Abstract
Physcion can induce plant resistance to disease, and is registered to control powdery mildew by spraying in China. Seed coating is a widely applied precision method for pest prevention and control. To explore its potential in disease control and yield increase by seed coating, mixtures containing physcion and commonly used fungicides were designed and applied in a field trial. Greenhouse experiments screened the optimal concentration of physcion for seed coating an found it to be 1:50, with excellent promotion of plant growth and powdery mildew control. In field trials, seeds coated with a combinations of physcion with validamycin and pyrimidine nucleotide (2#) at 1:50 exhibited the highest emergence rate, tillering number, control effect of wheat powdery mildew, enzyme activity of ascorbate peroxidase (APX), glutathione reductase (GR), and peroxidase (POD), photosynthetic pigment content, and yield. These results provide an effective approach to wheat disease control and yield increase in wheat fields, and can lay the basis for reasonable application of physcion in Huang-huai-hai plain in China.
1. Introduction
As a main staple crop, wheat is planted worldwide. In China, its planting area accounts for 20~30% of the national cultivated area [1], and Huang-huai-hai plain is the main planting area. There are more than 30 kinds of insects and diseases on wheat in Huang-huai-hai plain. The production of wheat suffers from various adverse stresses due to precipitation and winter temperatures, changes in the cropping system, and returning of straw to the field.
The yield and quality of wheat are highly susceptible to abiotic stresses such as salt, drought, and pesticide [2] and biotic stresses such as diseases and pests [3]. When plants are subjected to abiotic or biotic stresses, their redox homeostasis is disturbed, resulting in excessive accumulation of reactive oxygen species (ROS) in cells, which in turn triggers oxidative stress [4]. Plants rely on the enzymatic reactions of antioxidant enzymes, e.g., ascorbate peroxidase (APX), glutathione reductase (GR), and peroxidase (POD), to maintain the normalization of intracellular ROS levels and inhibit membrane lipid peroxidation. The ability to scavenge ROS is one of the key factors in determining the resistance of plants to adversity. Many studies have shown that an imbalance of ROS metabolism and resulting damage to cells is one of the main reasons for plant injury under stress [5]. One of the most important characteristics for improving plant stress resistance is the improvement of cell defense ability against ROS [6].
Physcion, a highly active antibacterial compound of anthraquinone, was created by the Hubei Academy of Agricultural Sciences, China [7]. Application of physcion as medicine has been extensively studied, and research on its application as fungicide has been gradually increasing [8]. Physcion has a broad fungicide spectrum, and has shown obvious activity against Magnaporthe grisea and Botrytis cinerea. Physcion can inhibit the germination and growth of plant fungi and induce the defense response of crops [9]. Physcion interferes with the biosynthesis of chitin in the cell wall of pathogenic fungi, causing local expansion and rupture of germ tubes and mycelia. The contents of the cells are then discharged, resulting in cell death. Physcion acts through the oxidation and dehydrogenation of bacterial sugar and sugar metabolism intermediate, inhibiting ammonia nitrogen assimilation and oxidation, dehydrogenation and deamination of amino acid, and the synthesis of protein and nucleic acid [10]. In China, physcion is registered to control powdery mildew on wheat, cucumber, and grapes by foliar spraying (http://www.chinapesticide.org.cn/, accessed on 26 November 2023).
Coating seeds with pesticide is a widely applied precision method for pest prevention and control. Compared with the use of pesticides for spraying, coating can dramatically increase the utilization rate and decrease the damage caused to non-target organisms. Mixtures containing pesticides with different modes of action should be adopted to increase the control spectrum and delay the development of resistance to fungicides. Azoxystrobin is a strobilurin fungicide effectively against a wide-range of pathogens, including ascomycetes, basidiomycetes, fungi imperfecti, and oomycetes. In China, azoxystrobin has been registered to control various wheat diseases, namely, powdery mildew, scab, stem rot, and rust. Fludioxonil is another fungicide commonly used on wheat; it exhibits excellent control effects against ascomycetes, basidiomycetes, and fungi imperfecti. It is widely applied on wheat as a coating or seed dressing. Validamycin is a broad-spectrum fungicide which induces host resistance responses in tomato and rice [11]. Its actives plant resistance responses by the induction of pathogenesis-related (PR) proteins [12]. Validamycin is registered to control wheat sharp eyespot. Pyrimidine nucleosides are broad-spectrum agricultural antibiotics widely used to control leaf diseases, including powdery mildew, anthracnose, ring spot, apple rot, rice sheath blight disease, and wheat scab in vegetables, fruit trees, flowers, and other crops [13].
To determine the effect in terms of disease control and wheat yields of mixtures containing physcion by seed coating, substances commonly used on wheat were designed and applied in a series of mixtures in field trials. The potential mechanisms of the mixtures in terms of increasing yield were explored by determining the antioxidant activity and chlorophyll content achieved with each treatment. The results can provide an alternative approach to increase the control effect against disease in wheat fields and lay the basis for reasonable application of physcion.
2. Materials and Methods
2.1. Seeds, Plant Pathogens, and Fungicides
The most widely planted wheat cultivars in Huang-huai-hai plain, Bainong 207 (207), Bainong 307 (307), and Bainong 4199 (4199), were used as provided by the Wheat Breeding Center of Henan Institute of Science and Technology (Xinxiang, China).
Blumeria graminis was kindly provided by Zhejiang A&F University. Before inoculation, conidia were produced on wheat leaf segments in a growth chamber set at 18 ± 1 °C and constant light of 72 cd m−2. Approximately 10 days later, fresh conidia produced on the wheat leaves were used as inoculum.
The pesticide mixtures used in this study are annotated as 1# (0.8% physcion colloidal suspension), 2# (0.8% physcion + 0.5% validamycin + 0.25% pyrimidine nucleotide colloidal suspension), 3# (0.8% physcion + 0.5% validamycin colloidal suspension), 4# (0.8% physcion + 1% azoxystrobin colloidal suspension), and 5# (0.8% physcion + 0.25% fludioxonil colloidal suspension), and were provided by Beijing Qingyuanbao Biotechnology Co., Ltd. (Beijing, China).
2.2. Greenhouse Experiment
Wheat cultivar 207 was used in the greenhouse experiment. The growth-promoting effect was determined by coating seeds with physcion at 1:25, 1:50, 1:100, and 1:200 (formulation: seed, w/w), respectively. The seeds were sown in plastic pots (200 mm in diameter, 10 plants per pot) containing sterilized soil and cultured in a growth chamber (25 ± 0.5 °C, 70% RH, 16:8 h light: dark photoperiod). After treatment for 10 d (the two-leaf stage), the fresh weight, root length, and stem length of wheat seedlings were investigated.
To evaluate the control effect against B. graminis in vivo, the plants in the two-leaf stage obtained in the last step were used to inoculate conidia of B. graminis according to Yang et al. [14]. Briefly, the treated wheat plants were inoculated at a density of 2 × 103~4 × 103 conidia/cm2 in a setting tower and transferred to a growth chamber (18 ± 1 °C, 70% RH, 16:8 h light: dark photoperiod). After 8 days, the disease index was investigated when fresh white powder spores could be shaken off.
