images of a leaf. Int. J. innov. 2007, 5, 6. [Google Scholar]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- Dubberstein, D.; Oliveira, M.G.; Aoyama, E.M.; Guilhen, J.H.; Ferreira, A.; Marques, I.; Ramalho, J.; Patelli, F.L. Diversity of leaf stomatal traits among Coffea canephora Pierre ex A. Froehner Genotypes. Agronomy 2021, 11, 1126. [Google Scholar] [CrossRef]
- Kouwenberg, L.L.R. Changes in stomatal frequency and size during elongation of Tsuga heterophylla Needles. Ann. Bot. 2004, 94, 561–569. [Google Scholar] [CrossRef]
- Cole, D.F.; Dobrenz, A.K. Stomate density of Alfalfa (Medicago sativa L.). Crop Sci. 1970, 10, 61–63. [Google Scholar] [CrossRef]
- Tanzarella, O.A.; De, P.C.; Filippetti, A. Stomatal frequency and size in Vicia faba L.1. Crop Sci. 1984, 24, 1070–1076. [Google Scholar] [CrossRef]
- Smith, H.B. Variation and correlation of stomatal frequency and transpiration rate in Phaseolus vulgaris. Am. J. Bot. 1941, 28, 722–725. [Google Scholar] [CrossRef]
- Kawamitsu, Y.; Agata, W.; Hiyane, S.; Murayama, S.; Nose, A. Relation between leaf gas exchange rate and stomate. I. Stomatal frequency and guard cell length in C3 and C4 grass species. Jpn. J. Crop Sci. 1996, 65, 626–633. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Chaloner, W.G.; Beerling, D.J.; Sun, Q.; Collinson, M.; Mitchell, P. Assessing the potential for the stomatal characters of extant and fossil Ginkgo leaves to signal atmospheric CO2 change. Am. J. Bot. 2001, 88, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Laza, M.R.C.; Kondo, M.; Ideta, O.; Barlaan, E.; Imbe, T. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica 2009, 172, 149–158. [Google Scholar] [CrossRef]
- Miyazawa, S.I.; Livingston, N.J.; Turpin, D.H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa×P. deltoides). J. Exp. Bot. 2005, 57, 373–380. [Google Scholar] [CrossRef]
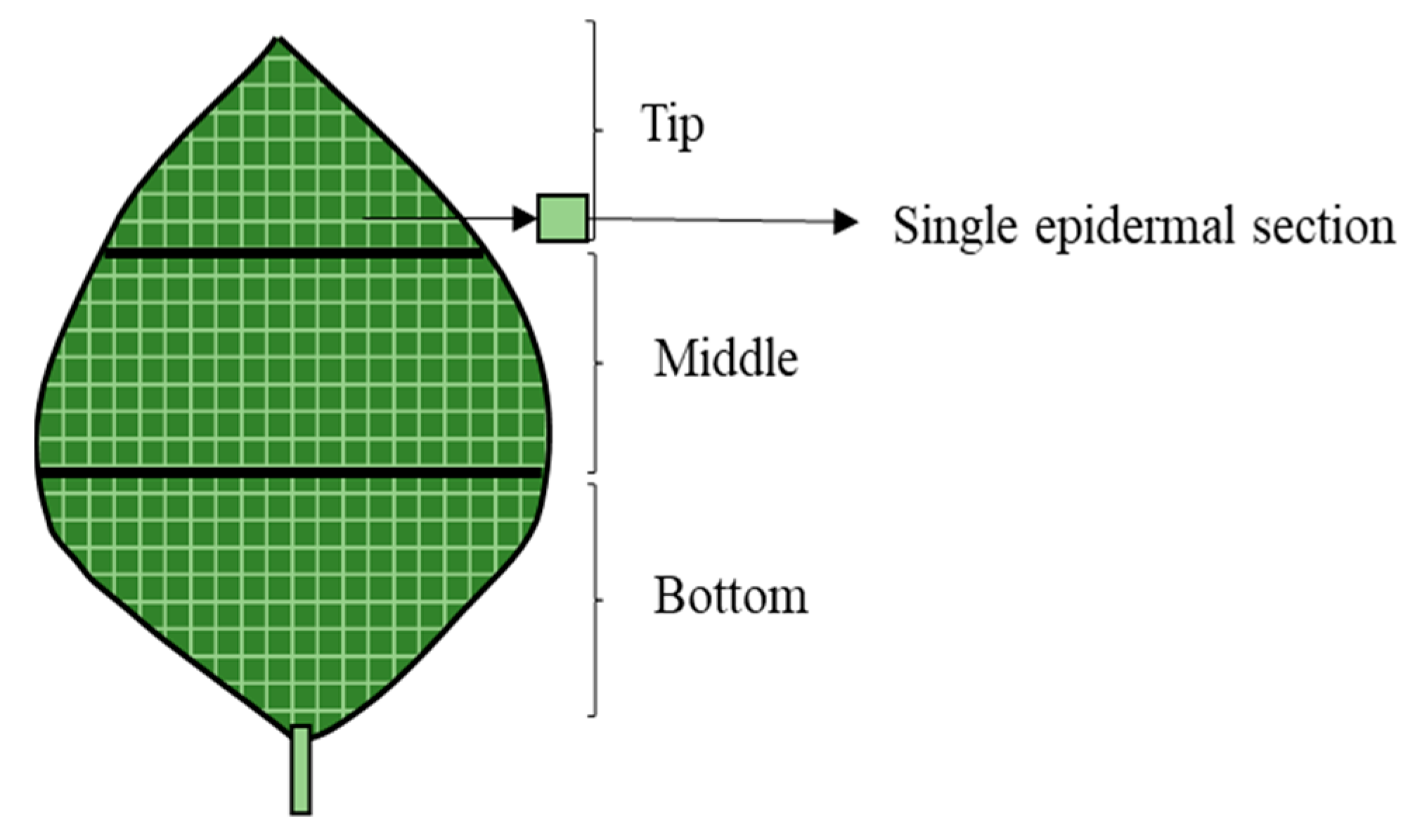
| No. Sections per Position | Number of Stomata/mm2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abaxial | Adaxial | |||||||||
| Tip | Middle | Bottom | Mean | LSD (x) | Tip | Middle | Bottom | Mean | LSD (x) | |
