Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Test Site
2.2. Design of the Experiment
2.3. Observation Indicators and Measurement Methods
2.3.1. Determination of Dry Matter
2.3.2. Determination of Chlorophyll Fluorescence Kinetic Parameters
2.3.3. Determination of Enzyme Activity and Content
2.3.4. Production and Production Structure
2.4. Analysis of Data
3. Results
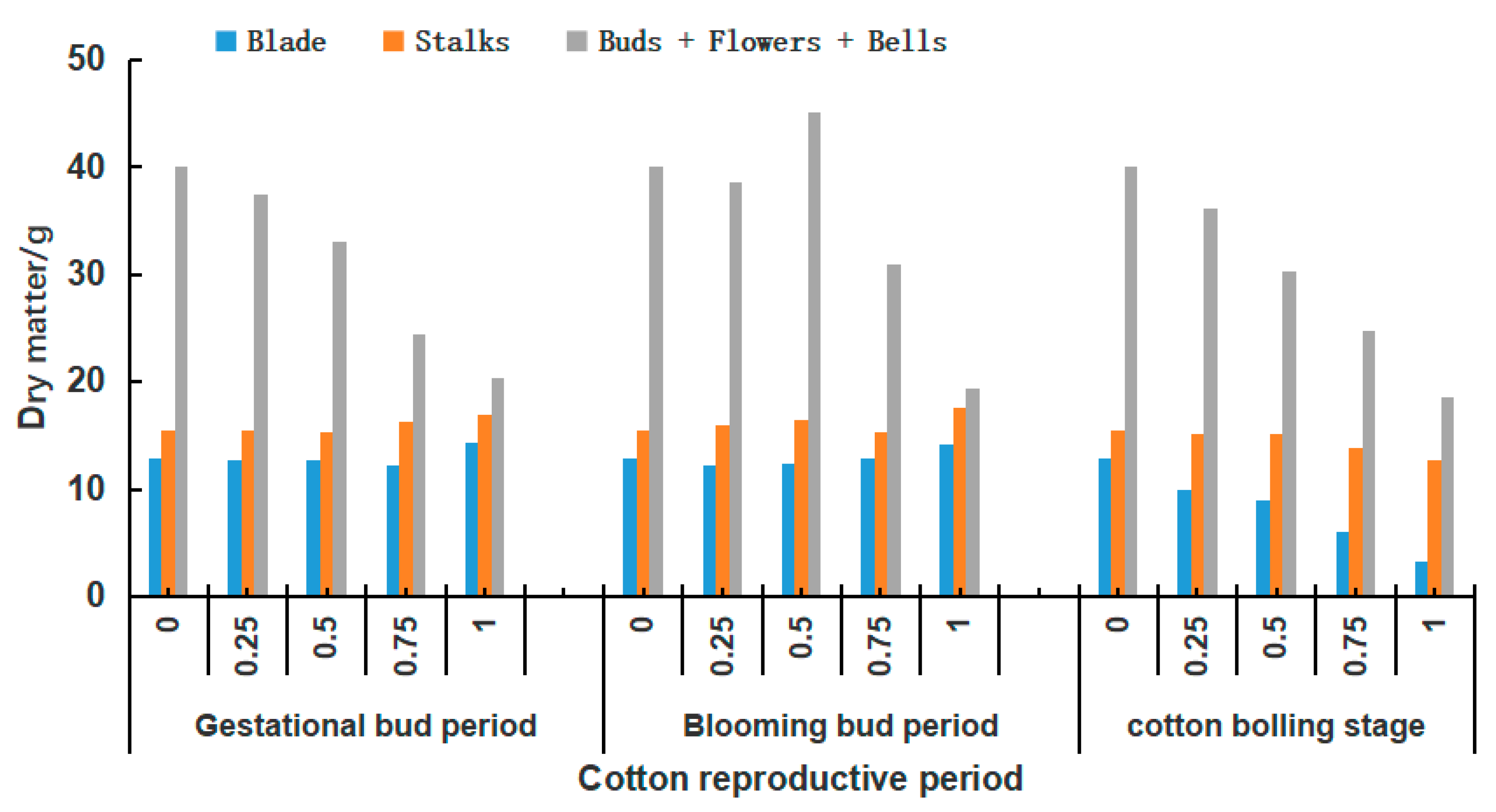
3.1. Effect of Defoliation at Different Periods on Dry Matter Changes in Cotton
3.2. Effect of Defoliation at Different Periods on Chlorophyll Fluorescence Parameters
3.2.1. Effect of Different Treatments on Kinetic Parameters of Chlorophyll Fluorescence Induction in Cotton at Full Bud Stage
3.2.2. Effect of Different Treatments on Kinetic Parameters of Chlorophyll Fluorescence Induction in Cotton at Boll Stage
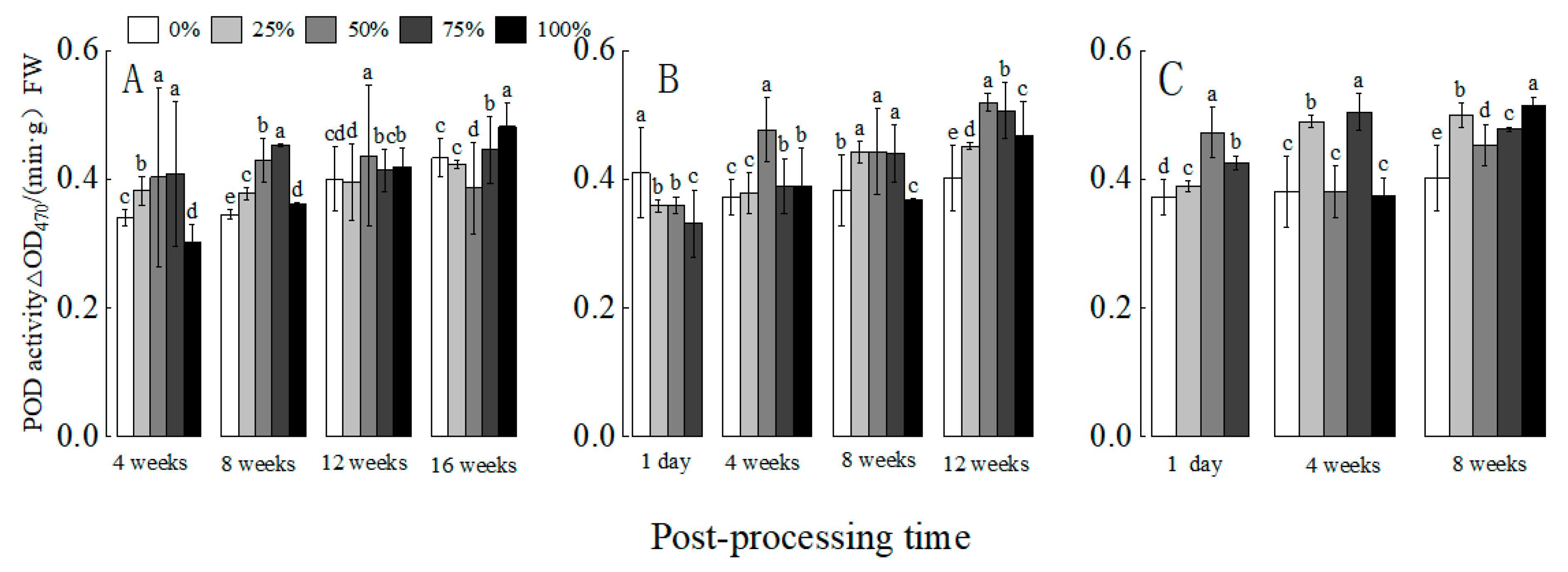
3.3. Effect of Defoliation on POD Enzyme Activity in Cotton at Different Stages
3.3.1. Effect of Defoliation at the Gestation Bud Stage on POD Enzyme Activity in Cotton
3.3.2. Effect of Defoliation at Full Bud Stage on POD Enzyme Activity in Cotton
3.3.3. Effect of Defoliation at Flowering Stage on POD Enzyme Activity in Cotton
3.4. Effect of Defoliation at Different Stages on the Activity of Cotton SOD Enzyme
3.4.1. Effect of Defoliation at the Gestation Bud Stage on the Activity of Cotton SOD Enzyme
3.4.2. Effect of Defoliation on Cotton SOD Enzyme Activity at Full Bud Stage
3.4.3. Effect of Defoliation at Flowering Stage on SOD Enzyme Activity in Cotton
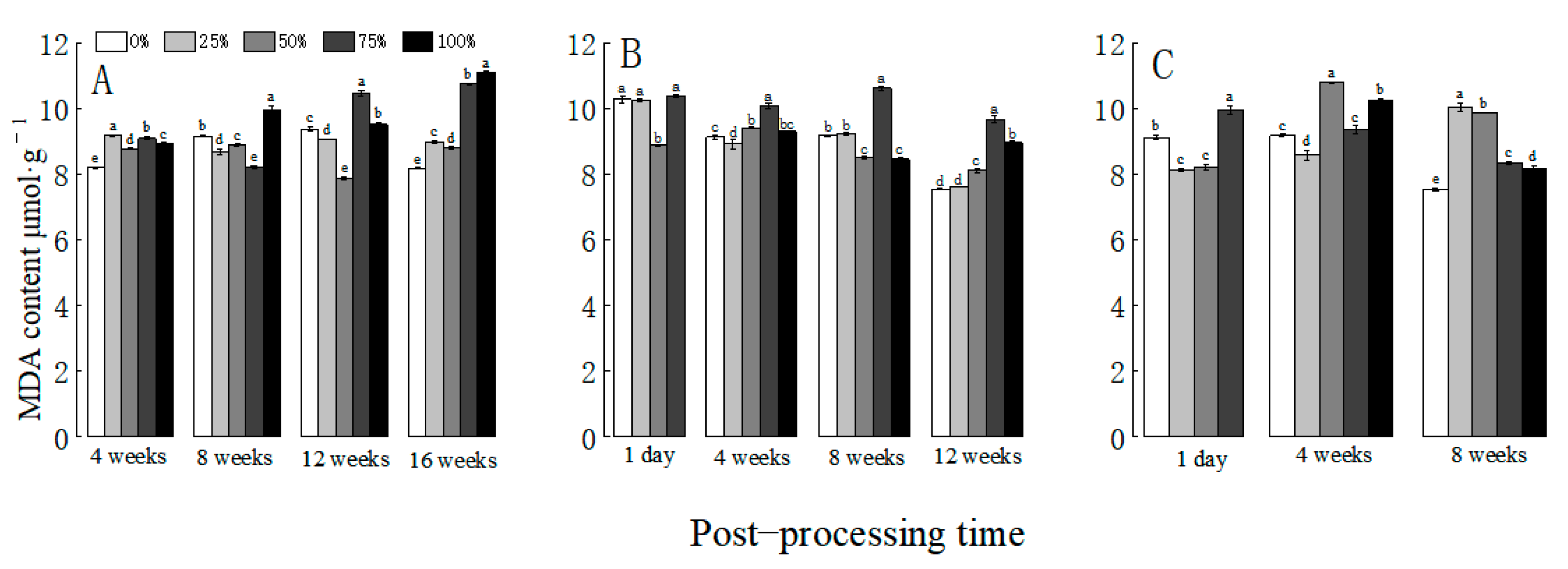
3.5. Effects of Defoliation at Different Growth Stages on MDA Content in Cotton Leaves
3.5.1. Effect of Defoliation on MDA Content in Cotton during Pregnancy Bud Stage
3.5.2. Effect of Defoliation on MDA Content in Cotton at Full Bud Stage
3.5.3. Effect of Defoliation at Flowering Boll Stage on MDA Content in Cotton
3.6. Effect of Defoliation at Different Times on Cotton Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zhang, J.; Zhang, L. Climatic characteristics of heavy hail weather in Aksu Prefecture of Xinjiang in the past 50 years. J. Desert Res. 2011, 31, 236–241. [Google Scholar]