2.3. Field Experimental Design
The field trial was performed at Xinlianxin Experimental Base, Langgongmiao Town, Xinxiang County, Henan Province (113°8′ N and 35°2′ E) during the farming seasons of 2021 and 2022. The meteorological conditions in recent years, including temperature and precipitation, are provided in Table S1. The soil physicochemical property is provided in Table S2. Wheat cultivars 207, 307, and 4199 were used in the field trial to enhance the general adaptability of the seed coating treatment. Seeds were coated with 1# to 5# manually at 1:50 and 1:100, respectively, and naturally dried. Seeds without coating were used as control and sown on 28 October 2021. The wheat was sown using a seeder, with rows spaced 10 cm apart to ensure accurate and uniform distribution of wheat seeds across the entire field. Before sowing, the foundational fertilizer Nutrient 301(N-P2O5-K2O, 25-13-7, Xinlianxin Chemical Industry Group Co., Ltd., Xinxiang, China was applied at 75 kg/km². At the jointing stage, urea topdressing with a nitrogen content of 46% was applied at 15 kg/km². A total of 108 plots (8 m × 10 m per plot, approximately 3.6 kg seeds sown per plot) were designed, and each treatment was separated by ridges at 0.4 m.
2.4. Survey of Seedling Emergence Rate and Tiller Number
The emergence rate of seedlings was investigated on 1 December 2021. The five points sampling method was used for the following investigation. Within each plot, a total of five points were selected randomly. At each point, the number of seedlings every 1 m length was counted in each row. The average spacing between two rows of wheat was determined and calculated. The effective seed number was determined by calculating the total amount of seeds sown. The emergence rate was calculated according to the following Formula (1).
The tillering number was investigated on 27 March 2022. A total of 10 wheat seedlings were dug continuously at each point, the total tillering number was counted, and the average value was calculated.
2.5. Disease Investigation
The disease indexes of wheat powdery mildew, wheat scab, and wheat stem rot were investigated on 15 May 2022, during the wheat filling period. At each point, the disease index of 100 plants were investigated. The control effect was calculated according to the grading method of powdery mildew. The standard of wheat powdery mildew classification was as indicated below.
Grade 0: no disease.
Grade 1: area of the disease spot ≤ 5%; diseased ear area ≤ 25%; first leaf sheath at the aboveground part of the ground shows brownness, but the stipes segments do not show lesion.
Grade 3: area of the lesion 6–15%; diseased ear area 25–50%; the first stipes at the aboveground part of the ground shows brownness.
Grade 5: area of the lesion 16–25%; diseased ear area 50–75%; the second stipes at the aboveground part of the ground shows brownness.
Grade 7: area of the lesion 26–50%; diseased ear area > 75%; brownness and lesions beyond the second stipes, but no white head.
Grade 9: area of the disease spot > 50%; diseased ear area = 100%; brownness and lesions beyond the second stipes; white head or missing ears due to disease.
The disease index and relative control effect were calculated using the following Formulas (2) and (3).
2.6. Yield
An investigation of the wheat spikes was conducted on 4 June 2022 (the wheat maturity stage). Briefly, five points (1 m × 1 m) were randomly chosen within each experimental plot. The spike count at each point was used to represent the effective number of spikes per unit area. Calculating the number of grains per spike involved randomly selecting 100 plants from each point and counting the average number of grains per plant. At each designated point, 1000 seeds were randomly selected and weighed using a sampler. This process was repeated five times, and the average value was calculated to obtain the 1000-grain weight. The total weight of the wheat with its spikes was measured (Table S3) and the theoretical yield was calculated.
The theoretical yield was calculated using the following Formula (4) [15].
2.7. Lipid Peroxidation
The turning green and filling stages in wheat represent the early and late stages after seed coating treatment, respectively. The turning green stage represents the concluding phase of the wheat seedling, and plays a pivotal role in root and leaf growth. During the filling stage, the accumulation of carbon dioxide (CO2) and subsequent accumulation of photosynthetic products are crucial processes contributing to the formation of healthy grains [16]. These stages were chosen for the determination of lipid peroxidation, antioxidant enzyme activities, and photosynthetic pigment content.
The flag leaves of wheat were sampled on 19 March 2022 (the turning green stage) and 15 May 2022 (the filling stage). At each point, twenty wheat flag leaves were randomly selected and used to determine the content of MDA, photosynthetic pigment, and antioxidant enzyme activities.
The extract was obtained referring to Zhang et al. [4]. Wheat leaf samples were ground under liquid nitrogen and a total of 0.2 g sample was added in phosphate buffer solution (PBS, 2 mL, 0.1 M, pH 7.5) containing 1 mM ethylene diamine tetraacetic acid and 1% polyvinylpyrrolidone. The samples were centrifuged at 15,000× g, 4 °C for 20 min, then the supernatant was collected to determine MDA content and the activity of POD, GR, and APX.
The MDA content was determined by the thiobarbituric acid (TBA) method [17]. The supernatant (0.2 mL) was mixed with TBA (0.1 mL, 0.75%) dissolved in 10% trichloroacetic acid (TCA), then transferred to a boiling water bath for 15 min. The sample was centrifuged at 4000× g, 4 °C for 20 min and the supernatant was collected. The absorbance of the supernatant was determined at 450, 532, and 600 nm. MDA content was calculated using the following Formula (5).
2.8. Activities of Antioxidant Enzymes
The activity of POD was determined by the guaiacol method [18]. The supernatant (10 μL) was mixed with guaiacol (150 μL, 1.92 mM) dissolved in 100 mM PBS (pH 6.5, 40 μL) of 50 mM H2O2. The absorbance of the mixture at 475 nm was measured rapidly for 5 min every 15 s, and the mixture was shaken for 5 s before each measurement. POD activity was measured by the change in absorbance of the mixture.
GR activity was measured using the method of Hasanuzzaman et al. [19]. The supernatant (30 μL) was mixed with 2.5 mM glutathione (40 μL) and 1 mM NADPH (40 μL). After mixing well, the absorbance at 340 nm was measured immediately and again every 15 s for 10 min, with shaking for 5 s before each measurement.
The APX enzyme activity was determined according to Nakano et al. [20]. The supernatant (40 μL) was mixed with 0.75 mM vitamin c (10 μL, dissolved in 50 mM PBS, pH = 7, protected from light) and 1 mM H2O2 (50 μL). The absorbance at 290 nm was rapidly measured every 30 s for 10 min with shaking for 5 s before each measurement.
Enzyme activity was calculated using the following Formula (6).
Here, ∆A is the change of absorbance during the reaction time, Vt is the total volume of the extract (mL), W is the fresh weight of sample (g), Vs is the volume of enzyme solution taken during determination (mL), and t is reaction time (min).
2.9. Photosynthetic Pigment Content
The contents of chlorophyll a, chlorophyll b, and carotene in leaves were determined by ethanol spectrophotometry. The leaves were cut into pieces and weighed. A total of 5 mL of 95% ethanol was added to seal the leaves. The leaves were stored and extracted at room temperature under dark conditions for 24 h. The absorbance of the supernatant was measured at 665, 649, and 470 nm, and the content of photosynthetic pigment was calculated using the formula reported by Pongprayoon et al. [21].
