Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacteria Additive on Quality, Aerobic Stability, and In Vitro Digestibility of Fermented Total Mixed Ration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Silage Production
2.3. Analysis of Nutrient Content of Experimental Silages
2.4. Determination of Fermentation Metabolites
2.5. Enumeration of Total Bacteria
2.6. Aerobic Stability Evaluation
2.7. Determination of Rumen Degradation Characteristics
2.8. Statistical Analysis
3. Results
3.1. Fermentation Quality of FTMR
3.2. Microbial Population of Fermented TMR
3.3. Nutrient Composition of Fermented TMR
3.4. Protein and Carbohydrate Fractions of Fermented TMR
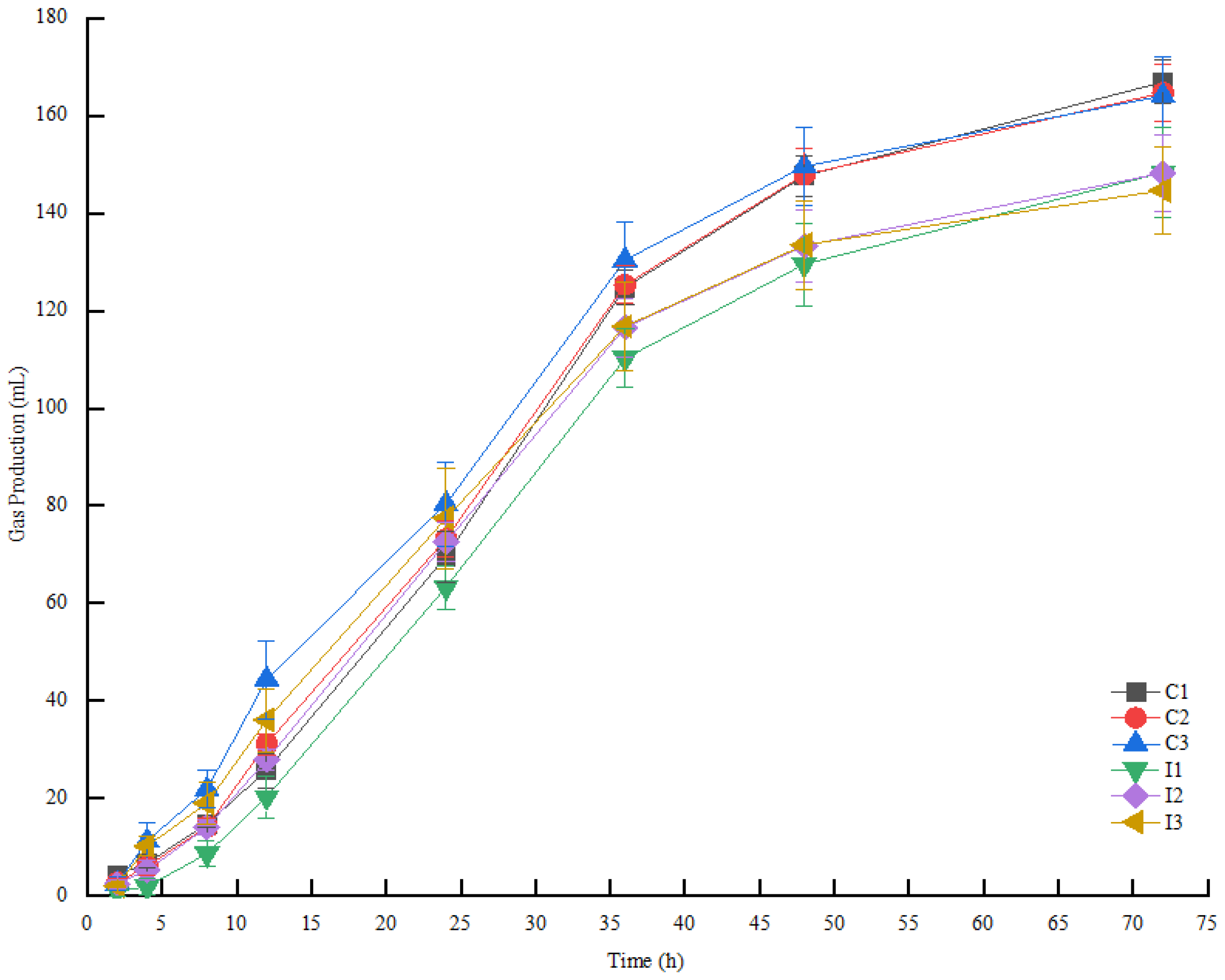
3.5. Rumen Degradation Characteristics of Fermented TMR
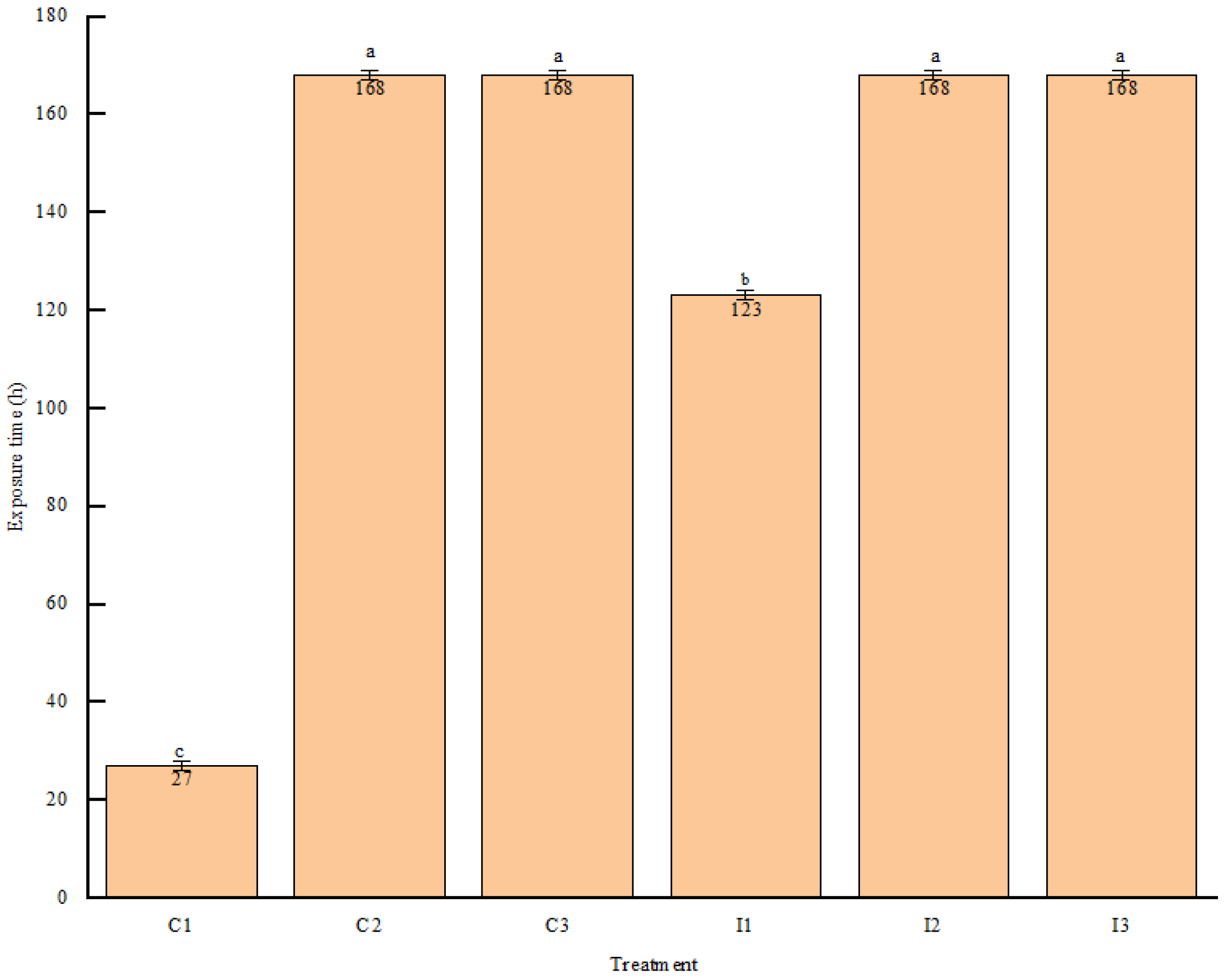
3.6. Influence of Roughage-to-Concentrate Ratio on the Aerobic Stability of Fermentation TMR
3.7. Fermentation Quality of Fermented TMR Exposed to Oxygen for 7 Days
3.8. Microorganism Number of Fermented TMR Fermented by Silage Exposed to Oxygen for 7 Days
4. Discussion
4.1. Fermentation Characteristics, Nutritional Quality, and Microbial Counts of Fermented TMR
4.2. Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacterial Agents on Carbohydrate Protein Fractions of Fermented TMR
4.3. In Vitro Digestive Properties of Fermented TMR
4.4. Aerobic Stability of Fermented TMR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Y.; Guo, C.; Wang, L.; Chen, F.; Dong, X.; Li, X.; Ni, K.; Yang, F. Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in hu lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Wang, G.G.; Wang, M.L.; Wang, J.M.; Yang, C.; Wang, W.J. Basis, Prospects and Suggestions for the Development of Pasture Industry in Southern China. Grassl. Sci. 2015, 32, 2114–2121. [Google Scholar]
- Xu, L.; Tang, G.; Wu, D.; Zhang, J. Yield and nutrient composition of forage crops and their effects on soil characteristics of winter fallow paddy in South China. Front. Plant Sci. 2024, 14, 1292114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Feng, Y.; Shi, Y.; Shen, H.; Hu, H.; Luo, Y.; Xu, L.; Kang, J.; Xing, A.; Wang, S.; et al. Yield and quality properties of silage maize and their influencing factors in China. Sci. China Life Sci. 2022, 65, 1655–1666. [Google Scholar] [CrossRef]
- Vlachostergios, D.N.; Lithourgidis, A.S.; Dordas, C.A. Agronomic, forage quality and economic advantages of red pea (Lathyrus cicera L.) intercropping with wheat and oat under low-input farming. Grass Forage Sci. 2018, 73, 777–778. [Google Scholar] [CrossRef]
- Xu, C.C. Modern Silage Theory and Technology; Science Publishing House: Beijing, China, 2013; p. 50. [Google Scholar]
- Gao, R.; Luo, Y.; Xu, S.; Wang, M.; Sun, Z.; Wang, L.; Yu, Z. Effects of Replacing Ensiled-Alfalfa with Fresh-Alfalfa on Dynamic Fermentation Characteristics, Chemical Compositions, and Protein Fractions in Fermented Total Mixed Ration with Different Additives. Animals 2021, 11, 572. [Google Scholar] [CrossRef]
- Paradhipta, D.; Seo, M.; Jeong, S.; Joo, Y.; Lee, S.; Seong, P.; Lee, H.; Kim, S. Antifungal and carboxylesterase-producing bacteria applied into corn silage still affected the fermented total mixed ration. Anim. Biosci. 2023, 36, 720–730. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Lazzari, G.; Jobim, C.C.; Daniel, J.L.P. Ensiling total mixed ration for ruminants: A review. Agronomy 2020, 10, 879. [Google Scholar] [CrossRef]