- Li, M.; Zhao, F. Effects of different degrees of hailstorm on cotton growth and yield. J. Anhui Agric. Sci. 2006, 34, 649–673. [Google Scholar]
- Zhao, Y. Hail disaster relief measures for cotton at different times. Rural. Sci. Technol. 2022, 2, 17–19. [Google Scholar]
- Feng, J.; Hu, X.; Mao, X. Application of chlorophyll fluorescence kinetics in the study of plant stress physiology. Econ. For. Res. 2002, 20, 14–18. [Google Scholar]
- Zhang, X.H.; Li, Y.; Yu, K.; Huo, Y.; Wang, Y.; Chen, B. Mechanism of Verticillium wilt stress affecting photosynthetic and chlorophyll fluorescence characteristics of cotton seedlings. Cotton Sci. 2018, 30, 136–144. [Google Scholar]
- Li, Q.; Li, Z.; Ji, J.; Zou, Q.; Yu, H. Chlorophyll fluorescence kinetics and its application in the study of plant stress resistance physiology. Hubei Agric. Sci. 2013, 52, 5399–5402. [Google Scholar]
- Meyer, G.A. Mechanisms promoting recovery from defoliation in goldenrod (Solidago altissima). Can. J. Bot. 1998, 76, 450–459. [Google Scholar]
- Sun, L.; Zhang, S.; Luo, X. Effects of deficit irrigation on the activities of protective enzymes in cotton leaves and roots. J. Tarim Univ. 2021, 33, 86–93. [Google Scholar]
- Wang, Q.; Yin, F.; Li, C. Progress of reactive oxygen radical metabolism in plants under water stress. Henan Agric. Sci. 2004, 10, 25–28. [Google Scholar]
- Smiroff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Weng, M.; Cui, L.; Liu, F. Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
- Zhang, R.; Zheng, Y.; Ma, G.; Zhang, X.; Lu, H. Effects of drought stress on photosynthesis and protective enzymes in maize seedling leaves. Acta Ecol. Sin. 2011, 31, 1303–1311. [Google Scholar]
- Lum, M.S.; Hanafi, M.M.; Rafii, Y.M.; Akmar, A.S.N. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Yin, Y.; Hu, Y.; Hu, S. Analysis of chlorophyll fluorescence parameters at flowering and boll stage of cotton under different cultivation modes. Agric. Technol. 2022, 42, 6–10. [Google Scholar]