2.10. Data Analysis
All treatments were repeated three times, and the data are expressed as the overall average of the replicates. SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was adopted for the analysis of statistical differences between treatments, using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (p < 0.05). The OriginPro 2022 system (OriginLab, Northampton, MA, USA) was applied for editing the figures.
3. Results
3.1. Coating with Physcion Increased the Fresh Weight and Plant Height of Wheat Seedling and Decreased the Disease Index of Powdery Mildew in the Greenhouse Experiment
As presented in Table 1, seeds coated with physcion at 1:50, 1:100, and 1:200 statistically increased fresh weight compared with CK, while seeds coated with physcion at 1:50 statistically increased the stem length. Seeds coated with physcion at 1:50 demonstrated the highest fresh weight (73%) and plant height (19%) increase compared to CK. Seeds coated with physcion at 1:25, 1:50, and 1:100 displayed significant inhibition of root length, reduced by 16%, 16%, and 11%, respectively, compared with CK (Table 1).

Table 1.
The influence of coating with physcion on the fresh weight, root length, and stem length of wheat seeds.
Coating with physcion statistically decreased the disease index of powdery mildew. The control effect of coated powdery mildew at 1:50 was significantly higher than that of other treatments, and the control effect was 93% compared with CK (Table 2).

Table 2.
Control effect of coating with physcion and its mixtures on wheat powdery mildew in the greenhouse experiment.
3.2. Coating with Physcion or Its Mixtures Increased the Emergence Rate of Wheat Seedlings and Tiller Number in the Field Trial
Seeds coated with 1# to 5# at 1:50 increased the seedling emergence rate and tiller number significantly compared with CK (Table 3). Compared to treatment at 1:100, seeds coated at 1:50 exhibited a higher emergence rate, although the difference was not statistically significant. For the tiller number, seeds of 207 coated with 2#, 3#, and 4# and seeds of 4199 coated with 2# and 3# showed a significantly higher number at 1:50 than at 1:100. Compared to CK, seeds coated with 1# at 1:50 exhibited significant promotion of both the emergence rate and tiller number. Compared to 1#, seeds coated with 2# to 5# at 1:50 exhibited promotion of the emergence rate and tiller number; however, the difference was not significant. Among all the treatment, seeds coated with 2# at 1:50 showed the highest emergence rate and tiller number. The emergence rates of 207, 307, and 4199 coated with 2# at 1:50 were increased by 40%, 49%, and 42%, respectively, compared to CK, while the tiller number was increased by 61%, 49%, and 47%, respectively (Table 3).

Table 3.
Effect of coating with physcion and its mixtures on the emergence rate of wheat seedlings and tiller number.
3.3. Coating with Physcion or Its Mixtures Decreased the Disease Index of Powdery Mildew in the Field Trial
Coating with 1# to 5# decreased the disease index of powdery mildew in 207 and 4199 significantly compared to CK. For 307, 1# to 5# only decreased the disease index of powdery mildew significantly at 1:50. Compared to 1:100, seeds coated at 1:50 showed a significant better control effect against wheat powdery mildew, except for 207 treated with 4# or 5# and 4199 treated with 4#. Compared to CK, seeds coated with 1# exhibited a significantly higher control effect against wheat powdery mildew. The control effect against wheat powdery mildew of 2# and 4# coated at 1:50 was significantly higher than that of 1#, with 2# showing the best control effect. Compared with CK, the control effect against powdery mildew of 2# coated at 1:50 was increased by 80.77%, 68.42%, and 81.45%, respectively, for plants of 207, 307, and 4199 (Table 4).

Table 4.
Control effect of coating with physcion or its mixtures against wheat powdery mildew.
The disease index of wheat scab and stem rot during the field trial was low (nearly 0), and could not accurately represent the control efficacy of the agents. The data are provided in Table S4.
3.4. Coating with Physcion or Its Mixtures Increased the Yield of Wheat
The spike number, grain number per spike, and 1000-grain weight are the three primary yield components for wheat. Coating with 1# at 1:50 increased the grain number per spike significantly compared to CK. Compared to 1#, only seeds of 307 coated with 2# exhibited a significantly higher grain number per spike. Seeds coated at 1:50 showed a higher grain number per spike than those coated at 1:100, although only 2# showed a significant difference in all three cultivars, and for 3# to 5# the difference was only significant in 207 (Table 5).

Table 5.
Effect of coating with physcion or its mixtures on the theoretical yield of wheat.
Seeds coated with 1# to 5# at 1:50 resulted in significant increases in the 1000-grain weight and spike number as compared to CK. The 1000-grain weight of 2# to 5# coated at 1:50 was higher than 1#, and the difference was significant for 207 coated with 2#. Seeds coated at 1:50 showed higher 1000-grain weight than those coated at 1:100, and the difference was significant for seeds of 207 coated with 2# and 4# and 4199 coated with 2#. The spike number of 2# to 5# was higher than that of 1# when coated at 1:50, and the difference was significant for 207 and 4199 coated with 2#. The spike number of seeds coated at 1:50 was higher than that in seeds coated at 1:100, and significant differences were observed in 207 coated with 1# and 2#, 307 coated with 1#, 2#, and 5#, and 4199 coated with 1# to 5#.
Seeds coated with 1# to 5# at 1:50 had significantly increased yield of wheat compared to CK. Seeds coated with 2# to 5# at 1:50 had increased yield compared with 1#, and significant differences were observed in 207 coated with 2#, 307 coated with 2# and 4#, and 4199 coated with 2# (Table 5).
Among all the treatments, coating with 2# showed the highest increase in grain number per spike, 1000-grain weight, spike number, and substantial yield. The grain number per spike of 207, 307, and 4199 treated by 2# at 1:50 was increased by 10%, 12%, and 15%, respectively, compared to CK, while the 1000-grain weight was increased by 7%, 8%, and 10%, the spike number by 12%, 9%, and 11%, and the yield by 25%, 21%, and 32%, respectively.