| 5 | 143.15± 3.39 | 134.11 ± 3.80 | 129.61 ± 3.98 | 135.63 | 4.58 | 53.45 ± 5.22 | 55.83 ± 2.94 | 55.47 ± 4.30 | 54.92 | 5.26 |
| 7 | 143.84 ± 2.27 | 133.25 ± 3.50 | 129.65 ± 3.75 | 135.58 | 3.98 | 53.05 ± 4.81 | 56.28 ± 3.98 | 55.64 ± 2.38 | 55.00 | 4.74 |
| 10 | 143.47 ± 2.56 | 133.40 ± 2.04 | 129.31 ± 1.78 | 135.40 | 2.64 | 53.7 ± 2.92 | 55.28 ± 3.16 | 54.64 ± 2.58 | 54.55 | 3.56 |
| 15 | 144.14 ± 2.10 | 133.85 ± 1.41 | 129.12 ± 1.41 | 135.71 | 2.05 | 53.04 ± 1.67 | 54.75 ± 2.91 | 54.87 ± 2.20 | 54.22 | 2.85 |
| 20 | 143.66 ± 1.40 | 134.13 ± 1.40 | 129.17 ± 0.89 | 135.66 | 1.54 | 53.62 ± 1.26 | 54.76 ± 1.42 | 54.97 ± 1.40 | 54.45 | 1.67 |
| 25 | 143.41 ± 0.70 | 133.82 ± 0.96 | 129.14 ± 1.01 | 135.46 | 1.11 | 53.53 ± 0.81 | 54.65 ± 0.62 | 55.03 ± 1.27 | 54.41 | 1.15 |
| 30 | 143.65 ± 0.00 | 133.56 ± 0.00 | 129.70 ± 0.00 | 135.64 | 1.41 | 54.04 ± 0.00 | 54.92 ± 0.00 | 55.05 ± 0.00 | 54.68 | 1.05 |
| LSD (y) | 2.43 | 2.65 | 2.69 | 1.71 | 3.54 | 2.98 | 2.78 | 2.31 | ||
| Growth Stages | Leaflet | Abaxial (Number of Stomata/mm2) | Adaxial (Number of Stomata/mm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tip | Middle | Bottom | LSD (y) | Tip | Middle | Bottom | LSD (y) | ||
| VC | Left | 193.99 | 175.45 | 173.16 | 13.79 | 67.63 | 66.71 | 62.40 | 8.24 |
| Right | 195.64 | 172.79 | 166.46 | 12.75 | 69.83 | 69.28 | 61.48 | 6.97 | |
| LSD (x) | 14.09 | 11.99 | 14.39 | 6.51 | 8.05 | 8.60 | |||
| V1 | Left | 368.34 | 331.50 | 319.71 | 39.58 | 134.62 | 138.57 | 125.17 | 22.83 |
| Right | 366.69 | 327.88 | 300.81 | 20.11 | 144.89 | 126.36 | 120.39 | 15.71 | |
| Central | 374.77 | 324.94 | 300.53 | 24.67 | 140.03 | 120.39 | 116.63 | 15.57 | |
| LSD (x) | 35.25 | 29.51 | 24.16 | 17.56 | 17.62 | 16.57 | |||
| V2 | Left | 314.11 | 284.19 | 285.39 | 26.68 | 77.54 | 83.51 | 84.33 | 13.92 |
| Right | 322.73 | 296.31 | 294.93 | 28.60 | 87.73 | 85.71 | 90.20 | 12.57 | |
| Central | 310.99 | 288.05 | 274.56 | 30.27 | 81.30 | 91.67 | 86.34 | 11.79 | |
| LSD (x) | 29.65 | 25.84 | 30.00 | 11.14 | 12.03 | 14.89 | |||
| V3 | Left | 332.00 | 288.78 | 289.52 | 34.77 | 66.25 | 62.31 | 69.28 | 9.46 |
| Right | 314.20 | 291.26 | 283.37 | 20.67 | 65.43 | 68.92 | 65.34 | 11.32 | |
| Central | 330.99 | 317.41 | 287.68 | 23.24 | 69.92 | 78.37 | 73.32 | 10.92 | |