- Yuan, J.; Ma, C.; Feng, Y.; Zhang, J.; Yang, H.; Li, Y. Responses of rapid chlorophyll fluorescence-induced kinetic curves of wheat with different drought tolerances to drought and rewatering. Chin. J. Plant Physiol. 2018, 54, 1119–1129. [Google Scholar]
- Liu, L.; Li, C.; Sun, H.; Liu, L.; Gao, X.; Feng, L. Effects of drought on cell membrane damage, protective enzyme activity and yield of cotton leaves with different boll weight genotypes. Cotton Sci. 2009, 21, 296–301. [Google Scholar]
- Wu, H.; Zhang, J.; Shi, J.; Fan, Z.; Aliyan, L.; Rou, Z. Physiological responses of cotton seedlings to different degrees of low temperature stress. Northwest Bot. 2013, 33, 74–82. [Google Scholar]
- Li, Q.; Zhao, Y.; Li, P.; Zhu, Y.M.; Yang, C.G.; Cao, J.L.; Wang, Z.Q.; Yang, J.C.; Gu, J.F. Research progress of crop source-store relationship and its physiological regulation pathway. Jiangsu Agric. Sci. 2020, 48, 50–56. [Google Scholar]
- Kerns, D.L.; Fromme, D.D.; Baugh, B.A.; Doederlein, T. Ability of cotton on the Texas High Plains to compensate for pre-bloomsquare loss and impact on yield and fiber quality. J. Cotton Sci. 2016, 20, 103–115. [Google Scholar] [CrossRef]
- Sheng, C. Analysis of the compensatory effect on early bud loss in cotton. J. Ecol. 1988, 8, 97–103. [Google Scholar]
- Li, Y.; Sheng, C. Changes in protective enzyme activity in cotton leaves under leaf shape overcompensation. Acta Entomol. Sin. 2004, 6, 780–786. [Google Scholar]
- Mukhtar, M.; Muminjian, A. Simulation experiment on yield compensation capacity after boll stage damage in cotton. Rural. Sci. Technol. 2013, 7, 21–22. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, F. Effects of Low Temperature Stress on Photosynthetic Fluorescence and Physiological Characteristics of Cotton Seedlings; Shihezi University: Shihezi, China, 2010. [Google Scholar]
- Zhong, X.N. Effects of Low Temperature Stress at Bud Stage on Cotton Growth and Development, Photosynthetic and Chlorophyll Fluorescence Characteristics; Shihezi University: Shihezi, China, 2022. [Google Scholar]