3.5. Coating with Physcion or Its Mixtures Reduced the MDA Content
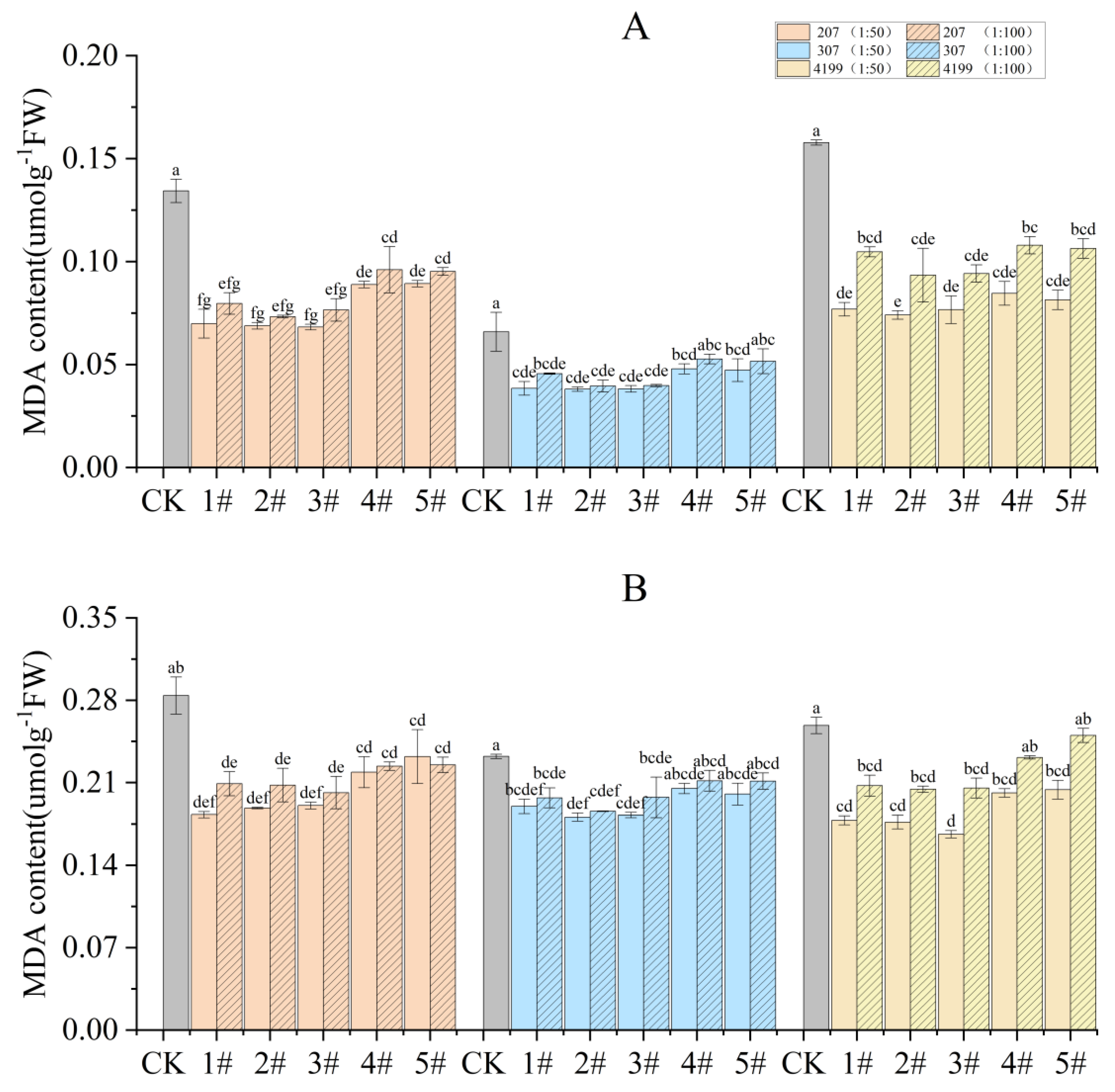
The concentration of MDA in wheat flag leaves was analyzed during the turning green stage and grain filling stage. The results indicate that the MDA content during the turning green stage was significantly reduced in seeds coated with 1# to 5# at 1:50 compared to CK. Compared to 1#, seeds coated with 2# to 5# at 1:50 had decreased MDA content, and the difference was significant for 207 coated with 2# and 5# (Figure 1A). During the grain filling stage, the only significant decrease in MDA content compared to CK was observed for seeds coated with 1# to 5# at 1:50, except for 4# and 5# in 307. Compared with 1#, seeds coated with 2# to 5# at 1:50 had decreased MDA content; however, the difference was not significant. (Figure 1B). Seeds coated with 1# to 5# at 1:50 had lower MDA content than those coated at 1:100; again, however, the difference was not significant. Among all the treatments, seeds coated with 2# at 1:50 showed the lowest MDA content. Compared with CK, the MDA content during the turning green stage was decreased by 34%, 22%, and 24%, 207, 307, and 4199, respectively, while the MDA content during the grain filling stage was decreased by 49%, 42%, and 53%, respectively.
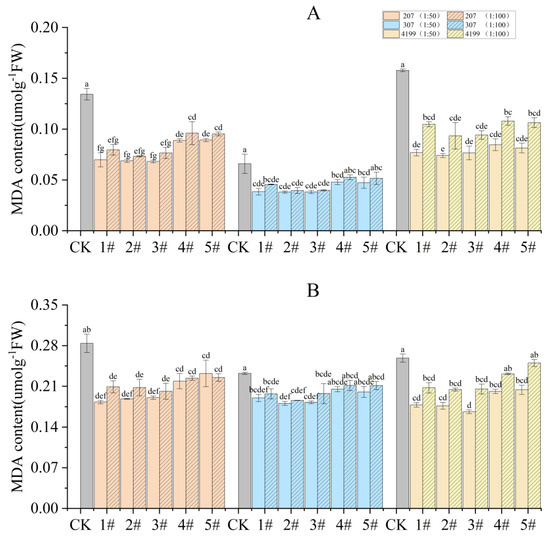
Figure 1.
Decreased MDA content in flag leaves of wheat after coating with physcion or its mixtures during the turning green stage (A) and the grain filling stage (B). Different lowercase letters indicate significant differences (p < 0.05). Error bars indicate standard errors calculated with three replications.
