Abstract
Treatment of plants with mineral-organic concentrates has developed intensively in recent years. Fertilizers containing, among others, humus, L-amino acids, vitamins, chelates and metal complexes, macro-, micro- and meso-elements, organic matter and humic acids from the top organic layer of leonardite coal have a positive effect on plant growth and quality and seed yield. An experiment was conducted between 2019 and 2022 at the University of Agriculture in Kraków, in which the impact of mineral-organic concentrate on the seed yield and quality of perennial ryegrass (Lolium perenne L.) 2N Bokser (Hodowla Roślin Grunwald Ltd. Grupa IHAR, Grunwa, Poland) was determined. As part of the experiment, plants were sprayed with a mineral-organic product in three different doses: 1.0, 2.0 and 3.0 L·ha−1. The following parameters were examined: the number of generative shoots, the length of the flag leaf, seed yield, germination capacity and the weight of one thousand seeds. It turned out that the application of the concentrate with 3 L·ha−1 and 2 L·ha−1 led to a significant increase in the number of generative shoots and in seed yield, compared to control. In addition, seed germination capacity of fertilized plants was higher, with the greatest increase after the application of 3 L·ha−1. Moreover, the average weight of one thousand seeds was significantly higher in fertilized plants, compared to the control, and the largest increase was recorded in response to the highest dose. The results indicated a positive effect of the organic-mineral concentrate on perennial ryegrass development parameters and seed quality.
1. Introduction
The necessity to reduce climate change impacts, carbon footprints, water consumption, and economic considerations restricts the use of mineral fertilizers, both macro (N, P, K) and micronutrients. Modern agricultural practices and new fertilization technologies have developed in recent years, including the application of balanced mineral organic concentrates that reduce soil degradation and water pollution [1]. Currently, plant biostimulation, particularly with amino acids and humic and fulvic acids, is becoming increasingly important as the most effective alternative to traditional fertilizer treatment of grasslands and seed crops [2,3,4]. The function of biostimulants is to support physiological processes in the plant, to improve its overall condition, and thus increase yield and seed production. They also possess protective and preventive properties against abiotic and biotic stress [5].
One of the innovative solutions in the treatment of seed crops, including grass grown for seeds, may be the application of humic substances. Their activity have been reported to be similar to that of phytohormones [6], and thus they have a stimulating effect on plant physiological processes and growth [7,8,9]. Their application positively influences nutrient uptake, improving the nutritional status of plants, among others, by increasing the activity of enzymes ensuring positive nutrient utilization, such as nitrate reductase, glutamine and glutamate synthetase or phosphoenolpyruvate carboxylase (PEP case) [10]. They enhance the expression of gene isoforms encoding plasma membrane proton pumps (PM H+-ATPase) and increase their activity [11,12]. Additionally, humic compounds improve ion transport in plant tissues. The application of humic substances has a beneficial effect on plant photosynthesis and respiration rates, increases protein synthesis and plant hormone activity and improves the absorption of nutrients such as phosphorus and potassium [13].
The positive effects of amino acids are due to their participation in the biosynthesis of multiple organic compounds [14]. Most notably, they are the building blocks of proteins and take an active part in plant growth and development [15]. They are also involved in the composition of enzymes and nucleic acids. The use of amino acid products can prevent and mitigate the harmful effects of stress conditions [16].
Vitamin C, which is a powerful antioxidant, also plays an important role in plant development and promotes iron uptake. It ensures proper functioning of photosynthesis and is also involved in the synthesis of the plant hormone ethylene [17]. In addition, it acts as an inducer of disease resistance in various crop-plant-pathogen interactions [18,19].
An additional benefit of those substances is that they are applied in low doses to leaves. This effectively limits the demand for soil-applied mineral fertilizers, thus reducing adverse environmental impacts resulting from leaching their nutrients [20].
Considering the positive effect of the application of bio products to grass seed crops, an experiment was carried out to determine the effect of a product in a concentrated liquid form, combining the action of its biologically active substances and mineral components in a chelated form, free L-amino acids, vitamins and organic matter and humic acids from leonardite coal, on perennial ryegrass production, morphological characteristics and seed yield.
Perennial ryegrass (Lolium perenne L.) is a significant subject of research due to its unique chemical composition, which makes it important in agriculture. It is recognized as a key forage plant in temperate zones due to its high yield and feed quality [21]. The soluble carbohydrate content in perennial ryegrass feed contributes to the efficiency of meat and milk production in cattle, which may also assist in reducing nitrogen oxide emissions [22,23]. High-sugar varieties of grass supporting this increased productivity have been success-fully developed [24]. This plant serves as a substantial source of protein, carotenoids, minerals, water-soluble carbohydrates, and fatty acids. With its unique combination of mineral components, perennial ryegrass stands out for high digestibility and excellent palatability, gaining recognition among various livestock species. This forms a solid foundation for further in-depth analysis of the potential of perennial ryegrass as a significant element in animal nutrition systems.
2. Materials and Methods
2.1. Study Site
The field experiment was conducted between 2019 and 2022 at the Experimental Station of the University of Agriculture in Kraków, Poland (50°07′ N, 20°05′ E, 272 m.a.s.l.). It was set up with the randomized block method, in four replications and experimental plots of 10 m2. The experiment was conducted on degraded black soil derived from loess and of very good quality (Class I). The chemical properties of soil are shown in Table 1.

Table 1.
Chemical properties of soil in the study site.
2.2. Experiment Design and Factor
The mineral-organic product was applied at three doses constituting an experimental factor: 1.0, 2.0 and 3.0 L·ha−1. On the control plot no stimulator was applied. Spraying plants with the product was conducted twice during the growing season, the first time at its start, the second 14 days later. Spray solutions were prepared by dissolving an appropriate amount of the mineral-organic product in such an amount of water that the volume of the spray liquid was 300 L·ha−1.
The concentrate contained organic carbon, salt, amino acids, minerals, macro-elements and micro-elements. The detailed composition was as follows: total nitrogen (N) content: 5.0%; total phosphorus expressed as phosphorus pentoxide (P2O5): 2.0%; total potassium expressed as potassium oxide (K2O): 3.5%; total calcium expressed as calcium oxide (CaO): 0.22%, total magnesium expressed as magnesium oxide (MgO): 0.02%, total sulphur expressed as sulphur trioxide (SO3): 1.5%; total boron (B): 1.15%; total copper (Cu): 0.7%; total iron (Fe): 0.6%; total manganese (Mn): 1.3%; total molybdenum (Mo): 0.05%; total zinc (Zn): 0.5%; total titanium (Ti): 0.008%; dry matter (DM) content: 55.4%; organic matter: 53.0%DM; humic acids: 2.8%; fulvic acids: 1.2%; amino acid content: 2.2%DM. The mineral-organic concentrate was produced by the QULTIVO Ltd. in Charsznica (ul. Młyńska 21, 32-250 Charsznica—Poland).
The research was conducted on perennial ryegrass (Lolium perenne L.) 2N Bokser. This variety has been in the National Register since 14 March 2005 and was bred by the Hodowla Roślin Grunwald Ltd. Grupa IHAR. The sowing rate of perennial ryegrass was 15 kg·ha−1, with a distance between rows of 12 cm. Seeds were sown using a precision sowing machine (Wintersteiger AG, Ried im Innkreis, Austria). Grass seeds were sown without an intercrop on 5 September 2019. Mineral fertilization was applied to the soil in the following doses: before sowing, 40 kg N∙ha−1 in the form of ammonium nitrate, 26.2 kg P∙ha−1 in the form of enriched superphosphate, and 66.4 kg K∙ha−1 in the form of 57% potassium salt. Additionally, in the autumn, phosphorus and potassium were applied to the main field in the same amounts and forms as pre-sowing. Similarly, in years of full utilization, the same doses of phosphorus and potassium were applied in the same form during the autumn. The nitrogen fertilization for perennial ryegrass was divided into three parts. The first portion of nitrogen was applied after harvesting the perennial ryegrass seeds at a dose of 40 kg N∙ha−1, the second part in spring at the beginning of vegetation at a rate of 20 kg N∙ha−1, and the third part between April and May at a dose of 40 kg N∙ha−1. At the tillering stage, plots were sprayed to eliminate dicotyledonous weeds. In case of heavy weed infestation, Starane 250 SL (DOW, Midland, MI, USA) was applied at a rate of 0.6 L·ha−1. Single stubborn weeds were controlled with Roundup (Bayer AG, Leverkusen, Germany), while monocotyledonous weeds were treated at the beginning of September and in the spring, immediately after the start of the growing period, with Stomp 330 EC (BASF, Ludwigshafen, Germany) at a rate of 5 L·ha−1 dissolved in 300 L of water.
At the beginning of ripening, the length of 30 shoots and spikes and the length of flag leaf blades were measured on each plot. Prior to the seed harvest, the number of generative shoots in each plot was recorded in a randomly determined area of 1 m2. The seed harvest of perennial ryegrass was in mid-July (14 July 2020; 12 July 2021; 15 July 2022). It was conducted with a Wintersteiger NM-ELITE field harvester. After the harvest, straw and postharvest residues were removed from the plots. Following threshing, the seeds were cleaned and dried to a moisture content of 14%. Seed sowing value was determined four months after the harvest, based on a sample of 100 seeds in four replicates from each plot. According to the standards, seeds producing normal seedlings (normally germinating) and healthy but non-germinating seed are considered to possess germination capacity. Thousand-seed weight and germination capacity were determined separately for each plot in samples collected after threshing, re-drying, and recleaning. Germination energy was calculated after 5 days and germination capacity after 14 days on a Jacobsen germinator (Rumded Rubarth Apparate GmbH, Laatzen, Germany). The seed-related tests was conducted according to ISTA standards [25].