- Xing, S.; Li, Z.; Tang, L.; Zhao, R.; Wei, Y. Effects of different temperatures on chlorophyll fluorescence in leaves of different genotypes of cotton. Xinjiang Agric. Sci. 2017, 54, 403–408. [Google Scholar]
- Wang, N.; Huang, F.; Chao, H.; Chen, D.; Zhang, T.; Jiang, N.; Tu, C.; Li, Y.; Yang, D. Effects of mowing on the growth, gas exchange and fluorescence of the invasive alien plant A. xanthocarpus. Acta Ecol. Sin. 2012, 32, 2943–2952. [Google Scholar] [CrossRef]
- Wang, T. Physiological and Ecological Response Characteristics of Dominant Plant Sheep’s Hoof to Defoliation Stress in Riparian Zone; Jiangxi Normal University: Nanchang, China, 2018. [Google Scholar]
- Li, J.; Huang, X.; Huang, H. Comparison of cell wall metabolic enzyme activities in the pericarp of litchi cultivars with different susceptibility to fruit splitting. J. Plant Physiol. Mol. Biol. 2003, 29, 141–146. [Google Scholar]
- Guo, H.; Li, Z.; Lin, Y.; Li, Z.; Li, J.; Huang, G.; Cui, S.; Pan, X. Effect of Verticillium wilt on the activity and photosynthetic properties of SOD and POD enzymes in cotton leaves. Chin. Agric. Sci. 1995, 28, 40–45. [Google Scholar]
- Liu, G.; Han, S.; Liu, X.; He, J. Environmental effects of algal bloom aggregation: Effects on antioxidant enzyme activity in floating plant water hyacinth (Eichharnia crassipes). J. Lake Sci. 2016, 28, 31–39. [Google Scholar]
- Carballo, I.G.; Llerena, J.L.; Sanchez, M.E.V. Effects of defoliation and water restriction total phenols and antioxidant activities in grapes during ripening. J. Int. Sci. Vigne Vin 2014, 48, 31–42. [Google Scholar]
- Liu, T.; Xu, C.; Gu, L.; Dong, S. Regulation of deleafing on photosynthetic performance of summer maize populations and single leaves under high planting density conditions. Acta Agron. Sin. 2014, 40, 143–153. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Xu, J.; Li, J.; Jiang, W. Physiological Responses of Different Lotus Cultivars to Low Temperature Stress after Low Temperature Exercise. J. Plant Resour. Environ. 2015, 24, 76–82. [Google Scholar]