3.6. Coating with Physcion or Its Mixtures Enhanced Antioxidant Enzyme Activity
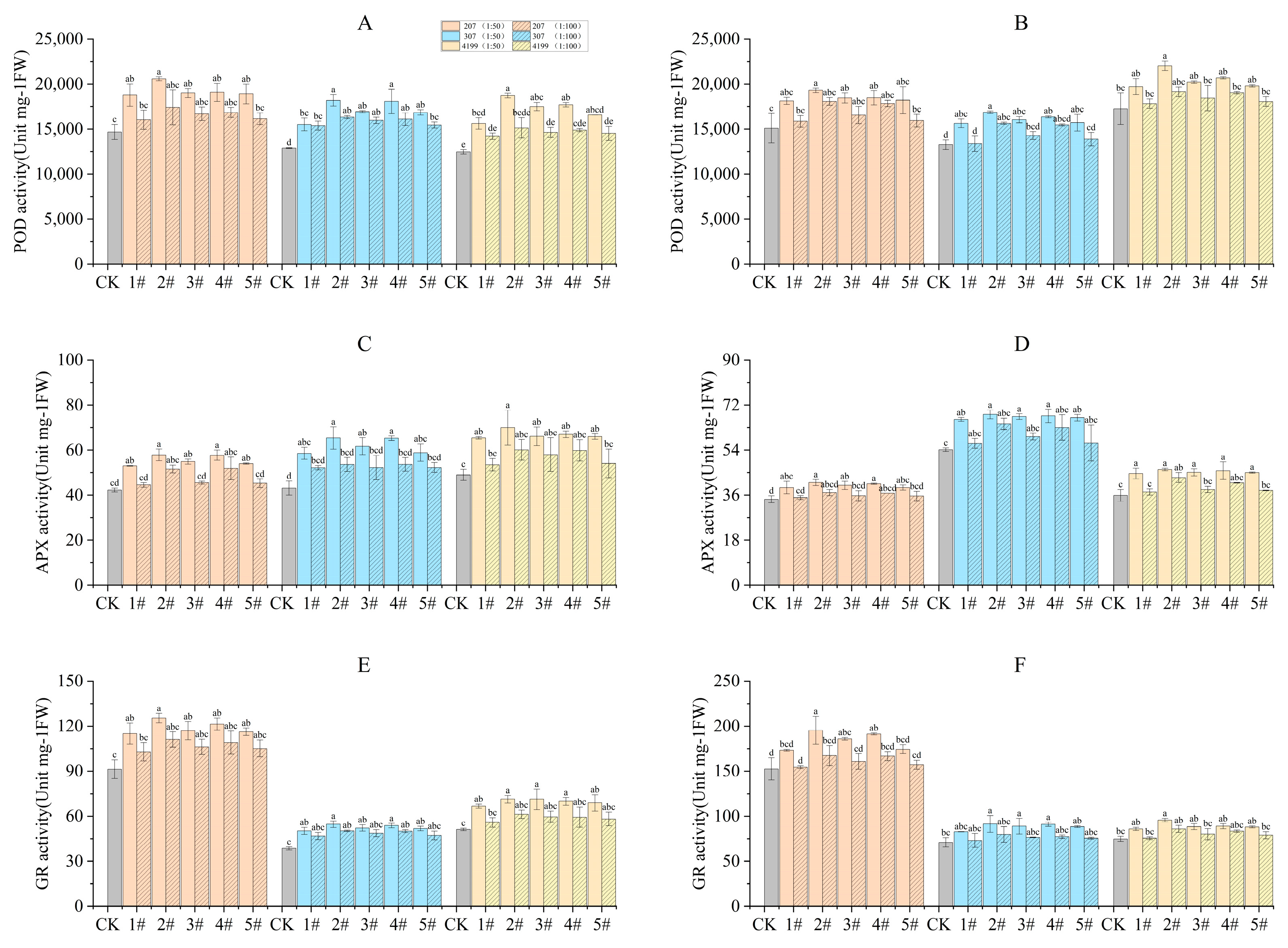
The antioxidant enzyme activity in wheat flag leaves during the turning green stage and grain filling stage were measured. Compared to CK, the antioxidant enzyme activity was significantly increased in seeds coated with 1# to 5# at 1:50 during the turning green stage (Figure 2). Compared with 1#, the activity of antioxidant enzymes in 307 coated with 2# and 4# and 4199 coated with 2# showed significant increases at 1:50. During the grain filling stage, POD enzyme activity was increased significantly compared to CK for 207 seeds coated with 2# to 4#and 307 seeds coated with 1# to 5# at 1:50. However, only seeds coated with 2# resulted in a significant increase in POD enzyme activity of 4199 compared to CK. Seeds coated with 1# to 5# at 1:50 significantly increased APX enzyme activity compared to CK. Seeds coated with 2# to 4# at 1:50 significantly increased GR enzyme activity compared to 1#, while coating with 3# and 4# only resulted in significant increases in GR enzyme activity in 207 and 4199 compared to CK. Compared with 1#, only seeds coated with 2# significantly increased GR enzyme activity in 207.
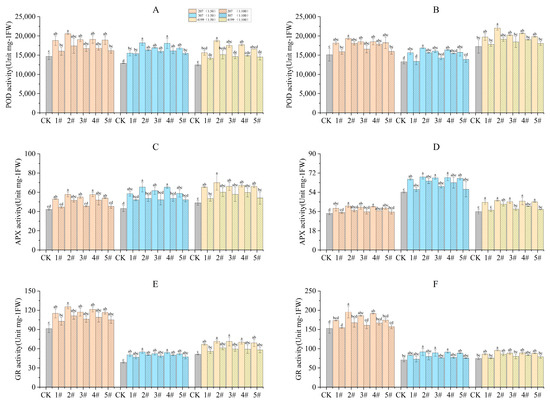
Figure 2.
Improved antioxidant enzyme activity in flag leaves of wheat after coating seeds with physcion or its mixtures. POD enzyme activity in flag leaves of wheat during the turning green stage (A) and the grain filling stage (B); GR enzyme activity in flag leaves of wheat during the turning green stage (C) and the grain filling stage (D); APX enzyme activity in flag leaves of wheat during the turning green stage (E) and the grain filling stage (F). Different lowercase letters indicate significant differences (p < 0.05). Error bars indicate standard errors calculated with three replications.
Coating with 2# at 1:50 was found to be the most effective in improving the amount of antioxidant enzyme activity as compared with CK. Specifically, POD enzyme activity was increased by 40%, 41%, and 50% in 207, 307, and 4199, respectively. During the turning green stage, APX enzyme activity exhibited increases of 37%, 52%, and 43%, while GR enzyme activity was increased by 37%, 42%, and 39%. During the grain filling stage, POD enzyme activity in 207, 307, and 4199 was increased by 28%, 27%, and 28% respectively, while APX enzyme activity was increased by 19%, 26%, and 29% and GR enzyme activity by 28%, 29%, and 28%.
3.7. Coating with Physcion or Its Mixture Improved Chlorophyll Content
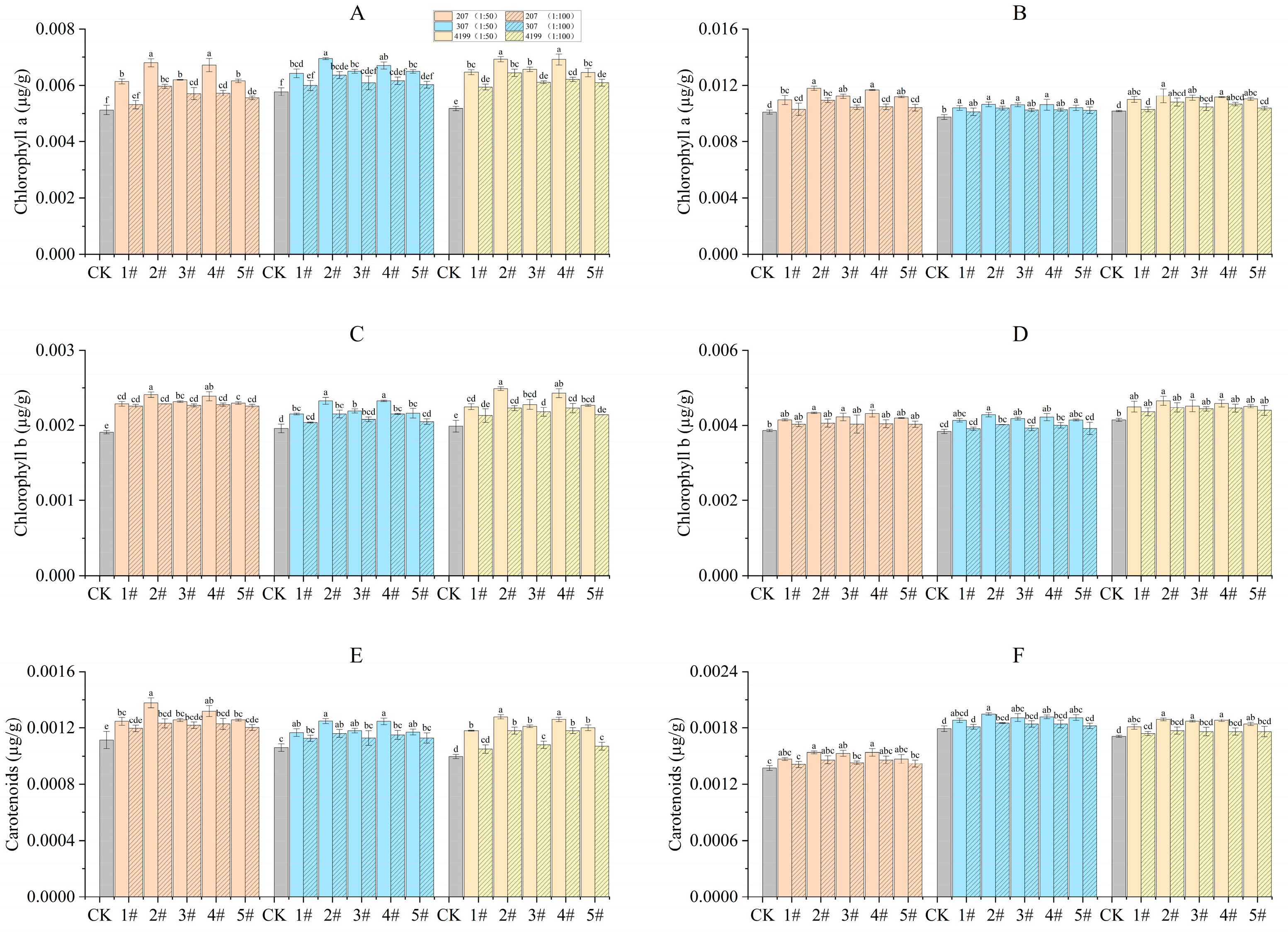
During the turning green stage, seeds coated with 1# to 5# at 1:50 had significantly increased chlorophyll content compared to CK (Figure 3). Seeds of 207 and 4199 coated with 2# and 4# and seeds of 307 coated with 2# had significantly increased chlorophyll a content when compared to the same seeds coated with 1#. seeds of 207, 307, and 4199 coated with 2# and 4# had significantly increased chlorophyll b content compared with 1#. Finally, seeds of 4199 coated with 2# and 4# and seeds of 207 coated with 2# had significantly increased carotenoids content when compared with 1#. During the grain filling stage, seeds coated with 1# at 1:50 had significantly increased chlorophyll a content compared to CK. Compared with 1#, the chlorophyll a content was significantly higher for 207 coated with 2# and 4#. Seeds coated with 1# at 1:50 increased chlorophyll b and carotenoid content significantly compared to CK, except for the carotenoids content in seeds of 4199 coated with 1#.
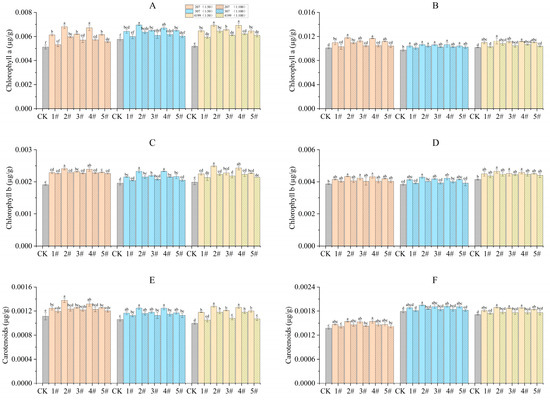
Figure 3.
Improved chlorophyll content in flag leaves of wheat after coating with physcion or its mixtures. Chlorophyll A content in flag leaves of wheat during the turning green stage (A) and the grain filling stage (B); chlorophyll B content in flag leaves of wheat during the turning green stage (C) and the grain filling stage (D); carotenoids content in flag leaves of wheat during the turning green stage (E) and the grain filling stage (F). Different lowercase letters indicate significant differences (p < 0.05). Error bars indicate standard errors calculated with three replications.