2.3. Statistical Analysis
In order to find statistically significant differences between treatment variants, one-way analysis of variance (ANOVA) was applied together with Tukey’s HSD post hoc test. Statistical analysis was performed using the Statistica 14.0 program (TIBCO Software Inc., Palo Alto, CA, USA) at the significance level of p = 0.05. The normal distribution was assessed with the Shapiro–Wilk test. In the case of germination rate (this parameter expresses probability, it does not have a normal distribution), calculations were made after data transformation, using the Bliss method. The growing period was used as a random factor in the mixed model of the total variance. A simple linear regression analysis and Pearson’s correlation coefficient were applied to find the relationship between ryegrass morphological and productive variables.
2.4. Weather Conditions
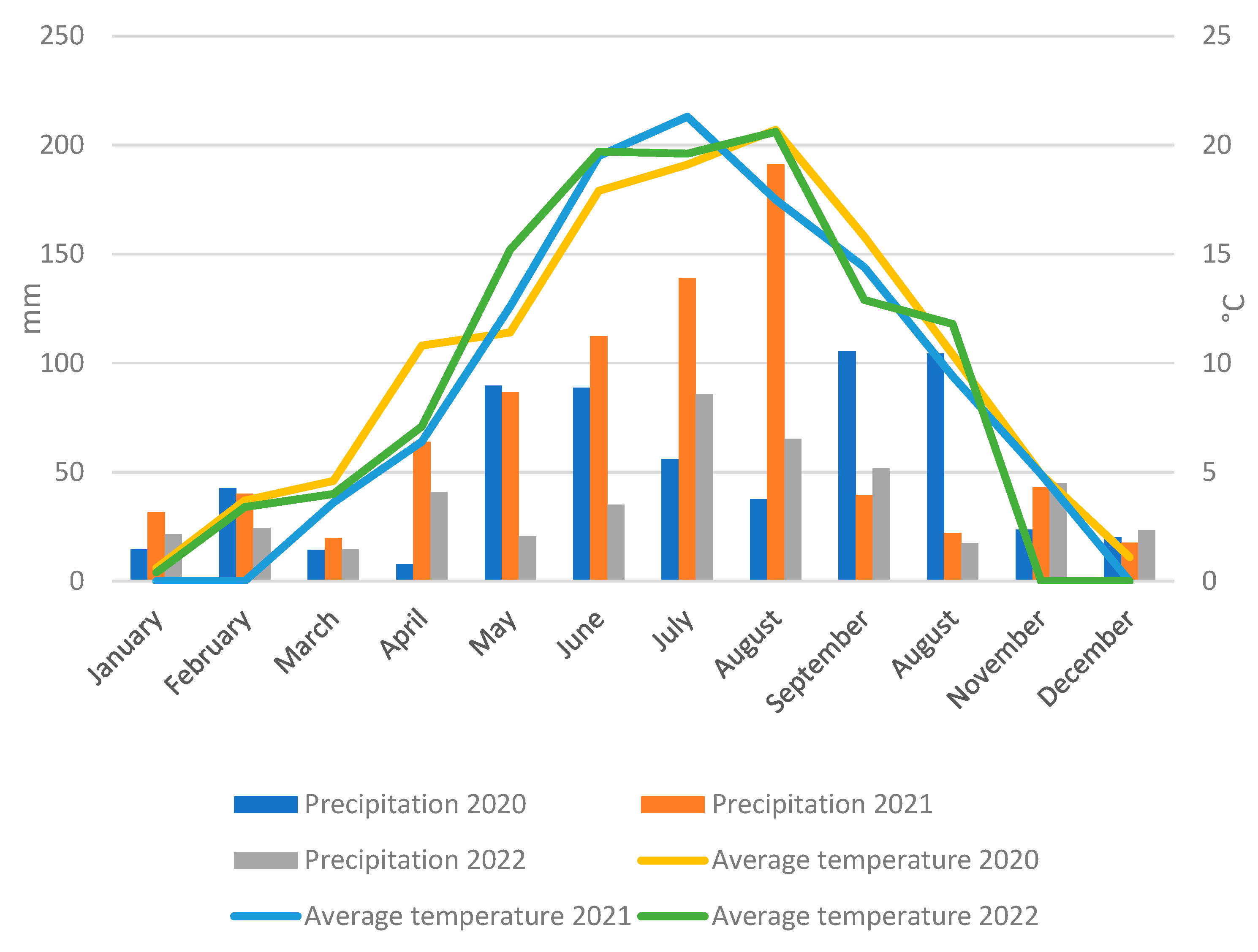
From 2020 to 2022, weather conditions were favorable for the growth and development of perennial ryegrass seeds. Rainfall in those years was as follows: in 2020—605 mm, in 2021—807 mm and in 2022—446 mm (Figure 1). During the growing season, from April to September, rainfall was 385 mm in 2020, 633 mm in 2021 and 299 mm in 2022. The average air temperature was 10.1 °C (2020), 8.9 °C (2021) and 9.0 °C (2022). During the growing season (April–September), the average temperature was: 16.0 °C (2020), 15.3 °C (2021), and 15.8 °C (2022) (Figure 1). Comparing these data, there were large differences in precipitation between years, with 2021 standing out for its particularly high precipitation, which was almost double that of 2022.
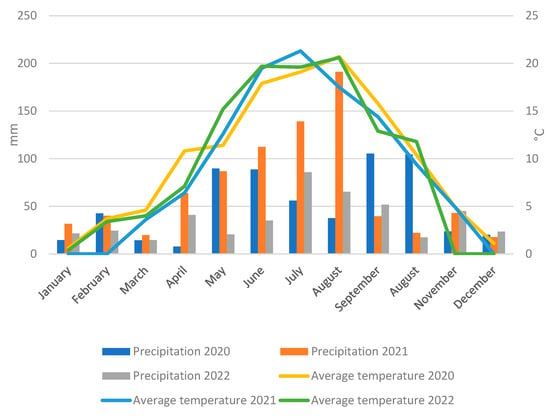
Figure 1.
Total precipitation and average air temperature at the Experimental Station in Prusy, University of Agriculture in Kraków, between 2020 and 2022.
3. Results
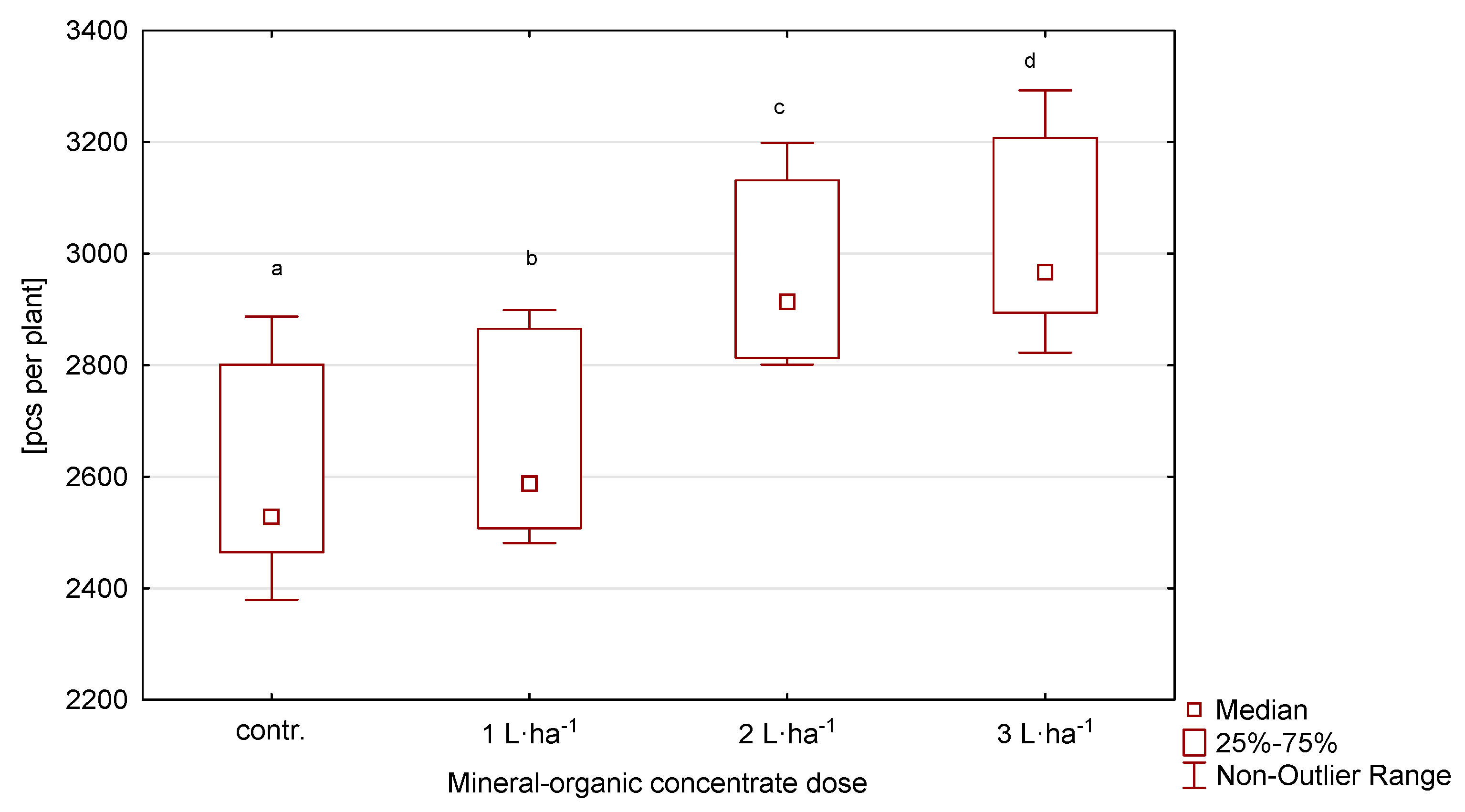
The highest number of generative shoots was developed by plants in the first year, while in the following years it was lower (Table 2, Figure 2). The applied treatment significantly affected the number of generative shoots. In the variant with the highest dose of the organic-mineral concentrate at 3 L·ha−1, the number of shoots was the highest. Similarly, in the second variant, with 2 L·ha−1, a statistically significant higher number of shoots was recorded than on the control plot. The number of shoots showed a variation of 9.0%.