- Li, D.; Tu, E.; Wang, L.; Mai, W.; Wang, L. Relationship between Verticillium wilt resistance and leaf protective enzyme activity and malondialdehyde content in cotton. J. Xinjiang Agric. Univ. 2014, 37, 131–136. [Google Scholar]
- Zhang, J. Response of maize cell protective enzyme activity to drought at seedling stage. North China Agric. Dly. 1990, 5, 19–23. [Google Scholar]
- Zhen, S.; Zhang, X.; Wang, L.; Shang, X. Effects of Pb~(2+),Cd~(2+) stress on protective enzyme and malondialdehyde content in cotton. J. Henan Agric. Sci. 2007, 8, 43–45+63. [Google Scholar]

| Terms and Equations | Definitions |
|---|---|
| Fo | Minimum fluorescence intensity after dark adaptation |
| Fj | Fluorescence intensity at point J (2 ms) |
| Fi | Fluorescence intensity at point I (30 ms) |
| Fp | Fluorescence intensity at the point of maximum fluorescence (point P) |
| Fm = Fp | Maximum fluorescence intensity after dark adaptation |
| Fv = Ft − Fo | Variable fluorescence intensity at t |
| Vt = (Ft − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at time t |
| Vi = (Fi − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at time I |
| Vj = (Fj − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at point J |
| Mo = 4 (F300μs − Fo)/(Fm − Fo) | Initial slope of the OJIP fluorescence induction curve |
| φPo ≡ TRo/ABS = 1 − (Fo/Fm) | Maximum photochemical efficiency of PSII |
| φEo ≡ ETo/ABS = [1 − (Fo/Fm)]ψo | Quantum yield for electron transfer |
| ψo ≡ ETo/TRo = (1 − Vj) | Probability that an exciton captured in the reaction centre will transfer an electron to other electron acceptors after the primary quinone acceptor (QA) |
| Period | Treatment | Mo | Vj | φpo | ψo | φEo | φDo |
|---|---|---|---|---|---|---|---|
| Gestational bud period | 0% | 0.59 ± 0.02 a | 0.47 ± 0.01 a | 0.63 ± 0.01 b | 0.53 ± 0.03 c | 0.34 ± 0.04 e | 0.37 ± 0.02 a |
| 25% | 0.29 ± 0.01 d | 0.28 ± 0.02 e | 0.79 ± 0.02 a | 0.72 ± 0.01 a | 0.57 ± 0.02 b | 0.21 ± 0.04 d | |
| 50% | 0.27 ± 0.02 d | 0.24 ± 0.03 d | 0.81 ± 0.02 a | 0.76 ± 0.02 a | 0.61 ± 0.01 a | 0.19 ± 0.05 e | |
| 75% | 0.34 ± 0.01 c | 0.33 ± 0.02 c | 0.75 ± 0.02 a | 0.67 ± 0.02 b | 0.5 ± 0.03 c | 0.25 ± 0.02 c | |