Compared with CK, 2# was the most effective in increasing chlorophyll content during the turning green stage, by 33%, 20%, and 34% for chlorophyll a content in 207, 307, and 4199, respectively, by 26%, 19%, and 25% for chlorophyll b content, and by 24%, 18%, and 28% for carotenoids content. During the grain filling stage, chlorophyll a content was increased by 17%, 9%, and 11%, respectively in 207, 307, and 4199, by 12%, 11%, and 12% for chlorophyll b content, and by 12%, 9%, and 11% for carotenoids content in comparison to CK (Figure 3).
4. Discussion
In China, Physcion has been registered to control wheat powdery mildew by spraying and wheat sharp eyespot by coating. To explore its scientific application in disease control, wheat seeds coated with physcion or mixtures of physcion with several fungicides commonly used on wheat (validamycin, pyrimidine nucleotide, azoxystrobin, and fludioxonil) were explored.
Physcion is reported to function as a plant growth inducer. As reported by Xiang et al. [22], physcion can enhance the defense ability of tomatoes against B. cinerea by promoting the endogenous defense response, promoting the growth of tomatoes as a growth inducer. Our greenhouse study suggested that physcion showed growth promotion in wheat at low concentrations and growth inhibition at high concentrations. Coating seeds with physcion at 1:50 showed the best growth promotion effect. However, physcion coating inhibited the root development of wheat seedlings, which is in agreement with the findings of inhibition in maize roots by Li et al. [23]. As studied by Li et al. [24], this might be attributed to enhancement of the respiration rate after coating with physcion.
Many studies have proven that physcion can control powdery mildew caused by B. graminis, P. xanthii, S. fuliginea, etc. [25,26,27,28]. According to Zhao and Xia [28], 0.05% physcion SC can effectively control powdery mildew in strawberry, tomato, cucumber, and grapes with a control effect of 70~80%. An indoor pot biological activity assay of physcion showed that the control effect against cucumber powdery mildew was more than 95% [25]. Our greenhouse experiment demonstrated that physcion coating showed an excellent control effect (93%) against wheat powdery mildew. In the field trial, the application of physcion combined with validamycin, pyrimidine nucleotide (2#), or azoxystrobin (4#) was more effective in controlling wheat powdery mildew compared to application of physcion alone (Table 4), suggesting that these mixtures can be applied to effectively control wheat powdery mildew. While the mixtures of physcion, azoxystrobin (3#) and physcion, fludioxonil (5#) showed considerable control effects against wheat powdery mildew compared with physcion alone, azoxystrobin is a systemic fungicide and is persistent in fields (DT50 = 180.7 d, http://sitem.herts.ac.uk/aeru/iupac/Reports/54.htm, accessed on 26 November 2023). Fludioxonil is only slightly systemic and is non-persistent in fields (DT50 = 16 d, http://sitem.herts.ac.uk/aeru/iupac/Reports/330.htm, accessed on 26 November 2023). Both act directly on fungal cells to inhibit diseases. In contrast, validamycin and pyrimidine nucleoside function by inducing plant resistance, acting as preventive measures against diseases. The different control effects of mixtures containing physcion (2#, 3#, 4#, 5#) might result from the long interval between seed coating and the late occurrence of wheat powdery mildew. In addition, the induction of wheat growth by physcion is combination with validamycin or pyrimidine nucleoside increases its resistance to plant diseases. The control effect against wheat powdery mildew in 307 was lower than that in 207 and 4199. According to Ou et al. [29], 307 is middle-tolerant to wheat powdery mildew, while 207 and 4199 are susceptible to wheat powdery mildew; thus, this finding might be related to the different tolerances to powdery mildew among the three cultivars.