Table 2.
Selected biometric features of perennial ryegrass in response to the dose of the organic-mineral concentrate. Different letters indicate the significant differences (p < 0.05).
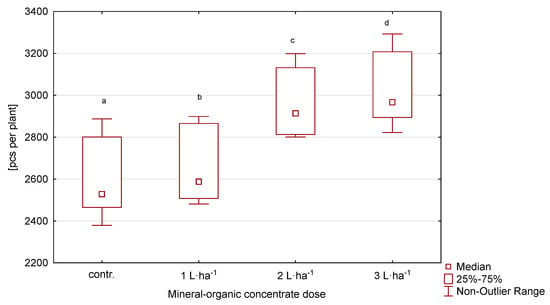
Figure 2.
Number of generative shoots of perennial ryegrass in response to the dose of the organic-mineral concentrate, average of 2020–2022. The same letters (a, b, c, d) mean no statistically significant changes (p ≥ 0.05).
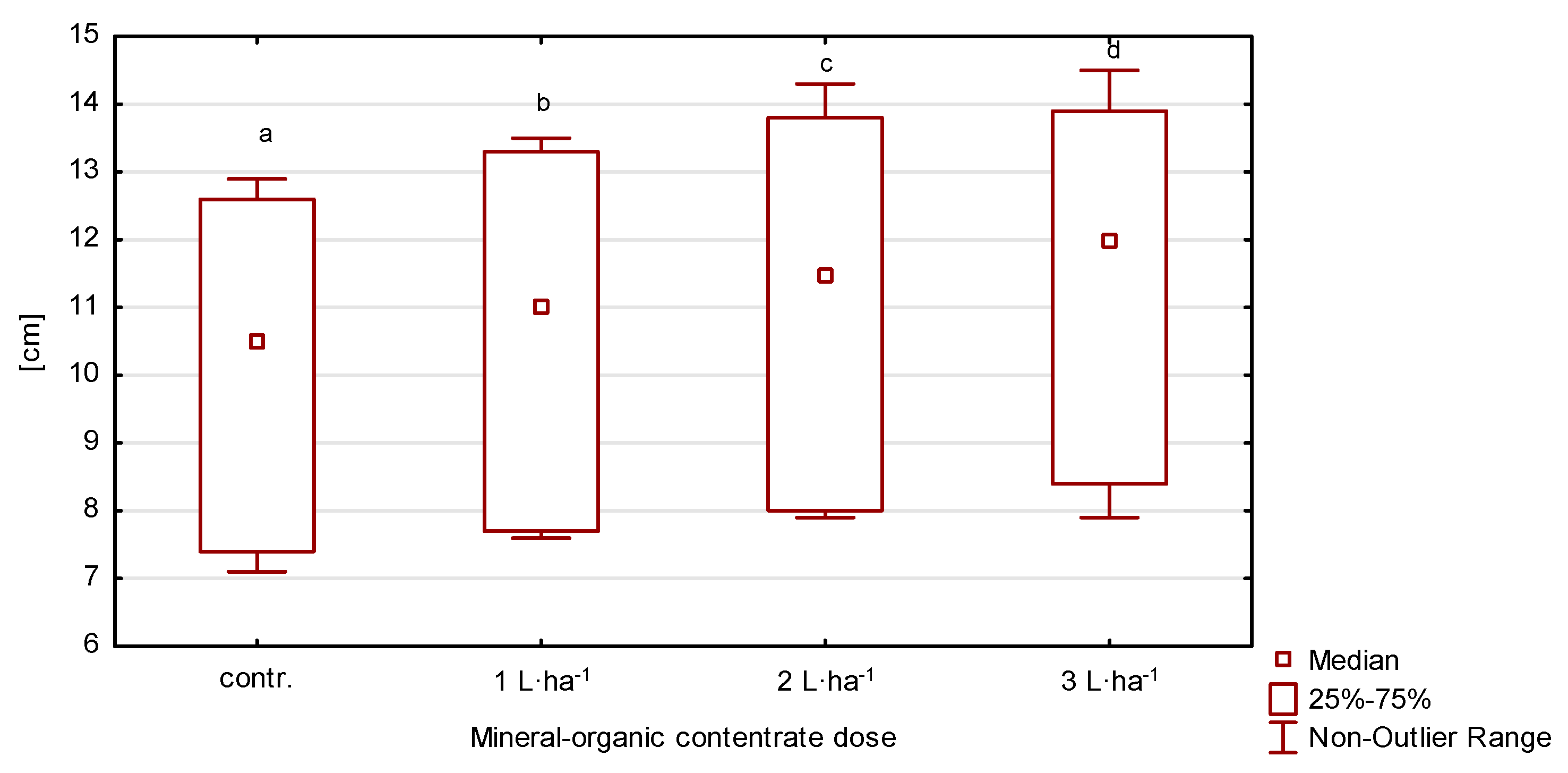
Depending on the year of the experiment and fertilizer treatment, the length of the flag leaf ranged from 7.26 to 14.24 cm (Table 2, Figure 3). The lowest values were recorded in plants from the control plot and in those fertilized with the organic-mineral concentrate at 1 L·ha−1. Plants from plots fertilized with higher doses had significantly higher (p ≤ 0.05) values in relation to plants from the control facility. Across the growing seasons, the year 2020 had the greatest variation (V = 23.1%) with low growing season rainfall of 385.2 mm.
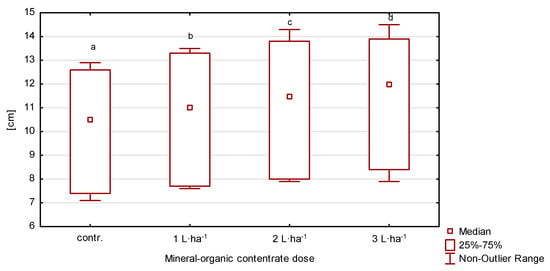
Figure 3.
Flag leaf length of perennial ryegrass in response to the dose of the organic-mineral concentrate, average of 2020–2022. The same letters (a, b, c, d) mean no statistically significant changes (p ≥ 0.05).