| 100% | 0.51 ± 0.02 b | 0.44 ± 0.01 b | 0.66 ± 0.03 c | 0.56 ± 0.04 c | 0.37 ± 0.02 d | 0.34 ± 0.02 b | |
| Blooming bud period | 0% | 0.43 ± 0.02 a | 0.37 ± 0.02 a | 0.73 ± 0.02 b | 0.63 ± 0.05 b | 0.46 ± 0.05 b | 0.27 ± 0.02 b |
| 25% | 0.1 ± 0.05 c | 0.13 ± 0.05 b | 0.73 ± 0.02 b | 0.87 ± 0.01 a | 0.63 ± 0.02 a | 0.28 ± 0.02 a | |
| 50% | 0.12 ± 0.02 c | 0.14 ± 0.04 b | 0.75 ± 0.01 a | 0.86 ± 0.02 a | 0.65 ± 0.02 a | 0.25 ± 0.02 d | |
| 75% | 0.13 ± 0.02 b | 0.14 ± 0.03 b | 0.75 ± 0.02 a | 0.86 ± 0.02 a | 0.64 ± 0.01 a | 0.25 ± 0.01 cd | |
| 100% | 0.14 ± 0.01 b | 0.16 ± 0.02 b | 0.76 ± 0.02 a | 0.85 ± 0.01 a | 0.64 ± 0.02 a | 0.25 ± 0.03 bc |
| Period | Treatment | Mo | Vj | φpo | ψo | φEo | φDo |
|---|---|---|---|---|---|---|---|
| Gestational bud period | 0% | 0.37 ± 0.01 b | 0.39 ± 0.03 a | 0.68 ± 0.02 c | 0.61 ± 0.04 a | 0.41 ± 0.04 e | 0.32 ± 0.02 a |
| 25% | 0.27 ± 0.03 d | 0.3 ± 0.05 c | 0.68 ± 0.03 c | 0.7 ± 0.03 a | 0.48 ± 0.03 b | 0.32 ± 0.01 ab | |
| 50% | 0.28 ± 0.04 c | 0.3 ± 0.03 c | 0.7 ± 0.01 a | 0.7 ± 0.01 a | 0.49 ± 0.01 a | 0.30 ± 0.03 ab | |
| 75% | 0.37 ± 0.03 b | 0.38 ± 0.01 a | 0.69 ± 0.03 b | 0.62 ± 0.03 a | 0.43 ± 0.03 d | 0.31 ± 0.03 ab | |
| 100% | 0.39 ± 0.03 a | 0.35 ± 0.03 b | 0.71 ± 0.01 ab | 0.65 ± 0.05 a | 0.46 ± 0.03 c | 0.29 ± 0.03 b | |
| Blooming bud period | 0% | 0.26 ± 0.04 b | 0.31 ± 0.03 ab | 0.74 ± 0.03 b | 0.69 ± 0.02 d | 0.51 ± 0.05 d | 0.26 ± 0.04 ab |
| 25% | 0.32 ± 0.03 a | 0.33 ± 0.01 a | 0.72 ± 0.02 c | 0.67 ± 0.03 e | 0.48 ± 0.03 e | 0.28 ± 0.03 a | |
| 50% | 0.22 ± 0.02 c | 0.24 ± 0.03 c | 0.74 ± 0.03 b | 0.76 ± 0.01 b | 0.56 ± 0.01 b | 0.26 ± 0.02 ab | |
| 75% | 0.21 ± 0.03 cd | 0.28 ± 0.04 b | 0.75 ± 0.03 b | 0.72 ± 0.03 a | 0.54 ± 0.03 c | 0.25 ± 0.03 b | |
| 100% | 0.20 ± 0.03 d | 0.21 ± 0.05 d | 0.8 ± 0.01 a | 0.79 ± 0.01 c | 0.63 ± 0.02 a | 0.2 ± 0.03 c | |
| cotton bolling stage | 0% | 0.18 ± 0.05 d | 0.23 ± 0.05 d | 0.81 ± 0.01 a | 0.8 ± 0.01 a | 0.64 ± 0.01 a | 0.19 ± 0.05 d |
| 25% | 0.30 ± 0.03 b | 0.36 ± 0.01 a | 0.72 ± 0.03 d | 0.64 ± 0.05 e | 0.46 ± 0.04 d | 0.28 ± 0.03 b | |
| 50% | 0.24 ± 0.02 c | 0.27 ± 0.03 c | 0.77 ± 0.01 bc | 0.73 ± 0.03 c | 0.57 ± 0.02 b | 0.23 ± 0.02 c | |