Seedling emergence rate and tiller number are important indicators for evaluating the early growth and yield of wheat. Numerous studies have demonstrated that seed coating can effectively prevent pests, especially soil-borne diseases [30], while improving seedling rates and enhancing seedling growth. Mnasri et al. [31] showed that seed coating protects the crop against seed-borne and soil-borne pathogens and promotes active plant defense responses. In this study, seeds coated with physcion, valiomycin, and pyrimidine nucleotide (2#) at 1:50 showed the highest increase in both seedling emergence rate and tiller number. This might be a result of the induction of endogenous defense responses by physcion, valiomycin, and pyrimidine nucleoside, which could lay the foundation for resistance to disease and increased yields.
Yield is a crucial parameter for evaluating the control effect of fungicides on pests. Although all treatments increased the grain number per spike, 1000-grain weight, and spike number significantly when compared with CK, only 2# showed a significant increase in these indexes compared to physcion alone. To explore the potential mechanism of this increase in wheat yields, the antioxidant activity and the chlorophyll content of wheat leaves following each treatment were determined. Wheat powdery mildew results in the accumulation of ROS and an increase in MDA content [32]. MDA is a marker of membrane lipid peroxidation. Physcion has been preliminarily shown to inhibit MDA production in plant cells [22]. Ma et al. [33] showed that physcion effectively controls powdery mildew in barley by enhancing the expression of defense-related genes, particularly thionin genes specific to leaves. Here, the combination of physcion, validamycin, and pyrimidine nucleotide (2#) exhibited reduced MDA content compared to combinations of physcion and azoxystrobin (4#) and physcion and fludioxonil, as did the combination of physcion and validamycin (3#) (5#). Liu et al. [34] previously confirmed that several chemical pesticides are capable of causing physiological and biochemical toxicity to plants, leading to an accumulation of ROS and MDA. The difference in MDA content between 2#, 3# and 4#, 5# is likely a result of the potential stress caused by chemical fungicides. Physcion may reduce MDA content by relieving oxidative stress caused by powdery mildew (Figure 1 and Figure 2). The reduction in MDA content may also be associated with an elevation in antioxidant enzyme activity. Mixtures containing physcion and other fungicides (2#, 3#, 4#, 5#) showed increased antioxidant enzyme activity compared to physcion (1#) alone, while 2# showed significant induction of POD, GR, and APX activity in all the cultivars. The POD, GSHR, and APX enzymes are crucial antioxidant enzymes in plants, playing important roles in the scavenging of ROS [35]. Li et al. [23] showed that physcion can enhance antioxidant enzyme activity in maize. In this study, treatments with physcion or its mixtures significantly increased the activities of POD, GR, and APX in wheat leaves. The activity levels of these antioxidant enzymes are closely related to tolerance to oxidative stress, thereby improving adaptability. This correlation is further confirmed by the disease control results. The antioxidant enzyme levels increased more during the turning green stage than during the grain filling stage, while the MDA content decreased more during the turning green stage than during the grain filling stage. This might be due to the much longer interval of the grain filling stage following seed coating compared to the turning green stage.
The energy metabolism in plants is primarily governed by photosynthesis, a vital process for plant growth and development [36]. The levels of chlorophyll a, chlorophyll b, and carotenoids play significant roles in regulating photosynthesis. Photosynthesis in the flag leaf accounts for approximately one-third of dry matter accumulation in wheat grains, which is vital to enhancing the yield [37]. Here, treatments 2# and 4# showed the highest enhancement in chlorophyll content and the best control effect against powdery mildew. Wheat powdery mildew causes damage to infected leaves by chlorosis and yellowing, elevating respiration and transpiration rates, decreasing photosynthesis levels, and reducing nutrient accumulation [38]. Thus, the effective control of wheat powdery mildew can increase photosynthesis in plants. Chloroplasts function as a vital source of ROS production in plants [39]. Excessive accumulation of ROS causes damage to cellular membrane structures, notably the chloroplast membrane, significantly impeding the process of photosynthesis [40]. Physcion or its mixtures enhanced the activity of antioxidant enzymes and alleviated the accumulation of reactive oxygen species, thereby contributing to the improvement of photosynthetic processes.
5. Conclusions
In summary, wheat seeds coated with physcion or its mixtures showed enhancement in the spike number by facilitating both the emergence rate and tiller number. Seeds treated at 1:50 with physcion, validamycin, and pyrimidine nucleotide or physcion and azoxystrobin showed the best control effect against wheat powdery mildew by enhancing the defense capability of wheat leaves under stress and improving photosynthetic efficiency, ultimately fostering the accumulation of dry matter and leading to increased yields. Consequently, coating seeds with the combination of physcion, validamycin, and pyrimidine nucleotide is recommended for wheat production in the Huang-Huai-Hai plain in China.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14020237/s1. Table S1. The annual average temperature and annual average precipitation. Table S2. The physicochemical properties of soil in the field trial site. Table S3. Effect of coating with physcion or its mixture on the total weight of wheat with its spikes. Table S4. Control effect to wheat scab and wheat stem rot by coating with physcion or its mixture.