- Ribeiro, G.O.; Gruinger, R.J.; Badhan, A.; McAllister, T.A. Mining the rumen for fibrolytic feed enzymes. Anim. Front. 2016, 6, 206. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- Wang, F.J.; Nishino, N.; Wang, J.Y. Comparison of aerobic stability between normal and fermented total mixed diets. LVT 2011, 43, 27–30. [Google Scholar]
- Weinberg, Z.G.; Chen, Y.; Miron, D.; Raviv, Y.; Nahim, E.; Bloch, A.; Yosef, E.; Nikbahat, M.; Miron, J. Preservation of total mixed rations for dairy cows in bales wrapped with polyethylene stretch film—A commercial scale experiment. Anim. Feed Sci. Technol. 2011, 164, 125–129. [Google Scholar] [CrossRef]
- Dineen, M.; McCarthy, B.; Ross, D.; Ortega, A.; Dillon, B.; Van Amburgh, M.E. Characterization of the nutritive value of perennial ryegrass (Lolium perenne L.) dominated pastures using updated chemical methods with application for the Cornell Net Carbohydrate and Protein System. Anim. Feed Sci. Technol. 2021, 272, 114752. [Google Scholar] [CrossRef]
- Nie, H.; Wang, Z.; You, J.; Zhu, G.; Wang, H.; Wang, F. Comparison of in vitro digestibility and chemical composition among four crop straws treated by Pleurotus ostreatus. Asian Austral. J. Anim. 2020, 33, 24–34. [Google Scholar] [CrossRef]
- André, S.; Oliveira, Z.G.; Weinberg, I.M.; Ogunade, A.A.P.; Cervantes, K.G.; Arriola, Y.J.; Donghyeon, K.; Xujiao, L.; Mariana, C.M.; Gonçalves, D.V.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar]
- Tahir, M.; Li, J.; Xin, Y.; Wang, T.; Chen, C.; Zhong, Y.; Zhang, L.; Liu, H.; He, Y.; Wen, X.; et al. Response of fermentation quality and microbial community of oat silage to homofermentative lactic acid bacteria inoculation. Front. Microbiol. 2023, 13, 1091394. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Ferrero, F.; Piano, S.; Tabacco, E.; Borreani, G. Effects of conservation period and Lactobacillus hilgardii inoculum on the fermentation profile and aerobic stability of whole corn and sorghum silages. J. Sci. Food Agr. 2019, 99, 2530–2540. [Google Scholar] [CrossRef]
- Silva, E.B.; Smith, M.L.; Savage, R.M.; Polukis, S.A.; Drouin, P.; Kung, L. Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the bacterial community, fermentation and aerobic stability of high-moisture corn silage. J. Appl. Microbiol. 2021, 130, 1481–1493. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 1995. [Google Scholar]
- Li, F.; Ding, Z.; Ke, W.; Xu, D.; Zhang, P.; Bai, J.; Mudassar, S.; Muhammad, I.; Guo, X. Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 2019, 282, 211–221. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrtion. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Thomas, T.A. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Yu, J.D.; Liu, J.J.; Yang, H.Y.; Gao, L.J.; Yuan, X.F.; Cui, Z.J.; Wang, X.F. Material and microbial changes during corn stalk silage and their effects on methane fermentation. Bioresour. Technol. 2016, 222, 89–99. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The relationship of silage temperature with the microbiological status of the face of corn silage bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L.J. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Menci, R.; Coppa, M.; Torrent, A.; Natalello, A.; Valenti, B.; Luciano, G.; Priolo, A.; Niderkorn, V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 2021, 278, 114977. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Chen, L.; Guo, G.; Yu, C.; Zhang, J.; Shimojo, M.; Shao, T. The effects of replacement of whole-plant corn with oat and common vetch on the fermentation quality, chemical composition and aerobic stability of total mixed ration silage in Tibet. Anim. Sci. J. 2015, 86, 69–76. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wei, X.J. Rumen degradation rate of corn stover silage with different additives and the effect of feeding fattened frame cattle. CJAH 2002, 38, 25–26. [Google Scholar]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Tan, Z.; Li, Z.; Li, Y.; Lv, H.; Zhang, B.; Jin, Q. Microorganism profile, fermentation quality and rumen digestibility in vitro of maize-stalk silages produced at different maturity stages. Crop. Pasture Sci. 2017, 68, 225–233. [Google Scholar] [CrossRef]
- Haigh, P.M.; Parker, J.W.G. Effect of silage additives and wilting on silage fermentation, digestibility and intake, and on liveweight change of young cattle. Grass Forage Sci. 2010, 40, 429–436. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Li, X.; Macadam, J.W.; Zhang, Y. Interaction between plants and epiphytic lactic acid bacteria that affect plant silage fermentation. Front. Microbiol. 2023, 14, 1164904. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.; Ke, W.; Xu, D.; Wang, M.; Huang, W.; Zhang, Y.X.; Fang, L.; Guo, X.S. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Naserian, A.A.; Yari, M.; Jonker, A.; Yu, P. Crude protein fractionation, in situ ruminal degradability and FTIR protein molecular structures of different cultivars within barley, corn and sorghum cereal grains. Anim. Feed Sci. Technol. 2021, 275, 114855. [Google Scholar] [CrossRef]
- Damiran, D.; Yu, P.Q. Metabolic characteristics in ruminants of the proteins in newly developed hull-less barley varieties with altered starch traits. J. Cereal Sci. 2012, 55, 351–360. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Du, W.; Huang, W.M.; Li, S.L. The rumen degradation characteristics of whole sugarcane for dairy cows and its application in substituting alfalfa, oat hay and concentrate in dairy cows’ diets. AVZS 2020, 51, 2743–2756. [Google Scholar]
- Liu, C.L.; Jiang, W.B.; Fu, Q.; Liu, D.; Fu, X.K.; Li, Z.Q. Effects of soybean flavones and genistein on rument fermentation in cows. Czech J. Anim. Sci. 2011, 47, 60–63. [Google Scholar]
- Calsamiglia, S.; Ferret, A.; Devant, M. Effects of pH and pH fluctuations on microbial fermentation and nutrient flow from a dual flow continuous culture system. J. Dairy Sci. 2002, 85, 574–579. [Google Scholar] [CrossRef]
- Kennelly, J.J.; Robinson, B.; Khorasani, G.R. Influence of carbohydrate source and buffer on rumen fermentation characteristics, milk yield, and milk composition early-lactation Holstein cows. J. Dairy Sci. 1999, 82, 2486–2496. [Google Scholar] [CrossRef]
- Grummer, R.R.; Clark, J.H.; Davis, C.L.; Murphy, M.R. Effect of ruminal ammonia-nitrogen concentration on protein degradation in situ. J. Dairy Sci. 1984, 67, 2294–2301. [Google Scholar] [CrossRef]
- Hu, L.Q.; Guo, W.T.; Zhao, X.J.; Li, Y.; Shen, Y.Z.; Cao, Y.F. Effect of different stubble heights on in vitro fermentation indexes of whole-plant corn silage as assessed by in vitro gas production. FR 2022, 45, 61–66. [Google Scholar]