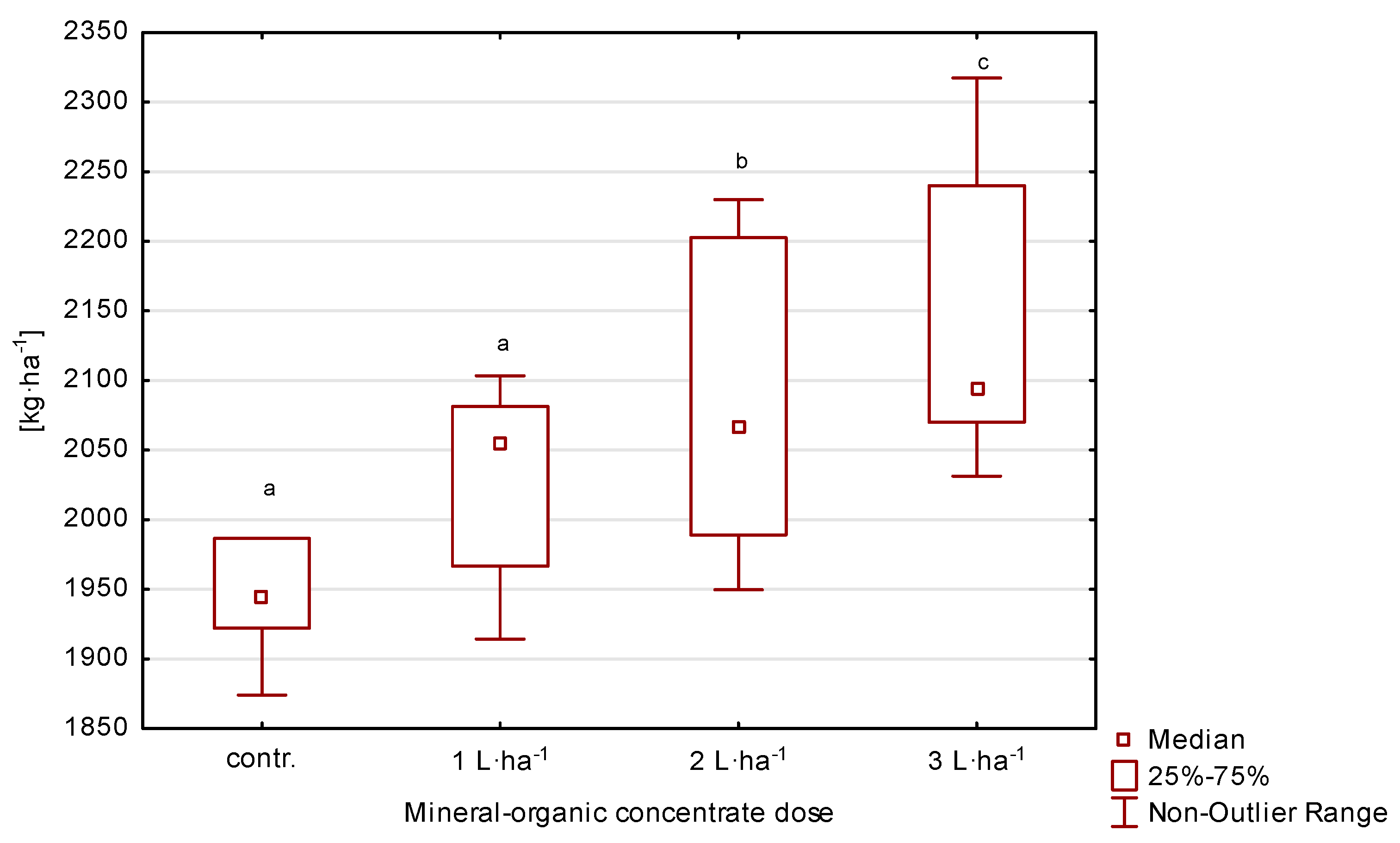
Seed yield varied depending on the treatment and year and ranged from 1898.4 to 2288.7 kg·ha−1 (Table 2, Figure 4). Over the three years the treatments of 2 and 3 L·ha−1 resulted in a significant growth (p ≤ 0.05) in seed yield compared to control, without additional fertilization. The increase in yield from the plot with 3 L·ha−1 in the first year was 11%, in the second 7%, and in the third 9%, compared to the values recorded on the control plot. On the other hand, on the plot where 2 L·ha−1 was applied, it increased by 8, 5, and 5%, respectively, compared to the control.
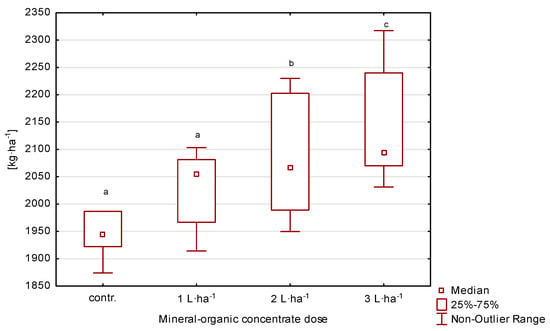
Figure 4.
Seed yield of perennial ryegrass in response to the dose of organic-mineral concentrate, average of 2020–2022. The same letters (a, b, c) mean no statistically significant changes (p ≥ 0.05).
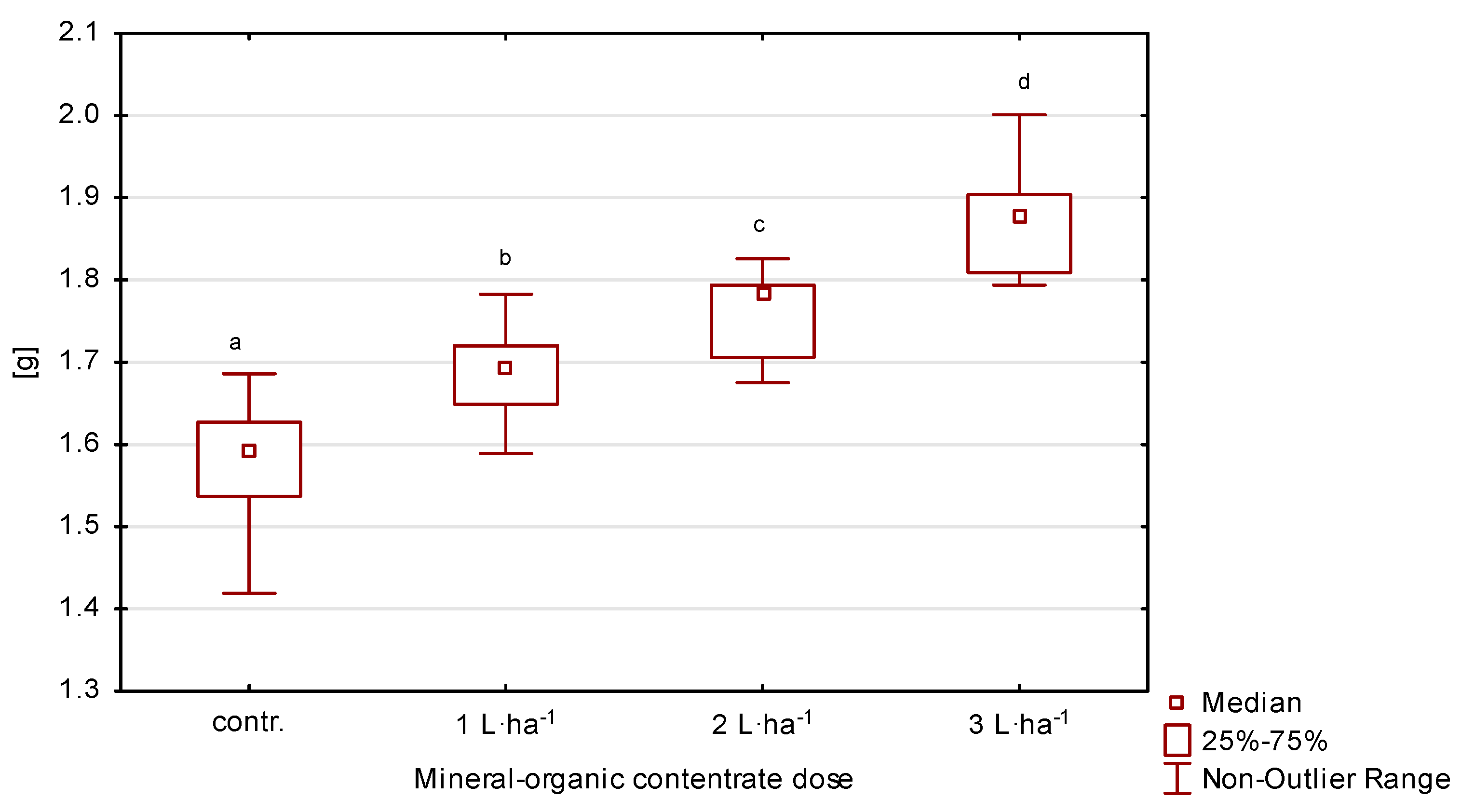
The average value (2020–2022) of thousand-seed weight was in the range of 1.49–1.93 g (Table 2, Figure 5). When the organic-mineral concentrate was applied to leaves, the weight of one thousand seeds on all plots was higher compared to the values obtained without concentrate fertilization. However, significant differences (p ≤ 0.05) were recorded for seeds from the plots with 2 and 3 L·ha−1. On the plot where 3 L·ha−1 was applied, an increase in the first year was 17%, in the second 16% and in the third 24%, compared to the control.
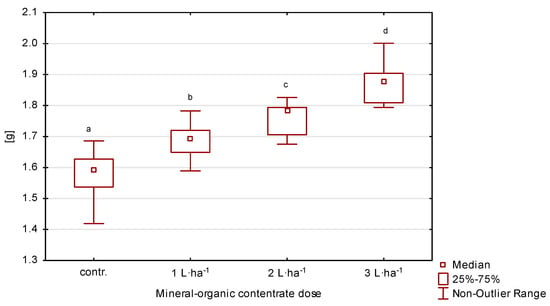
Figure 5.
Thousand-seed weight of perennial ryegrass in response to the dose of organic-mineral concentrate; average of 2020–2022. The same letters (a, b, c, d) mean no statistically significant changes (p ≥ 0.05).