Author Contributions
Conceptualization, R.L. and H.W.; investigation, Z.T. and J.L.; writing—original draft preparation, Z.T.; writing—review and editing, J.L. and L.X.; supervision, L.Z. and F.Z. (Feng Zhou); project administration, F.Z. (Fulong Zhang); funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henan provincial science and technology major project (No. 221100110100), the special fund project for central guiding Henan province local development (No. Z20221343034), the leading talents of science and technology in the central plain of China (No. 234200510007), and the Young Backbone Teacher Training Project of Henan Province (2020GGJS166).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Author Fulong Zhang was employed by the company Beijing Qingyuanbao Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, H.; Xiong, W.; Pequeño, D.N.L.; Hernández-Ochoa, I.M.; Krupnik, T.J.; Burgueño, J.; Xu, Y. Exploring the uncertainty in projected wheat phenology, growth and yield under climate change in China. Agric. Forest Meteorol. 2022, 326, 109187. [Google Scholar] [CrossRef]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.; Jamshed, M.; Samuel, M.A. Abiotic stress signaling in wheat–an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Todorovska, E.; Christov, N.; Slavov, S.; Christova, P.; Vassilev, D. Biotic stress resistance in wheat—Breeding and genomic selection implications. Biotekhnol. Biotekh. 2009, 23, 1417–1426. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Hu, Z.; Wang, S.; Zhang, J.; Wang, X.; Wang, Q.; Zhang, B. Lack of K-dependent oxidative stress in cotton roots following coronatine-induced ROS accumulation. PLoS ONE 2015, 10, e0126476. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Zhang, L.; Feng, T.; Zhang, Z.; Zhang, B. Fungicide difenoconazole induced biochemical and developmental toxicity in wheat (Triticum aestivum L.). Plants 2021, 10, 2304. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Wang, S.; Yu, D.; Ni, H. Synergistic interaction of physcion and chrysophanol on plant powdery mildew. Pestic. Sci. 2007, 63, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yuan, S.; Zhou, Y.; Liu, Y. Study on the photolysis characteristics of botanic pesticide physcion. Mod. Pestic. 2020, 19, 35–38. [Google Scholar]
- Wang, N.; Cai, M.; Wang, X.; Xie, Y.; Ni, H. Inhibitory action of biofungicide physcion on initial and secondary infection of Magnaporthe oryzae. J. Phytopathol. 2016, 164, 641–649. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Marsell, A.; Riederer, M. Direct effects of physcion, chrysophanol, emodin, and pachybasin on germination and appressorium formation of the barley (Hordeum vulgare L.) powdery mildew fungus Blumeria graminis f. sp. hordei (DC.) speer. J. Agric. Food Chem. 2018, 66, 3393–3401. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhao, C.; Cao, L.; Huang, Q.; Wu, Y. Enhanced germicidal efficacy by co-delivery of Validamycin and Hexaconazole with Methoxy Poly (ethylene glycol)-Poly (lactideco-glycolide) nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 152–159. [Google Scholar] [CrossRef]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J.; Zhou, M. Effects of validamycin in controlling Fusarium head blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pestic. Biochem. Physiol. 2019, 153, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Kang, Q.; Lin, S.; Zhu, C.; Yu, D.; Deng, Z. lsolation and Structural ldentification of a Tetraene Macrolide Produced by Streptomyces hygrospinosus var. Beijingensis. J. Shanghai Jiaotong Univ. 2011, 45, 88–91. [Google Scholar]
- Yang, X.; Yang, L.; Yu, D.; Ni, H. Effects of physcion, a natural anthraquinone derivative, on the infection process of Blumeria graminis on wheat. Can. J. Plant Pathol. 2008, 30, 391–396. [Google Scholar] [CrossRef]
- Ren, M.; Wang, F.; Zhang, H.; Talib, K.M.; Mujtaba, K.G.; Jiang, X.; Zhou, C.; Kang, X. Effects of reducing base fertilizer and herbicide on yield and nitrogen use efficiency of winter. Guizhou Agirc. Sci. 2021, 49, 56–64. [Google Scholar]
- Xiaojuan, L.; Honggang, W.; Hanbing, L.; Lingyun, Z.; Nianjun, T.; Qingqing, L.; Jian, W.; Tingyun, K.; Zhensheng, L.; Bin, L.; et al. Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiol Plantarum. 2006, 127, 701–709. [Google Scholar]
- Abdullahil Baque, M.; Lee, E.J.; Paek, K.Y. Medium salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia: The role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep. 2010, 29, 685–694. [Google Scholar] [CrossRef]
- Pec, P.; Frebort, I. Competition of homologous substrates, putrescine and cadaverine, in the reaction catalyzed by pea diamine oxidase. Biochem. Int. 1991, 24, 633–640. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Xiang, L.; Xue, M.; Yang, L.; Gong, S.; Yu, D. Bionic fungicide physcion controls gray mold in tomato: Possible modes of action. J. Gen. Plant Pathol. 2019, 85, 57–65. [Google Scholar] [CrossRef]
- Li, J.; Han, A.; Zhang, L.; Meng, Y.; Xu, L.; Ma, F.; Liu, R. Chitosan oligosaccharide alleviates the growth inhibition caused by physcion and synergistically enhances resilience in maize seedlings. Sci. Rep. 2022, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Z.; Li, J.; Askari, K.; Han, A.; Ma, J.; Liu, R. Physcion and chitosan-Oligosaccharide (COS) synergistically improve the yield by enhancing photosynthetic efficiency and resilience in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 203, 107993. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Lee, S.W.; Jang, K.S.; Kim, J.S.; Cho, K.Y.; Kim, J.C. Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews. Crop Prot. 2004, 23, 1215–1221. [Google Scholar] [CrossRef]
- Yang, L.; Gong, S.; Yang, X.; Yu, D. Activities of botanical fungicide physcion on several plants pathogenic fungi. Agrochemicals 2010, 49, 133–135. [Google Scholar]
- Zhang, C.; Li, J.; Su, Y.; Wu, X. Association of Physcion and Chitosan Can Efficiently Control Powdery Mildew in Rosa roxburghii. Antibiotics 2022, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, L. Field control efficacy of 0.05% physcion SC against powdery mildew on four crops. Pestic. Sci. Manag. 2021, 42, 44–49. [Google Scholar]
- Ou, X.; Li, X.; Qiao, H.; Ou, Y.; Wang, Z.; Zhang, S.; Xu, P. Breeding of a new wheat variety bainong 307 with dwarf, multi-resistance and high yield. Newsl. Agric. Sci. Technol. 2021, 08, 291–292. [Google Scholar]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Mnasri, N.; Chennaoui, C.; Gargouri, S.; Mhamdi, R.; Hessini, K.; Elkahoui, S.; Djébali, N. Efficacy of some rhizospheric and endophytic bacteria in vitro and as seed coating for the control of Fusarium culmorum infecting durum wheat in Tunisia. Eur. J. Plant Pathol. 2017, 147, 501–515. [Google Scholar] [CrossRef]
- Li, Y.; Roychowdhury, R.; Govta, L.; Jaiwar, S.; Wei, Z.Z.; Shams, I.; Fahima, T. Intracellular Reactive Oxygen Species-Aided Localized Cell Death Contributing to Immune Responses Against Wheat Powdery Mildew Pathogen. Phytopathology 2023, 113, 884–892. [Google Scholar] [CrossRef]
- Ma, X.; Yang, X.; Zeng, F.; Yang, L.; Yu, D.; Ni, H. Physcion, a natural anthraquinone derivative, enhances the gene expression of leaf-specific thionin of barley against Blumeria graminis. Pest Manag. Sci. 2010, 66, 718–724. [Google Scholar] [CrossRef]
- Liu, T.; Li, T.; Zhang, L.; Li, H.; Liu, S.; Yang, S.; An, Q.; Pan, C.; Zou, N. Exogenous salicylic acid alleviates the accumulation of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2021, 208, 111654. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Iqbal, N. Chemo-priming with mannose, mannitol and H2O2 mitigate drought stress in wheat. Cereal Res. Commun. 2014, 42, 450–462. [Google Scholar] [CrossRef]
- Liu, J.; Friebe, V.M.; Frese, R.N.; Jones, M.R. Polychromatic solar energy conversion in pigment-protein chimeras that unite the two kingdoms of (bacterio) chlorophyll-based photosynthesis. Nat. Commun. 2020, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, C.; Li, H.; Zheng, Q.; Li, B.; Li, Z. An analysis of the genetic relation between photosynthesis and yield-related traits in Wheat. Agriculture 2022, 12, 560. [Google Scholar] [CrossRef]
- Yang, M.J.; Huang, K.Y.; Han, Q.D. Research progresses on wheat powdery mildew and its resistance. Mol. Plant Breed 2016, 14, 1244–1254. [Google Scholar]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Responses of plants to pesticide toxicity: An overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).