| LSD (x) | 31.95 | 27.06 | 23.89 | 8.42 | 11.05 | 12.00 | |||
| V4 | Left | 406.97 | 377.06 | 357.52 | 22.09 | 119.84 | 132.88 | 120.76 | 20.11 |
| Right | 397.43 | 367.24 | 366.33 | 19.91 | 141.32 | 134.62 | 129.94 | 23.56 | |
| Central | 380.92 | 362.01 | 330.54 | 25.82 | 137.83 | 128.38 | 127.19 | 22.98 | |
| LSD (x) | 24.73 | 22.22 | 21.11 | 23.08- | 22.15 | 21.55 | |||
| Mean | LSD (w) | LSD (w) | |||||||
| VC | 194.82 | 174.12 | 169.81 | 10.19 | 68.73 | 67.99 | 61.94 | 5.08 | |
| V1 | 369.93 | 328.11 | 307.02 | 20.83 | 139.85 | 128.44 | 120.73 | 13.29 | |
| V2 | 315.95 | 289.52 | 284.96 | 26.21 | 82.19 | 86.96 | 86.26 | 9.53 | |
| V3 | 325.73 | 299.15 | 286.86 | 23.40 | 67.20 | 69.86 | 69.31 | 7.34 | |
| V4 | 395.11 | 368.77 | 351.46 | 19.62 | 132.99 | 131.96 | 125.96 | 19.61 | |
| LSD (z) | 59.22 | 44.40 | 53.72 | 31.53 | 29.88 | 30.77 | |||
| Abaxial Side | Adaxial Side | ||||
|---|---|---|---|---|---|
| Source of Variation | Df | F-Value | Pr (>F) | F-Value | Pr (>F) |
| Growth stage (S) | 4 | 432.14 | <2.2 × 10−16 *** | 286.17 | <2.2 × 10−16 *** |
| Leaflet location (L) | 2 | 1.38 | 0.25 | 1.52 | 0.22 |
| Leaf position (P) | 2 | 76.15 | <2.2 × 10−16 *** | 3.61 | 0.03 * |
| S × L | 7 | 2.50 | 0.02 * | 1.67 | 0.11 |
| S × P | 8 | 1.81 | 0.07 | 2.28 | 0.02 * |
| L × P | 4 | 1.16 | 0.33 | 1.02 | 0.39 |
| S × L × P | 14 | 0.43 | 0.96 | 0.8 | 0.67 |
| Residuals | 588 | ||||
| Total | 629 | ||||
| Abaxial Side (Number of Stomata/mm2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivars | VC | Index | V1 | Index (a) | V2 | Index (a) | V3 | Index (a) | V4 | Index (a) | LSD (y) |
| Daepung | 150.00 | 81.16 | 236.81 | 76.07 | 218.88 | 76.29 | 260.00 | 76.99 | 456.8 | 94.63 | 16.49 |
| Hosim | 158.99 | 86.03 | 293.28 | 94.21 | 249.53 | 86.97 | 301.11 | 89.17 | 422.42 | 87.50 | 17.79 |
| Pungsannamul | 159.48 | 86.29 | 274.19 | 88.07 | 230.45 | 80.32 | 246.54 | 73.01 | 298.84 | 61.90 | 8.94 |
| Seonpung | 174.84 | 94.61 | 343.62 | 110.38 | 280.25 | 97.68 | 297.37 | 88.06 | 329.13 | 68.18 | 13.63 |
| Williams 82 | 184.81 | 100.00 | 311.32 | 100.00 | 286.91 | 100.00 | 337.69 | 100.00 | 482.74 | 100.00 | 13.39 |
| LSD (x) | 6.46 | 20.16 | 12.73 | 11.66 | 16.95 | ||||||