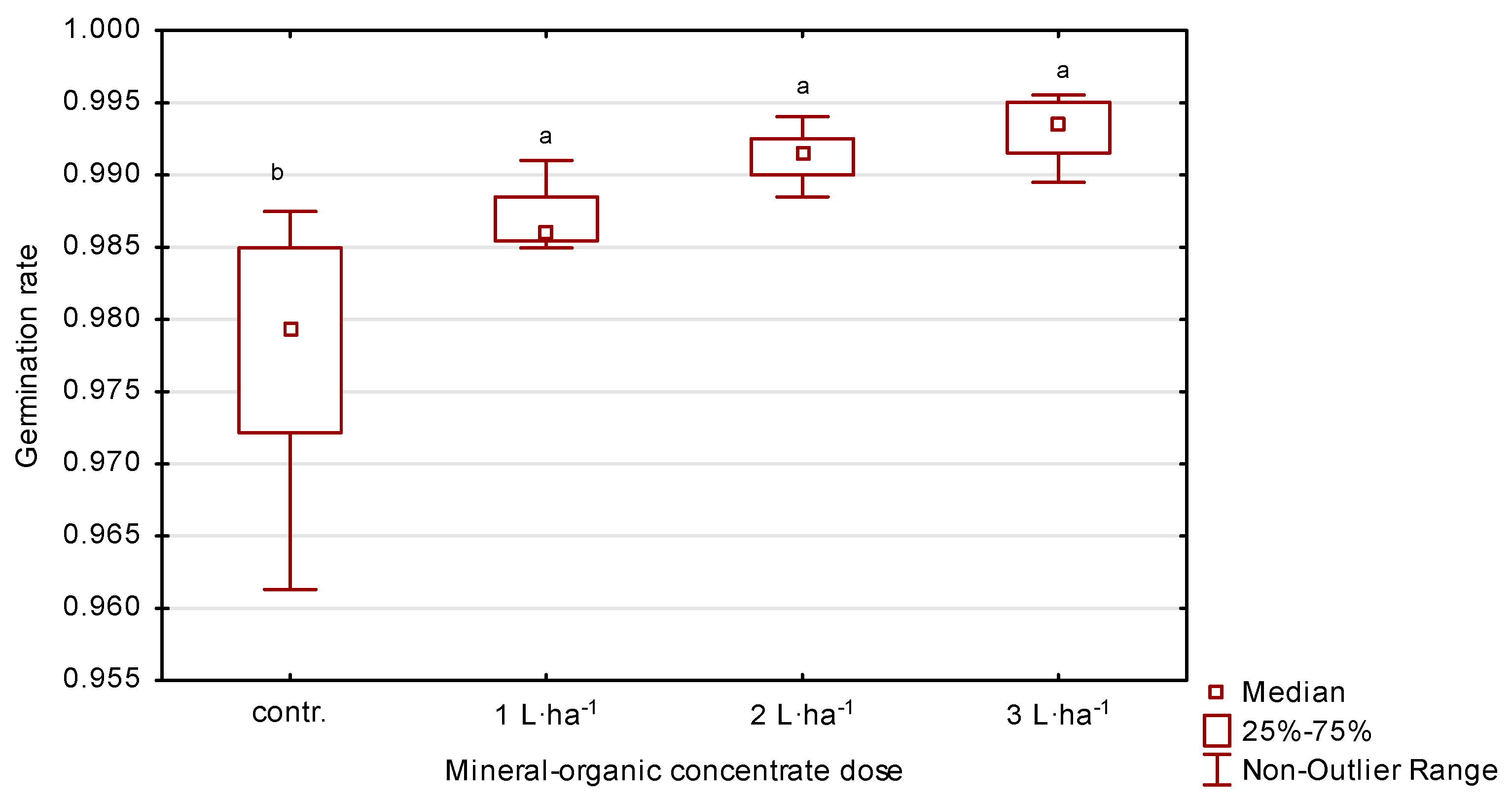
The average germination capacity of perennial ryegrass seeds ranged from 94 to 99% (Table 2, Figure 6). Spraying plants with 3 L·ha−1 of the organic-mineral concentrate resulted in an increase of 2% in the first year, 3% in the second and 4% in the third, compared to the control. On the other hand, application of 2 L·ha−1 in the following years resulted in an increase in germination capacity of 1, 3 and 4%, respectively, compared to the control.
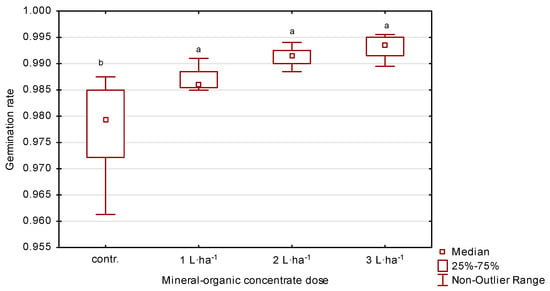
Figure 6.
Germination capacity of perennial ryegrass in response to the dose of organic-mineral concentrate, average of 2020–2022. The same letters (a, b) mean no statistically significant changes (p ≥ 0.05).
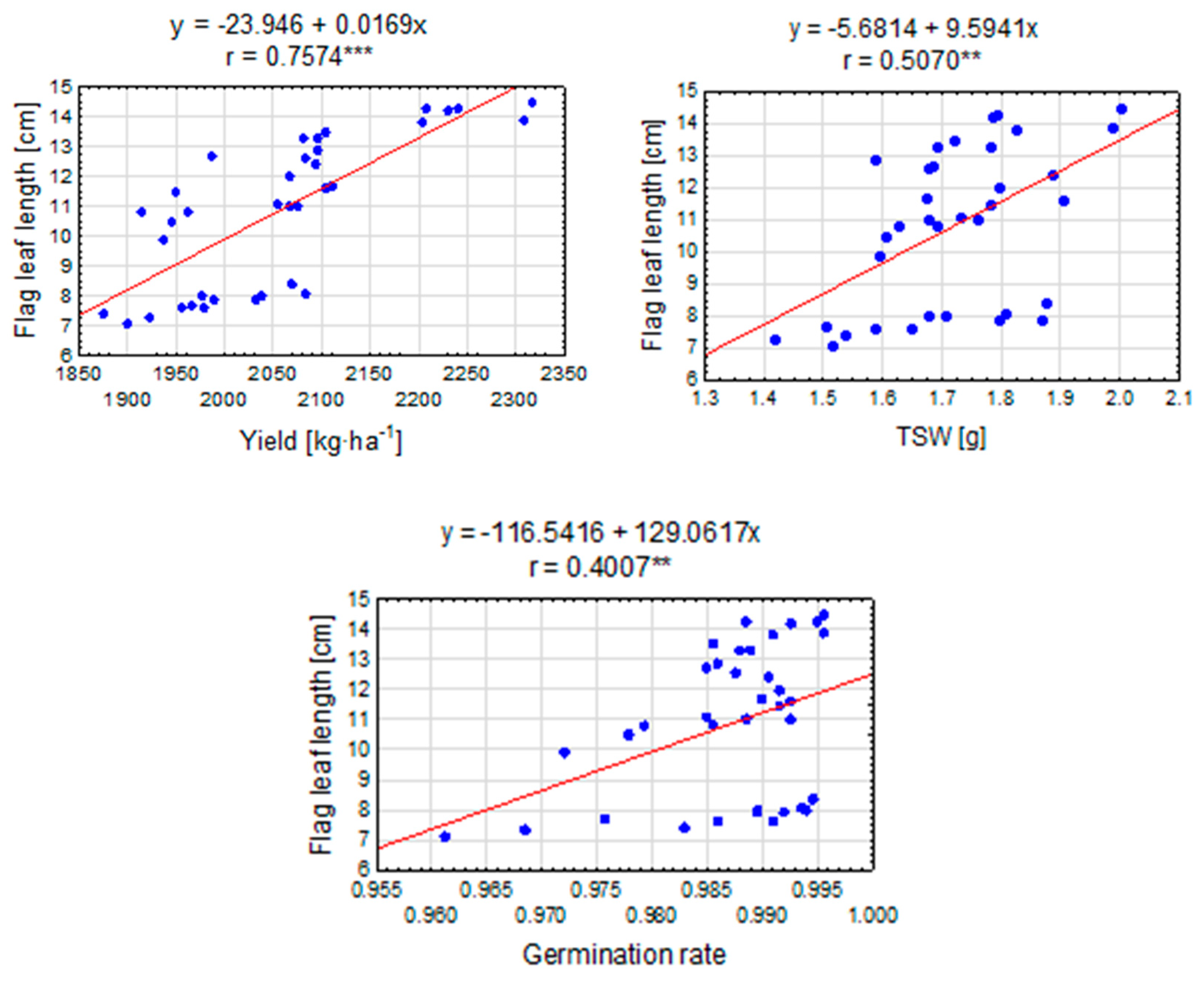
Pearson correlation coefficient analysis indicates a moderately strong positive linear relationship between seed yield and the flag leaf length (Figure 7). A result of r = 0.7574 means that as the seed yield increases, a simultaneous increase in the length of the flag leaf can be expected. This positive correlation suggests that there is some relationship between the number of seeds and the distribution of leaves on the plant. Similarly, the weight of a thousand seeds (TSW) and the flag leaf length show a moderate positive linear relationship. The value of Pearson’s correlation coefficient indicates that an increase in the weight of a thousand seeds is correlated with the flag leaf length. This result may suggest that plants with a higher seed weight tend to develop longer flag leaves. A value of r = 0.4007 indicates a moderate positive linear relationship between germination capacity and the flag leaf length. This means that, as germination capacity increases, an increase in the length can be expected too.
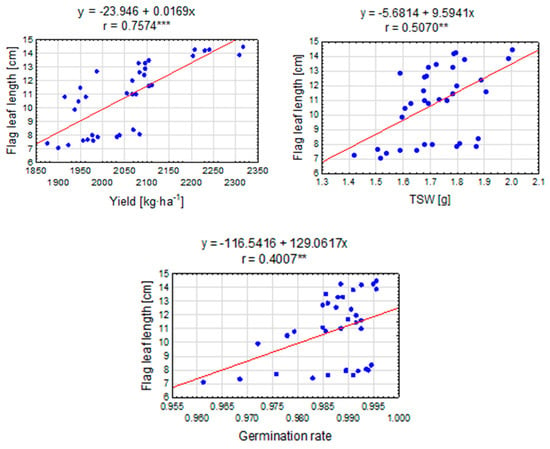
Figure 7.