- Rodriguez, M.P.; Mariezcurrena, M.D.; Mariezcurrena, M.A.; Lagunas, B.C.; Elghandour, M.M.M.Y.; Kholif, A.M.; Kholif, A.E.; Almaráz, E.M.; Salem, A.Z.M. Influence of live cells or cells extract of Saccharomyces cerevisiae on in vitro gas production of a total mixed ration. Ital. J. Anim. Sci. 2015, 14, 3713. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Gou, C.L.; Chen, L.M.; Liao, Y.C.; Zhang, H.; Luo, L.L.; Ji, J.H.; Qi, Y. Solid-state fermentation with white rot fungi (Pleurotus species) improves the chemical composition of highland barley straw as a ruminant feed and enhances in vitro rumen digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef]
- Zhang, W.J. Study on the Production of Ruminant Feed by Mixed Solid-State Fermentation of Soybean Dregs with Prion Yeast and White Ground Mold. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2015. [Google Scholar]
- Wang, L.; Wang, J.; Wang, P.; Liu, C.; Li, X.; Chang, J.; Jin, S.; Yin, Q.; Zhu, Q. Effect of Corn Straw Treated with Lactobacillus plantarum and Cellulase on Ruminal Fermentation and Microbiota of Hu Sheep. Fermentation 2024, 10, 402. [Google Scholar] [CrossRef]
- Zhang, J.M.; Guan, H.; Li, H.P.; Jia, Z.F.; Ma, X.; Liu, W.H.; Chen, Y.J.; Chen, S.Y.; Jiang, Y.M.; Gan, L.; et al. Effects of mixing ratio and lactic acid bacterial agent on the quality and rumen degradation characteristics of oat-fed pea fermented TMR. J. Grass Industry 2024, 33, 169–181. [Google Scholar]
- Na, R.H.; Dong, H.M.; Tao, X.P.; Ma, R.J.; Xi, J.L. Comparison of in vitro digestive performance and methane production in dairy cows on different types of diets. JAES 2010, 29, 1576–1581. [Google Scholar]
- King, K.J.; Bergen, W.G.; Sniffen, C.J.; Grant, A.L.; Grieve, D.B.; King, V.L.; Ames, N.K. An assessment of absorbable lysine requirements in lactating cows. J. Dairy Sci. 1991, 74, 2530–2539. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, T.; Ma, S.; Chen, X.; Wang, Z.; Zhao, S.; Ren, Y. Effects of different proportions of stevia stalk on nutrient utilization and rumen fermentation in ruminal fluid derived from sheep. PeerJ 2023, 11, e14689. [Google Scholar] [CrossRef]
- Ma, J.; Sun, G.Q.; Shah, A.M.; Fan, X.; Li, S.L.; Yu, X. Effects of different growth stages of amaranth silage on the rumen degradation of dairy cows. Animals 2019, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, A.; Zhu, L.; Guo, W.; Guo, X.; Zhu, B.; Yang, M. Effect of additive cellulase on fermentation quality of whole-plant corn silage ensiling by a Bacillus inoculant and dynamic microbial community analysis. Front. Microbiol. 2024, 14, 1330538. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.J.; Cao, W.R. Effects of complex microbial preparations on fermentation quality of sorghum straw and its rumen degradation characteristics. Feed. Res. 2023, 46, 109–113. [Google Scholar]
- Li, M.; Zhou, H.L.; Zi, X.J.; Cai, Y.M. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef]
- Guan, H.; Shuai, Y.; Ran, Q.; Hong, Y.Y.; Wang, X.; Li, D.D.; Cai, Y.M.; Zhang, X.Q. The microbiome and metabolome of Napier grass silages prepared with screened lactic acid bacteria during ensiling and aerobic exposure. Anim. Feed Sci. Technol. 2020, 269, 114673. [Google Scholar] [CrossRef]