| Adaxial Side (Number of Stomata/mm2) | |||||||||||
| Cultivars | VC | Index | V1 | Index (a) | V2 | Index (a) | V3 | Index (a) | V4 | Index (a) | LSD (y) |
| Daepung | 60.87 | 95.77 | 114.09 | 93.11 | 71.69 | 79.13 | 91.03 | 93.53 | 146.39 | 91.58 | 11.78 |
| Hosim | 59.58 | 93.74 | 148.11 | 120.87 | 87.84 | 96.95 | 104.48 | 107.35 | 146.70 | 91.77 | 10.77 |
| Pungsannamul | 61.42 | 96.63 | 114.16 | 93.16 | 86.87 | 95.88 | 87.84 | 90.25 | 86.07 | 53.84 | 7.08 |
| Seonpung | 71.14 | 111.93 | 129.45 | 105.64 | 95.00 | 104.86 | 98.98 | 101.70 | 116.05 | 72.60 | 8.46 |
| Williams 82 | 63.56 | 100.00 | 122.54 | 100.00 | 90.60 | 100.00 | 97.33 | 100.00 | 159.85 | 100.00 | 9.21 |
| LSD (x) | 5.54 | 12.86 | 8.46 | 6.96 | 12.15 | ||||||
| Abaxial Side | Adaxial Side | ||||
|---|---|---|---|---|---|
| Source of Variation | Df | F-Value | Pr (>F) | F-Value | Pr (>F) |
| Cultivar (C) | 4 | 158.33 | <2.2 × 10−16 *** | 32.19 | <2.2 × 10−16 *** |
| Stage (S) | 4 | 1301.64 | <2.2 × 10−16 *** | 330.17 | <2.2 × 10−16 *** |
| C × S | 16 | 57.81 | <2.2 × 10−16 *** | 17.61 | <2.2 × 10−16 *** |
| Residuals | 1100 | ||||
| Total | 1124 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, S.N.; Jo, H.; Song, J.T.; Kim, K.; Lee, J.-D. Stomatal Density Variation Within and Among Different Soybean Cultivars Across Various Growth Stages. Agriculture 2024, 14, 2028. https://doi.org/10.3390/agriculture14112028
Sultana SN, Jo H, Song JT, Kim K, Lee J-D. Stomatal Density Variation Within and Among Different Soybean Cultivars Across Various Growth Stages. Agriculture. 2024; 14(11):2028. https://doi.org/10.3390/agriculture14112028
Chicago/Turabian StyleSultana, Syada Nizer, Hyun Jo, Jong Tae Song, Kihwan Kim, and Jeong-Dong Lee. 2024. "Stomatal Density Variation Within and Among Different Soybean Cultivars Across Various Growth Stages" Agriculture 14, no. 11: 2028. https://doi.org/10.3390/agriculture14112028
APA StyleSultana, S. N., Jo, H., Song, J. T., Kim, K., & Lee, J. -D. (2024). Stomatal Density Variation Within and Among Different Soybean Cultivars Across Various Growth Stages. Agriculture, 14(11), 2028. https://doi.org/10.3390/agriculture14112028