The linear relationship between the flag leaf length [cm] and the selected elements of the perennial ryegrass productive variables. The linear regression equation and the Pearson correlation coefficient (r) are shown when the r is significant (**, ***—at 0.01 and 0.001 probability level). The points represent means over 2020–2022.
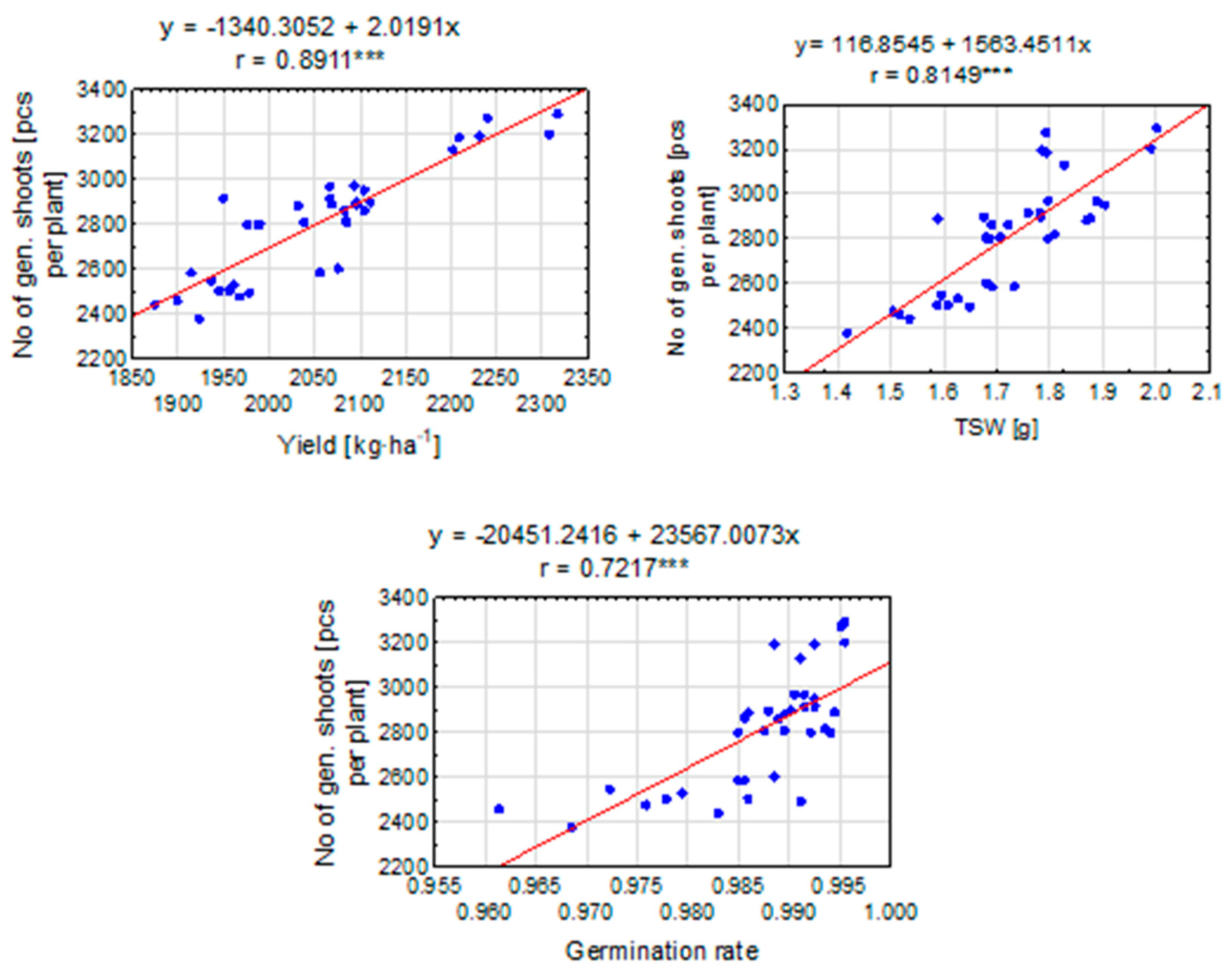
Pearson’s correlation coefficient (r = 0.8911) clearly indicates a very strong positive linear relationship between seed yield and the number of generative shoots of perennial ryegrass (Figure 8). This means that as the seed yield increases, a simultaneous increase in the number of generative shoots can be expected. Additionally, the proximity of the r-value to 1 confirms that this relationship is extremely strong. Similarly, a value of r = 0.8149 indicates a strong positive linear relationship between the weight of one thousand seeds and the number of generative shoots of perennial ryegrass. With an increase in the weight of a thousand seeds (TSW), the number of generative shoots is also likely to increase. Again, a high r-value confirms the strength of this relationship, providing strong evidence for the existence of a positive correlation between the two variables. On the other hand, for the value of r = 0.7217, a moderate positive linear relationship was observed between ryegrass germination capacity and the number of generative shoots. As germination capacity increases, an increase in the number of generative shoots can be expected. Despite the fact that the r-value is lower than in the previous cases, it still indicates a positive correlation between the variables.
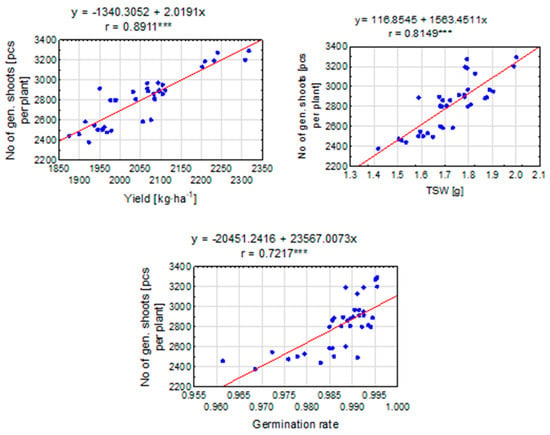
Figure 8.
The linear relationship between the number of generative shoots [pcs per plant] and the selected elements of the perennial ryegrass productive variables. The linear regression equation and the Pearson correlation coefficient (r) are shown when the r is significant (***—at 0.001 probability level). The points represent means over 2020–2022.
4. Discussion
The application of mineral-organic concentrates is currently one of the most important research directions in agrotechnology. Appropriate management of plant nutrition, growth, and stress tolerance (drought, salinity, soil depletion) is becoming a key element for increasing yields under changing environmental conditions [26,27,28,29]. Zhou et al. [30] claim that proper application of organic fertilizers is essential for optimizing agricultural production and improving soil health. The above authors conducted studies in which they analyzed the effects of different fertilization practices on yields and soil quality. According to those studies, the use of organic fertilizers leads to a significant increase in wheat and maize yields and an improvement in soil quality, with higher organic carbon and nitrogen content. Organic fertilizer also significantly affected soil enzyme activity and foraging activity of soil fauna. However, excessive use of inorganic fertilizers can have a negative impact on the activity of soil fauna. Therefore, the authors emphasize both the importance of balance in the use of fertilizers and the benefits of incorporating organic fertilizers into farming practices. Improvements in the overall condition of grasses following the application of a fertilizer consisting of amino acids and mineral additives were also reported by Zhang et al. [31].
The effects of amino acids, humic acids as well as macro- and micro-nutrients are the subject of numerous studies conducted on crop plants. However, there are few reports on the application of these components in a single formulation. The yield and morphological quality increase recorded in the present experiment is consistent with the beneficial effects of humic substances and amino acids reported by Kandil et al. [32], Akdağ [33] or Radkowski et al. [34].
Humus acids, a component of the fertilizer used in the present experiment, affect plant growth and yield by increasing the level of growth hormones, such as auxin and cytokinins, promoting stress resistance, nutrient metabolism, and photosynthesis [35,36,37,38]. It was found that the application of humus acids increased turf grass root mass growth, chlorophyll content, photochemical productivity, and improved antioxidant and hormone metabolism and plant tolerance to various abiotic stresses [39,40]. The application of humic products to Agrostis stolonifera L. increased dehydrogenase activity, root mass growth and antioxidant concentration, with beneficial effects on turf quality, germination capacity and turf coverage [41,42]. Similarly, the application of humic substances to tall fescue [Festuca arundinacea Scherb.] resulted in improved antioxidant concentration, better regeneration after heat stress damage or higher tolerance to it and in increased root mass [43,44].
A study by White et al. [45] showed that humic substance concentrations as low as 0.01% stimulated the roots of annual meadow grass (Poa annua L.), enabling better internalization of bacteria into root cells. Such substances also promote vertical root growth and trichome elongation, which may be partly due to their ability to enhance rhizophagy and endophytism.
On the other hand, a study by Regelink and Koopmans [46] reported that such biostimulants as Liqhumus, containing humic acid, and Fulvagra, containing fulvic acid, did not affect the development of rough-stalked meadow-grass (Poa trivialis) or the uptake of nitrogen (N) and phosphorus (P) by this plant. An explanation for this inconsistency is that the doses of humic substances used were low compared to the amount of organic carbon in the soil, causing them to be adsorbed on the soil surface and preventing interaction with plant roots. The expected benefits of humic substance addition in terms of orthophosphate (ortho-P) availability are only likely at high phosphorus levels [0.4–0.6 mol mol−1], whereas the soil had lower values [0.09 and 0.29 mol mol−1].