- Drouin, P.; Tremblay, J.; Renaud, J.; Apper, E. Microbiota succession during aerobic stability of maize silage inoculated with Lentilactobacillus buchneri NCIMB 40788 and Lentilactobacillus hilgardii CNCM-I-4785. Microbiol. Open 2021, 10, e1153. [Google Scholar] [CrossRef]
- Li, D.X.; Ni, K.K.; Zhang, Y.C.; Lin, Y.L.; Yang, F.Y. Influence of lactic acid bacteria, cellulase, cellulase-producing Bacillus pumilus and their combinations on alfalfa silage quality. J. Integr. Agr. 2018, 17, 2768–2782. [Google Scholar] [CrossRef]
- Qiu, C.; Yang, K.; Diao, X.; Zhang, W.; Lv, R.; He, L. Effects of kinds of additives on fermentation quality, nutrient content, aerobic stability, and microbial community of the mixed silage of king grass and rice straw. Front. Microbiol. 2024, 15, 1420022. [Google Scholar] [CrossRef]
- Li, H.; Guan, H.; Jia, Z.; Liu, W.; Ma, X.; Liu, Y.; Wang, H.; Ma, L.; Zhou, Q. Screening of freeze-thaw resistant lactic acid bacteria and their effects on silage quality and aerobic stability of oats. J. Grass Industry 2022, 31, 158–170. [Google Scholar]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. Effect of inoculation with Lactobacillus buchneri LB1819 and Lactococcus lactis O224 on fermentation and mycotoxin production in maize silage compacted at different densities. Anim. Feed Sci. Technol. 2018, 246, 36–45. [Google Scholar] [CrossRef]
| Treatments | Control | Inoculants | ||||
|---|---|---|---|---|---|---|
| Ratio | 65:35 | 70:30 | 75:25 | 65:35 | 70:30 | 75:25 |
| Number | C1 | C2 | C3 | I1 | I2 | I3 |
| Oat–vetch | 39 | 42 | 45 | 39 | 42 | 45 |
| Corn straw | 26 | 28 | 30 | 26 | 28 | 30 |
| Concentrate | 35 | 30 | 25 | 35 | 30 | 25 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Concentrate | ||||||
| Corn flour | 54.73 | |||||
| Soybean meal | 12.27 | |||||
| Corn lees | 8.33 | |||||
| Cottonseed meal | 10 | |||||
| Premix | 5.87 | |||||
| Sodium bicarbonate | 4.13 | |||||
| MgCl | 2.94 | |||||
| NaCl | 1.73 | |||||
| Name | Formula |
|---|---|
| CHO | 100−CP(%DM)−EE(%DM)−Ash(%DM) |
| NSC | 100−CB2(%CHO)−CC(%CHO) |
| CA | [100−STARCH(%NSC)] × [l00−CB2(%CHO)−CC(%CHO)]/100 |
| CB1 | STARCH(%NSC) × [100−CB2(%CHO)−CC(%CHO)]/100 |
| CB2 | 100 × [NDF(%DM)−NDIP(%CP) × 0.01 × CP(%DM)−CC]/CHO(%DM) |
| CC | 100 × [NDF(%DM) × 0.01 × ADL(%NDF) × 2.4]/CHO(%DM) |
| PA | NPN(%SCP) × 0.01 × SCP(%CP) |
| PB1 | SCP(%CP)−PA(%CP) |
| PB2 | 100−PA(%CP)−PB1(%CP)−PB3(%CP)−PC(%CP) |
| PB3 | NDIP(%CP)−ADIP(%CP) |
| PC | ADIP(%CP) |
| Treatments | pH | NH3-N/TN (%) | LA (%) | AA (%) | PA (%) | BA (%) | |
|---|---|---|---|---|---|---|---|
| Control | C1 | 4.65 ± 0.29 Aa | 4.91 ± 0.09 Aab | 3.44 ± 0.70 Aa | 1.28 ± 0.53 Aa | 0.53 ± 0.31 Aa | ND |
| C2 | 4.37 ± 0.01 Aa | 5.06 ± 0.11 Aa | 3.36 ± 0.30 Aa | 0.94 ± 0.23 Aa | 0.28 ± 0.08 Aa | ND | |
| C3 | 4.32 ± 0.02 Aa | 4.12 ± 0.69 Ab | 2.86 ± 1.14 Ba | 0.70 ± 0.42 Aa | 0.24 ± 0.06 Ba | ND | |
| SEM | 0.13719 | 0.32978 | 0.64605 | 0.33578 | 0.15438 | ND | |
| Inoculants | I1 | 4.39 ± 0.03 Aa | 2.65 ± 0.14 Ba | 3.57 ± 0.96 Aa | 0.89 ± 0.39 Aa | 0.55 ± 0.33 Aa | ND |
| I2 | 4.25 ± 0.02 Bb | 1.94 ± 0.07 Bb | 3.13 ± 0.40 Aa | 0.55 ± 0.21 Aa | 0.36 ± 0.13 Aa | ND | |
| I3 | 4.17 ± 0.01 Bc | 1.78 ± 0.26 Bb | 3.26 ± 0.17 Aa | 0.56 ± 0.01 Ba | 0.37 ± 0.05 Aa | ND | |
| SEM | 0.01466 | 0.14285 | 0.49652 | 0.20806 | 0.16819 | ND | |