| 75% | 0.31 ± 0.03 a | 0.33 ± 0.03 b | 0.74 ± 0.02 c | 0.67 ± 0.01 b | 0.5 ± 0.03 c | 0.26 ± 0.03 b | |
| 100% | 0.29 ± 0.03 ab | 0.24 ± 0.02 d | 0.79 ± 0.03 b | 0.77 ± 0.03 d | 0.54 ± 0.03 c | 0.33 ± 0.01 a |
| Defoliation Intensity | Gestational Bud Period | Blooming Bud Period | Cotton Bolling Stage | |||
|---|---|---|---|---|---|---|
| Number of Bells | Yield/(kg·hm−2) | Number of Bells | Yield/(kg·hm−2) | Number of Bells | Yield/(kg·hm−2) | |
| 0% | 6.67 a | 7427.93 a | 6.67 a | 7429.34 a | 6.67 a | 7427.93 a |
| 25% | 6.78 a | 7204.31 b | 6.33 a | 6927.11 b | 5.22 b | 5708.95 b |
| 50% | 6.67 a | 7034.86 c | 7.67 a | 7904.38 c | 4.54 bc | 4815.43 c |
| 75% | 6.44 a | 6372.78 d | 5.00 b | 5212.83 d | 3.78 c | 3995.57 d |
| 100% | 6.33 a | 5938.72 e | 3.22 c | 3105.56 e | 2.33 d | 2355.80 e |
| Source of Variation | Square Sum | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Intercept | 1,586,441,219 | 1 | 1,586,441,219 | 199,475.844 | 0.00 ** |
| Phase | 27,413,222.92 | 2 | 13,706,611.46 | 1723.441 | 0.00 ** |
| Deal with | 75,808,650.32 | 4 | 18,952,162.58 | 2383.006 | 0.00 ** |
| Phase * Deal with | 18,312,750.71 | 8 | 2,289,093.839 | 287.826 | 0.00 ** |
| Residual | 238,591.479 | 30 | 7953.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wu, B.; Hu, B.; Wan, Y.; Wang, J.; Jia, M. Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton. Agriculture 2024, 14, 258. https://doi.org/10.3390/agriculture14020258
Li W, Wu B, Hu B, Wan Y, Wang J, Jia M. Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton. Agriculture. 2024; 14(2):258. https://doi.org/10.3390/agriculture14020258
Chicago/Turabian StyleLi, Wenjun, Bingrong Wu, Bao Hu, Yanan Wan, Jichuan Wang, and Mengmeng Jia. 2024. "Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton" Agriculture 14, no. 2: 258. https://doi.org/10.3390/agriculture14020258
APA StyleLi, W., Wu, B., Hu, B., Wan, Y., Wang, J., & Jia, M. (2024). Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton. Agriculture, 14(2), 258. https://doi.org/10.3390/agriculture14020258