The present experiment provides valuable information on the effect of the tested product on seed cultivation of perennial ryegrass 2N Bokser. A 13% increase in seed yield, compared to control, is a significant proof of the benefits resulting from its application. It is also important to note that the application of even its lower dose (2.0 L·ha−1) brought significant benefits. This is important from the point of view of production economics, as lower doses of fertilizer can be more cost-effective. The noticeable increase in the number of generative shoots on plots treated with higher doses of the organic-mineral concentrate underlines its effect on plant development. Kandil et al. [31] also obtained an increase in the number of wheat blades after application of amino acids.
Research performed in New Zealand showed a beneficial effect of humus application on ryegrass-clover pasture productivity and on utilization of nitrogen applied as urea. The application of 10% and 20% of humus increased early spring production by 31% and 41% [47]. Similarly, Gerke et al. [48] reported a 150% increase in perennial ryegrass shoot yield on oxisols with added humus. Humic substances derived from Australian lignite increased the growth of Italian ryegrass (Lolium multiflorum Lam.) and alfalfa (Medicago sativa L.) [49]. An experiment investigating the effect of HA (humic acid) derived from lignite on the growth and macronutrient uptake of wheat (Triticum aestivum L.) showed significant differences with respect to its growth (plant height and shoot weight) and nitrogen uptake. On average, the highest increases in plant height and fresh and dry shoot weight were reported when HA was applied at 60 mg·kg−1 of soil and were 10%, 25% and 18%, respectively, compared to control (HA 0) [50]. Studies conducted by Osman et al. [51] showed an increase in the number of shoots per unit area; a thousand grain weight; grain and straw yield; and nitrogen, phosphorus and potassium content, with the best results obtained with combined HA + FA (humic acid and fulvic acid) and anhydrous ammonia fertilization. Similarly, studies by Alabdulla [52] evaluating foliar HS (humic substances) fertilization of oats (Avena sativa L. cv. Shaffaa) showed that HA fertilization (0, 3, 6, and 9 g·L) increased the number of panicles per m2, the number of grains per panicle and the content of nitrogen, phosphorus, potassium and crude protein in dry matter and the yields of grain and forage, in addition to reducing the weight of a thousand grains.
This study demonstrated an increase, in relation to control, in the germination capacity of perennial ryegrass seeds after application of the concentrate. This suggests that the concentrate not only promotes plant development, but also contributes to the production of better-quality seeds. Different results were obtained by Van Dyke [53], who reported no significant differences in ryegrass seed germination after application of a biostimulant based on, among others, amino acids.
Compared to control, plants fertilized with the concentrate had a higher thousand-seed weight, which is a key indicator of seed quality. Application of the formulation had a beneficial effect on the flag leaf length, which may be related to improved plant development and photosynthesis. This is particularly relevant in the context of abiotic stresses, such as drought observed in 2020. The positive role of biostimulants in stress mitigation is confirmed by De Luca et al. [54], Povero [55] or Drobek [56]. Akdağ et al. [33] reported a clear positive effect of biostimulants on plant growth, seed yield or the number of generative shoots, especially after the application of higher doses. Application of amino acids has a beneficial effect on the production of other compounds such as amines, purines, alkaloids, pyrimidines, enzymes, vitamins and terpenoids, enhancing photosynthesis and thus increasing grain yield [15]. Mohamed [57] reported that the application of an extract of amino acids and yeast produced a beneficial effect on the reproductive development of plants, which also affected grain yield. It was noted that by foliar application of amino acids and yeast during drought, higher numbers of spikes and grains could be obtained. A significant increase in those parameters after foliar application of amino acids has also been observed in wheat [58].
Although the results are promising, it would be useful to consider some limitations of this research. First of all, would the application of the formulation in other climatic and soil conditions produce similar results? Furthermore, would similar benefits be observed for other plants? How would the formulation affect other soil parameters, such as its structure or biological activity? In the context of the global challenges of securing sufficient healthy food for a growing population, these results are significantly important. The formulation could be a tool for farmers seeking to increase crop yields while maintaining soil health and quality. Further research is needed to fully understand the potential and limitations of this technology.
5. Conclusions
Fertilization technology utilizing a mineral-organic concentrate, composed of humus, L-amino acids, vitamins, chelates, metal complexes, macro-, micro-, and meso-elements, organic matter, and humic acids extracted from the upper organic layer of lignite, has undergone comprehensive evaluation to determine its impact on agricultural yields. In studies conducted by the Agricultural University of Cracow, a significant 13% increase in perennial ryegrass seed yield was observed in response to a specific fertilizer dose. Biostimulants, particularly amino acids, exhibit promising capabilities in alleviating plant stress and enhancing yields, especially under challenging environmental conditions. However, further research is necessary to fully understand the possibilities and limitations inherent to this innovative fertilizer technology. A key aspect of our findings was that the application of the concentrate at rates of 3 L·ha−1 and 2 L·ha−1 resulted in a significant increase in the number of generative shoots and seed yield compared to the control. Moreover, fertilized plants demonstrated enhanced seed germination capacity, with the most pronounced increase observed following the application of 3 L·ha−1. Additionally, the average weight of one thousand seeds was significantly higher in fertilized plants compared to the control, with the most substantial increase recorded in response to the highest dose. These results underscore the positive impact of the organic-mineral concentrate on the developmental parameters of perennial ryegrass and seed quality. In summary, our study emphasizes the potential of the mineral-organic concentrate in enhancing agricultural productivity and highlights the need for ongoing research to fully unlock its potential and address associated complexities.
Author Contributions
Conceptualization, A.R., K.W. and I.R.; methodology, A.R., K.W. and H.B.; software, P.K.; validation, A.R. and I.R.; formal analysis, P.K. and H.B.; investigation, A.R. and I.R.; resources, A.R. and K.W.; data curation, A.R. and I.R.; writing—original draft preparation, A.R., I.R. and H.B.; writing—review and editing, A.R., I.R. and P.K.; visualization, A.R. and P.K.; supervision, A.R. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to express their gratitude to QULTIVO LLC from Charsznica for their support in facilitating this experiment. Special thanks to Grzegorz Raczkiewicz, the co-owner of the company, for his invaluable assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Amiri Forotaghe, Z.; Souri, M.K.; Ghanbari Jahromi, M.; Mohammadi Torkashvand, A. Influence of Humic Acid Application on Onion Growth Characteristics under Water Deficit Conditions. J. Plant Nutr. 2022, 45, 1030–1040. [Google Scholar] [CrossRef]
- Najarian, A.; Souri, M.K.; Nabigol, A. Influence of Humic Substance on Vegetative Growth, Flowering and Leaf Mineral Elements of Pelargonium × Hortorum. J. Plant Nutr. 2022, 45, 107–112. [Google Scholar] [CrossRef]
- Wolski, K.; Biernacik, M.; Świerszcz, S.; Talar-Krasa, M.; Leshchenko, O. Effect of the Application of a Biostimulant and Mineral Fertilizers on the Concentration of Mineral Elements in the Sward of Forage Mixtures Cultivated on Light Soil. J. Elem. 2019, 24, 385. [Google Scholar] [CrossRef]
- Perminova, I.V.; García-Mina, J.M.; Knicker, H.; Miano, T. Humic Substances and Nature-like Technologies: Learning from Nature: Understanding Humic Substances Structures and Interactions for the Development of Environmentally Friendly, Nature-like Technologies. J. Soils Sediments 2019, 19, 2663–2664. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like Activity of Humic Substances in Fagus Sylvaticae Forests. New Phytol. 2001, 151, 647–657. [Google Scholar] [CrossRef]
- Yang, C.-M.; Wang, M.-C.; Lu, Y.-F.; Chang, I.-F.; Chou, C.-H. Humic Substances Affect the Activity of Chlorophyllase. J. Chem. Ecol. 2004, 30, 1057–1065. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A Combination of Biochar-Mineral Complexes and Compost Improves Soil Bacterial Processes, Soil Quality, and Plant Properties. Front. Microbiol. 2016, 7, 372. [Google Scholar] [CrossRef]
- Skamarokhova, A.; Petenko, A.; Gneush, A.; Yurina, N.; Yurin, D. The Role of Foschami Bio-Fertilizer in Increasing the Yield of Green Mass of Vetch-Wheat Grass Mixture. In Proceedings of the International Scientific Conference Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East (AFE-2021), Volozhenin, Russia, 21–22 June 2021; Springer International Publishing: Cham, Switzerland, 2022; Volume 354. [Google Scholar]