| Inoculant Ratio Inoculant × Ratio | ** | ** | ** | ** | ** | NS | |
| ** | ** | NS | NS | NS | NS | ||
| NS | NS | NS | NS | NS | NS | ||
| Treatments | LAB (Log cfu·g−1 FM) | Enterobacter (Log cfu·g−1 FM) | Yeast (Log cfu·g−1 FM) | Mold (Log cfu·g−1 FM) | |
|---|---|---|---|---|---|
| Control | C1 | 1.40 ± 0.05 Ba | ND | 3.33 ± 0.03 Aa | 2.40 ± 0.03 Aa |
| C2 | 1.42 ± 0.03 Aa | ND | ND | 0.60 ± 0.02 Ab | |
| C3 | 1.70 ± 0.06 Ba | ND | ND | ND | |
| SEM | 0.03791 | ND | ND | ND | |
| Inoculants | I1 | 3.21 ± 0.19 Aa | ND | 0.71 ± 0.05 Ba | 0.84 ± 0.09 Aa |
| I2 | 1.45 ± 0.03 Ac | ND | ND | 0.31 ± 0.02 Ab | |
| I3 | 1.97 ± 0.02 Ab | ND | ND | ND | |
| SEM | 0.09129 | ND | ND | ND | |
| Inoculant Ratio Inoculant × Ratio | ** | - | - | - | |
| ** | - | - | - | ||
| ** | - | - | - | ||
| Treatments | DM (%) | DM Loss (%) | NDF (%DM) | ADF (%DM) | EE (%DM) | CP (%DM) | WSC (%DM) | Starch (%DM) | Ash (%DM) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | C1 | 50.12 ± 2.14 Aa | 7.93 ± 2.34 Aa | 37.31 ± 2.43 Aa | 17.77 ± 1.00 Bb | 3.40 ± 0.34 Aa | 13.68 ± 0.04 Ba | 3.41 ± 0.47 Aa | 22.45 ± 0.12 Aa | 10.64 ± 0.09 Aa |
| C2 | 44.77 ± 2.43 Ab | 6.54 ± 0.80 Aab | 37.91 ± 2.95 Aa | 17.36 ± 1.23 Ab | 2.70 ± 0.12 Ab | 12.84 ± 0.12 Ab | 3.19 ± 0.14 Aa | 22.45 ± 0.12 Aa | 8.74 ± 0.14 Ab | |
| C3 | 38.34 ± 1.11 Ac | 4.23 ± 0.84 Ab | 40.54 ± 1.81 Aa | 20.86 ± 0.62 Aa | 1.53 ± 0.44 Ac | 12.95 ± 0.04 Bb | 3.24 ± 0.03 Ba | 20.81 ± 2.07 Aa | 8.51 ± 0.17 Ab | |
| SEM | 1.61558 | 1.22923 | 1.99208 | 0.80361 | 0.26685 | 0.06443 | 0.23174 | 1.37061 | 0.11584 | |
| Inoculants | I1 | 48.55 ± 0.41 Aa | 4.19 ± 1.59 Aa | 38.91 ± 2.19 Aa | 16.72 ± 0.59 Ab | 3.62 ± 0.11 Aa | 14.47 ± 0.24 Aa | 3.38 ± 0.20 Ab | 23.51 ± 0.36 Aa | 10.49 ± 0.17 Aa |
| I2 | 45.55 ± 0.04 Ab | 2.11 ± 0.18 Bb | 40.01 ± 2.53 Aa | 19.63 ± 0.64 Aa | 2.64 ± 0.58 Ab | 13.07 ± 0.07 Ac | 3.39 ± 0.15 Ab | 21.62 ± 0.67 Ab | 8.39 ± 0.06 Bb | |
| I3 | 39.31 ± 1.33 Ac | 2.55 ± 0.27 Bab | 41.57 ± 0.77 Aa | 19.87 ± 0.75 Aa | 1.42 ± 0.19 Ac | 13.60 ± 0.12 Ab | 3.84 ± 0.08 Aa | 21.35 ± 0.75 Ab | 8.07 ± 0.06 Bc | |
| SEM | 0.65889 | 0.7654 | 1.61855 | 0.5415 | 0.29243 | 0.1294 | 0.12532 | 0.50132 | 0.0918 | |
| Inoculant Ratio Inoculant × Ratio | NS | ** | NS | NS | NS | ** | * | NS | ** | |
| ** | ** | NS | ** | ** | ** | NS | NS | ** | ||
| NS | NS | NS | * | NS | ** | NS | NS | NS | ||
| Treatments | PA (%CP) | PB1 (%CP) | PB2 (%CP) | PB3 (%CP) | PC (%CP) | |
|---|---|---|---|---|---|---|
| Control | C1 | 5.33 ± 0.48 Ac | 49.56 ± 1.64 Aa | 16.46 ± 3.68 Ac | 12.18 ± 4.36 Aa | 16.47 ± 0.60 Aab |
| C2 | 6.15 ± 0.18 Ab | 47.51 ± 0.47 Ab | 21.73 ± 1.57 Ab | 7.49 ± 0.16 Aab | 17.11 ± 0.89 Aa | |
| C3 | 6.78 ± 0.12 Aa | 40.47 ± 0.46 Bc | 34.24 ± 0.18 Aa | 3.17 ± 1.15 Bb | 15.34 ± 0.95 Bb | |
| SEM | 0.24834 | 0.83283 | 1.88762 | 2.12604 | 0.6788 | |
| Inoculants | I1 | 5.78 ± 0.10 Ab | 49.65 ± 1.81 Aa | 17.74 ± 2.58 Ab | 9.57 ± 1.18 Aa | 17.26 ± 1.01 Aab |
| I2 | 6.54 ± 0.20 Aa | 48.30 ± 0.84 Aa | 22.10 ± 0.64 Aa | 6.76 ± 0.03 Bb | 16.31 ± 0.24 Ab | |
| I3 | 6.75 ± 0.14 Aa | 42.82 ± 0.11 Ab | 24.90 ± 0.60 Ba | 6.74 ± 0.91 Ab | 18.79 ± 1.58 Aa | |
| SEM | 0.12481 | 0.94023 | 1.2838 | 0.70133 | 0.88903 | |
| Inoculant Ratio Inoculant × Ratio | * | NS | * | NS | * | |
| ** | ** | ** | ** | NS | ||
| NS | NS | ** | * | ** | ||
| Treatments | CHO (%DM) | NSC (%CHO) | CA (%CHO) | CB1 (%CHO) | CB2 (%CHO) | CC (%CHO) | |