- Urrutia, O.; Fuentes, M.; Olaetxea, M.; Garnica, M.; Baigorri, R.; Zamarreño, A.M.; Movila, M.; De Hita, D.; Garcia-Mina, J.M. The Effect of Soil Organic Matter on Plant Mineral Nutrition. In Achieving Sustainable Crop Nutrition; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar]
- Tavares, O.C.H.; Santos, L.A.; Ferreira, L.M.; Sperandio, M.V.L.; da Rocha, J.G.; García, A.C.; Dobbss, L.B.; Berbara, R.L.L.; de Souza, S.R.; Fernandes, M.S. Humic Acid Differentially Improves Nitrate Kinetics under Low- and High-Affinity Systems and Alters the Expression of Plasma Membrane H+-ATPases and Nitrate Transporters in Rice. Ann. Appl. Biol. 2017, 170, 89–103. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Canellas, L.P.; Façanha, A.R. Indolacetic and Humic Acids Induce Lateral Root Development through a Concerted Plasmalemma and Tonoplast H+ Pumps Activation. Planta 2007, 225, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-Enriched Leonardite and Humic Acid Soil Amendments Enhance Tolerance to Drought and Phosphorus Deficiency Stress in Maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef]
- El-Said, M.A.S.; Mahdy, A.Y. Response of Two Wheat Cultivars to Foliar Application with Amino Acids under Low Levels of Nitrogen Fertilization. Middle East J. Agric. Res. 2018, 5, 462–472. [Google Scholar]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant Metabolites and Nutritional Quality of Vegetables. J. Food Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and Biochemical Studies on Drought Tolerance of Wheat Plants by Application of Amino Acids and Yeast Extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Fujiwara, A.; Shimura, H.; Masuta, C.; Sano, S.; Inukai, T. Exogenous ascorbic acid derivatives and dehydroascorbic acid are effective antiviral agents against Turnip mosaic virus in Brassica rapa. J. Gen. Plant Pathol. 2013, 79, 198–204. [Google Scholar] [CrossRef]
- Li, J.; Trivedi, P.; Wang, N. Field Evaluation of Plant Defense Inducers for the Control of Citrus Huanglongbing. Phytopathology 2016, 106, 37–46. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Influence of Foliar Fertilization with Amino Acids and Humic Acids on Productivity and Quality of Asparagus. Biol. Agric. Hortic. 2003, 21, 277–291. [Google Scholar] [CrossRef]
- Hegarty, M.; Yadav, R.; Lee, M.; Armstead, I.; Sanderson, R.; Scollan, N.; Powell, W.; Skøt, L. Genotyping by RAD Sequencing Enables Mapping of Fatty Acid Composition Traits in Perennial Ryegrass (Lolium perenne (L.)). Plant Biotechnol. J. 2013, 11, 572–581. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Jones, E.L.; Moorby, J.M.; Humphreys, M.O.; Theodorou, M.K.; Macrae, J.C.; Scollan, N.D. Production Responses from Lambs Grazed on Lolium Perenne Selected for an Elevated Water-Soluble Carbohydrate Concentration. Anim. Res. 2001, 50, 441–449. [Google Scholar] [CrossRef]
- Miller, L.A.; Moorby, J.M.; Davies, D.R.; Humphreys, M.O.; Scollan, N.D.; MacRae, J.C.; Theodorou, M.K. Increased Concentration of Water-Soluble Carbohydrate in Perennial Ryegrass (Lolium perenne L.): Milk Production from Late-Lactation Dairy Cows. Grass Forage Sci. 2001, 56, 383–394. [Google Scholar] [CrossRef]
- Humphreys, M.O. Water-Soluble Carbohydrates in Perennial Ryegrass Breeding. Grass Forage Sci. 1989, 44, 237–244. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; International Seed Testing Association (ISTA): Zurich, Switzerland, 2010. [Google Scholar]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant Biostimulants: Innovative Tool for Enhancing Plant Nutrition in Organic Farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Meddich, A.; Ait El Mokhtar, M.; Bourzik, W.; Mitsui, T.; Baslam, M.; Hafidi, M. Optimizing Growth and Tolerance of Date Palm (Phoenix dactylifera L.) to Drought, Salinity, and Vascular Fusarium-Induced Wilt (Fusarium oxysporum) by Application of Arbuscular Mycorrhizal Fungi (AMF). In Root Biology; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Tian, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. The Sewage Sludge Biochar at Low Pyrolysis Temperature Had Better Improvement in Urban Soil and Turf Grass. Agronomy 2019, 9, 156. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of Organic–Inorganic Compound Fertilizer with Reduced Chemical Fertilizer Application on Crop Yields, Soil Biological Activity and Bacterial Community Structure in a Rice–Wheat Cropping System. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Jiang, N.; Xiu, W.; Zhao, J.; Yang, D. Effects of Organic Fertilizer Incorporation Practices on Crops Yield, Soil Quality, and Soil Fauna Feeding Activity in the Wheat–Maize Rotation System. Front. Environ. Sci. 2022, 10, 2292. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological Effects of Liquid Applications of a Seaweed Extract and a Humic Acid on Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.M.; Seadh, S.E.; Altai, D.S.K. Role of Humic Acid and Amino Acids in Limiting Loss of Nitrogen Fertilizer and Increasing Productivity of Some Wheat Cultivars Grown under Newly Reclaimed Sandy soil. J. Adv. Res. Biol. Sci. 2016, 3, 123–136. [Google Scholar]
- Akdağ, N.; Avcı, S. The Impact of Sowing Time and Biostimulant Application on Seed Production in Italian Ryegrass. Turk. J. Agric. Food Sci. Technol. 2023, 11, 1260–1264. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Cyplik, A.; Wolski, K.; Bujak, H. Effect of Amino Acids and Effective Microorganisms on Meadow Silage Chemical Composition. Agronomy 2021, 11, 1198. [Google Scholar] [CrossRef]
- Laskosky, J.D.; Mante, A.A.; Zvomuya, F.; Amarakoon, I.; Leskiw, L. A Bioassay of Long-Term Stockpiled Salvaged Soil Amended with Biochar, Peat, and Humalite. Agrosyst. Geosci. Environ. 2020, 3, e20068. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Van Tol de Castro, T.A.; Berbara, R.L.L.; Tavares, O.C.H.; Mello, D.F.d.G.; Pereira, E.G.; Souza, C.d.C.B.d.; Espinosa, L.M.; García, A.C. Humic Acids Induce a Eustress State via Photosynthesis and Nitrogen Metabolism Leading to a Root Growth Improvement in Rice Plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Zhang, X.; Goatley, M. Evaluating Effects of Humic and Fulvic Acids for Improving Creeping Bentgrass Putting Green Quality and Root Growth during Summer Stress. Va. Turfgrass Counc. 2019, 1, 26–28. [Google Scholar]
- Van Dyke, A.; Johnson, P.G.; Grossl, P.R. Influence of Humic Acid on Water Retention and Nutrient Acquisition in Simulated Golf Putting Greens. Soil Use Manag. 2009, 25, 255–261. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D. Foliar Fertilization by Tank-Mixing with Organic Amendment on Creeping Bentgrass. Horttechnology 2012, 22, 157–163. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. Hormone-Containing Products’ Impact on Antioxidant Status of Tall Fescue and Creeping Bentgrass Subjected to Drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Seaweed Extract, Humic Acid, and Propiconazole Improve Tall Fescue Sod Heat Tolerance and Posttransplant Quality. HortScience 2003, 38, 440–443. [Google Scholar] [CrossRef]
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic Bacteria in Grass Crop Growth Promotion and Biostimulation. Grass Res. 2021, 1, 5. [Google Scholar] [CrossRef]
- Regelink, I.C.; Koopmans, G.F. Effects of Biostimulants and Fertilization on Nutrient Uptake by Grass and Composition of Soil Pore Water Versus 0.01 M CaCl2 Soil Extracts. Commun. Soil Sci. Plant Anal. 2021, 52, 2516–2532. [Google Scholar] [CrossRef]
- Espie, P.; Ridgway, H. Bioactive Carbon Improves Nitrogen Fertiliser Efficiency and Ecological Sustainability. Sci. Rep. 2020, 10, 3227. [Google Scholar] [CrossRef]
- Gerke, J.; Meyer, U.; Römer, W. Phosphate, Fe and Mn Uptake of N2 Fixing Red Clover and Ryegrass from an Oxisol as Affected by P and Model Humic Substances Application. 1. Plant Parameters and Soil Solution Composition. Z. Pflanzenernähr. Bodenkd. 1995, 158, 261–268. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do Lignite-Derived Organic Amendments Improve Early-Stage Pasture Growth and Key Soil Biological and Physicochemical Properties? Crop Pasture Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-Derived Humic Acid Effect on Growth of Wheat Plants in Different Soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Osman, E.A.M.; El-Masry, A.A.; Khatab, K.A. Effect of Nitrogen Fertilizer Sources and Foliar Spray of Humic and/or Fulvic Acids on Yield and Quality of Rice Plants. Adv. Appl. Sci. Res. 2013, 4, 174–183. [Google Scholar]
- Alabdulla, S.A. Effect of Foliar Application of Humic Acid on Fodder and Grain Yield of Oat (Avena sativa L.). Res. Crops 2019, 20, 880–885. [Google Scholar] [CrossRef]
- Dyke, A. Van Influence of Humic Acid on Kentucky Bluegrass Establishment. Available online: Humintech.com. (accessed on 4 December 2023).
- De Luca, V.; de Barreda, D.G.; Lidón, A.; Lull, C. Effect of Nitrogen-Fixing Microorganisms and Amino Acid-Based Biostimulants on Perennial Ryegrass. Horttechnology 2020, 30, 280–291. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress-a Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Mohamed, A.M. Effect of Some Bio-Chemical Fertilization Regimes on Yield of Maize. Ph.D. Thesis, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, 2006. [Google Scholar]
- Al-Khateeb, S.A. Promotive Effect of 5-Aminolevulinic Acid on Growth, Yield and Gas Exchange Capacity of Barley (Hordeum vulgare L.) Grown under Different Irrigation Regimes. J. King Saud Univ. 2006, 18, 103–111. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).