|---|---|---|---|---|---|---|---|
| Control | C1 | 73.11 ± 0.53 Ac | 56.35 ± 3.32 Aa | 56.13 ± 3.34 Aa | 21.87 ± 2.04 Aa | 37.24 ± 3.45 Aa | 6.41 ± 0.50 Bb |
| C2 | 74.87 ± 0.05 Ab | 53.05 ± 2.38 Aa | 52.83 ± 2.38 Aa | 22.45 ± 0.12 Aa | 38.90 ± 1.82 Aa | 8.05 ± 0.64 Aa | |
| C3 | 77.01 ± 0.50 Aa | 55.74 ± 3.60 Aa | 55.54 ± 3.62 Aa | 20.81 ± 2.07 Aa | 38.05 ± 3.01 Aa | 6.21 ± 0.59 Ab | |
| SEM | 0.34351 | 2.56674 | 2.57782 | 1.37061 | 2.32285 | 0.47085 | |
| Inoculants | I1 | 72.83 ± 0.23 Ac | 54.29 ± 2.55 Aa | 54.07 ± 2.55 Aa | 21.62 ± 0.67 Ab | 37.95 ± 1.92 Aa | 7.77 ± 0.63 Aa |
| I2 | 74.50 ± 0.41 Ab | 53.31 ± 3.65 Aa | 53.10 ± 3.64 Aa | 21.35 ± 0.75 Ab | 39.25 ± 3.64 Aa | 7.44 ± 0.18 Aa | |
| I3 | 76.91 ± 0.09 Aa | 52.30 ± 0.94 Aa | 52.07 ± 0.93 Aa | 23.51 ± 0.36 Aa | 41.55 ± 0.89 Aa | 6.15 ± 0.76 Ab | |
| SEM | 0.2245 | 2.14329 | 2.14272 | 0.50132 | 1.98372 | 0.47414 | |
| Inoculant Ratio Inoculant × Ratio | * | NS | NS | NS | NS | NS | |
| ** | NS | NS | NS | NS | ** | ||
| NS | NS | NS | NS | NS | * | ||
| Treatments | pH | NH3-N (mg·dL−1) | Crude Protein Degradation Rate (%) | Neutral Detergent Fiber Degradation Rate (%) | Acid Detergent Fiber Degradation Rate (%) | Dry Matter Degradation Rate (%) | |
|---|---|---|---|---|---|---|---|
| Control | C1 | 6.34 ± 0.03 Aa | 14.48 ± 0.34 Ab | 46.74 ± 0.36 Bb | 65.61 ± 2.41 Aa | 60.24 ± 2.70 Aa | 75.67 ± 0.23 Ab |
| C2 | 6.32 ± 0.01 Aa | 16.40 ± 0.65 Aa | 47.39 ± 0.61 Bb | 63.36 ± 1.72 Ba | 58.35 ± 2.00 Bab | 69.81 ± 0.13 Bc | |
| C3 | 6.15 ± 0.01 Ab | 13.72 ± 0.26 Bb | 57.47 ± 0.52 Ba | 62.21 ± 2.98 Ba | 54.19 ± 2.68 Ab | 79.26 ± 0.13 Aa | |
| SEM | 0.01361 | 0.36561 | 0.41332 | 1.97837 | 2.0267 | 0.13959 | |
| Inoculants | I1 | 6.30 ± 0.02 Aa | 12.33 ± 0.94 Bb | 51.21 ± 0.22 Ac | 64.45 ± 0.42 Ab | 58.73 ± 3.31 Ab | 77.63 ± 1.65 Aa |
| I2 | 6.21 ± 0.02 Bb | 17.54 ± 0.38 Aa | 54.86 ± 0.98 Ab | 68.52 ± 2.26 Aa | 64.48 ± 0.96 Aa | 77.43 ± 0.30 Aa | |
| I3 | 6.20 ± 0.07 Ab | 17.70 ± 0.30 Aa | 60.09 ± 1.06 Aa | 67.70 ± 0.66 Aa | 55.40 ± 1.65 Ab | 78.70 ± 0.21 Aa | |
| SEM | 0.03355 | 0.49766 | 0.68716 | 1.12641 | 3.24955 | 0.79716 | |
| Inoculant Ratio Inoculant × Ratio | * | ** | ** | ** | ** | ** | |
| ** | ** | ** | NS | * | ** | ||
| ** | ** | ** | * | * | ** | ||
| Treatments | 2 h | 4 h | 8 h | 12 h | 24 h | 36 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|---|---|---|
| Control | C1 | 4.35 ± 1.00 Aa | 6.72 ± 0.78 Ab | 14.78 ± 0.78 Ab | 25.72 ± 3.55 Ab | 69.63 ± 5.20 Ab | 124.75 ± 3.63 Aa | 147.60 ± 4.21 Aa | 166.88 ± 4.46 Aa |
| C2 | 2.73 ± 1.06 Ab | 6.13 ± 1.68 Ab | 14.22 ± 1.68 Ab | 31.15 ± 2.14 Ab | 73.23 ± 3.64 Aab | 125.32 ± 3.78 Aa | 147.88 ± 5.28 Aa | 164.68 ± 5.83 Aa | |
| C3 | 2.47 ± 1.36 Ab | 11.25 ± 3.83 Aa | 21.87 ± 3.83 Aa | 44.40 ± 8.01 Aa | 80.35 ± 8.60 Aa | 130.38 ± 7.84 Aa | 149.60 ± 7.89 Aa | 164.05 ± 8.10 Aa | |
| SEM | 0.66569 | 1.4178 | 2.34251 | 3.00586 | 3.56303 | 3.56303 | 3.6439 | 3.6439 | |
| Inoculants | I1 | 0.93 ± 0.87 Bb | 1.87 ± 1.75 Bc | 8.68 ± 2.67 Bc | 20.25 ± 4.42 Bc | 63.33 ± 4.45 Bb | 110.23 ± 5.96 Ba | 129.45 ± 8.37 Ba | 148.32 ± 9.18 Ba |
| I2 | 2.47 ± 0.83 Aab | 5.27 ± 1.99 Ab | 14.07 ± 1.51 Ab | 27.93 ± 2.19 Bb | 72.55 ± 4.09 Aa | 116.57 ± 5.98 Ba | 133.23 ± 7.43 Ba | 148.20 ± 7.93 Ba | |
| I3 | 1.98 ± 0.10 A | 10.17 ± 1.96 Aa | 19.02 ± 4.19 Aa | 36.05 ± 6.46 Aa | 77.48 ± 10.34 Aa | 116.78 ± 9.17 Ba | 133.48 ± 9.02 Ba | 144.67 ± 9.04 Ba | |
| SEM | 0.51643 | 1.09828 | 1.78386 | 2.70926 | 3.99098 | 4.15541 | 5.1772 | 5.04474 | |
| Inoculant Ratio Inoculant × Ratio | NS | ** | ** | ** | ** | * | NS | ** | |
| ** | NS | NS | NS | NS | ** | ** | ** | ||
| ** | NS | * | ** | NS | NS | NS | NS | ||
| Treatments | pH | NH3-N/TN (%) | LA (%DM) | AA (%DM) | PA (%DM) | BA (%DM) | |
|---|---|---|---|---|---|---|---|
| Control | C1 | 8.35 ± 0.02 Aa | 6.12 ± 0.15 Aa | 2.20 ± 0.80 Bb | 1.99 ± 0.58 Aa | 0.68 ± 0.36 Aa | ND |
| C2 | 4.33 ± 0.02 Ab | 6.18 ± 0.25 Aa | 4.59 ± 0.03 Aa | 1.29 ± 0.08 Ab | 0.54 ± 0.10 Aa | ND | |
| C3 | 4.26 ± 0.03 Ac | 5.24 ± 0.04 Ab | 4.16 ± 0.17 Aa | 1.05 ± 0.07 Ab | 0.31 ± 0.06 Ba | ND | |
| SEM | 0.01846 | 0.13708 | 0.38621 | 0.27746 | 0.17656 | ND | |
| Inoculants | I1 | 5.28 ± 0.03 Ba | 4.34 ± 0.13 Ba | 4.30 ± 0.15 Aa | 0.62 ± 0.11 Ba | 0.59 ± 0.11 Aa | ND |
| I2 | 4.19 ± 0.01 Bb | 2.48 ± 0.04 Bb | 4.16 ± 1.52 Aa | 0.79 ± 0.48 Aa | 0.56 ± 0.42 Aa | ND | |
| I3 | 4.13 ± 0.01 Bc | 2.58 ± 0.04 Bb | 4.36 ± 0.24 Aa | 0.74 ± 0.07 Ba | 0.58 ± 0.11 Aa | ND | |
| SEM | 0.01305 | 0.06558 | 0.72869 | 0.2368 | 0.21271 | ND | |
| Inoculant Ratio Inoculant × Ratio | ** | ** | NS | ** | NS | NS | |
| ** | ** | * | NS | NS | NS | ||
| ** | ** | * | * | NS | NS | ||
| Treatments | LAB (Log cfu·g−1 FM) | Enterobacter (Log cfu·g−1 FM) | Yeast (Log cfu·g−1 FM) | Mold (Log cfu·g−1 FM) | |
|---|---|---|---|---|---|
| Control | C1 | 1.07 ± 0.09 Ba | ND | 5.08 ± 0.15 Aa | 3.20 ± 0.11 Aa |
| C2 | ND | ND | 0.62 ± 0.04 Ab | 1.94 ± 0.06 Ab | |
| C3 | ND | ND | 0.58 ± 0.04 Ab | 1.80 ± 0.02 Ab | |
| SEM | 0.09129 | ND | 0.07628 | 0.05881 | |
| Inoculants | I1 | 2.85 ± 0.03 Aa | ND | 2.36 ± 0.18 Ba | 2.18 ± 0.00 Ba |
| I2 | ND | ND | 0.41 ± 0.03 Ab | 1.35 ± 0.05 Bc | |
| I3 | ND | ND | 0.37 ± 0.02 Ab | 1.21 ± 0.03 Bb | |
| SEM | 0.01557 | ND | 0.08828 | 0.02776 | |
| Inoculant Ratio Inoculant × Ratio | NS | NS | ** | ** | |
| NS | NS | ** | ** | ||
| NS | NS | ** | ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Wen, S.; Li, H.; Chen, S.; Chen, Y.; Huang, Y.; Guan, H. Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacteria Additive on Quality, Aerobic Stability, and In Vitro Digestibility of Fermented Total Mixed Ration. Agriculture 2024, 14, 2230. https://doi.org/10.3390/agriculture14122230
Bai R, Wen S, Li H, Chen S, Chen Y, Huang Y, Guan H. Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacteria Additive on Quality, Aerobic Stability, and In Vitro Digestibility of Fermented Total Mixed Ration. Agriculture. 2024; 14(12):2230. https://doi.org/10.3390/agriculture14122230
Chicago/Turabian StyleBai, Rui, Sisi Wen, Haiping Li, Shiyong Chen, Youjun Chen, Yanling Huang, and Hao Guan. 2024. "Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacteria Additive on Quality, Aerobic Stability, and In Vitro Digestibility of Fermented Total Mixed Ration" Agriculture 14, no. 12: 2230. https://doi.org/10.3390/agriculture14122230
APA StyleBai, R., Wen, S., Li, H., Chen, S., Chen, Y., Huang, Y., & Guan, H. (2024). Effect of Roughage-to-Concentrate Ratio and Lactic Acid Bacteria Additive on Quality, Aerobic Stability, and In Vitro Digestibility of Fermented Total Mixed Ration. Agriculture, 14(12), 2230. https://doi.org/10.3390/agriculture14122230





