Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Experimental Design
2.2. Plant Growth Parameters and Root Morphology
2.3. Determination of Photosynthetic Efficiency and Photosynthetic Pigments
2.4. Measurement of Relative Electrolyte Conductivity, MDA, AsA Content
2.5. Detection of Soluble Protein and Antioxidant Enzymes
2.6. Statistical Analysis
3. Result
3.1. Effect of Exogenous CTS on Biomass, Plant Height and Main Root Length of Maize Seedlings under Salt Stress
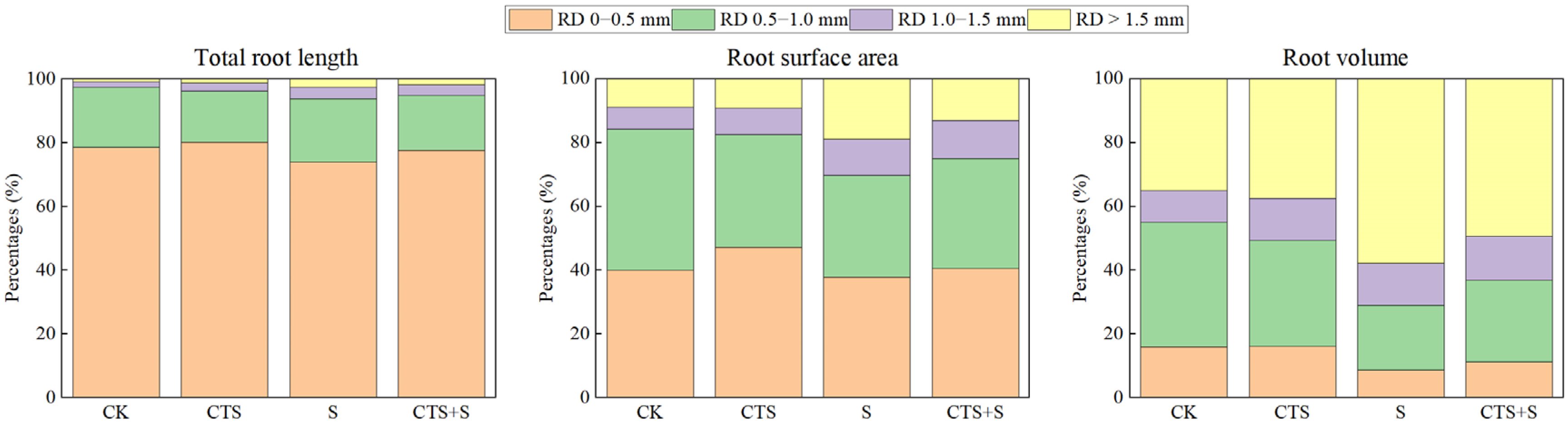
3.2. Effect of Exogenous CTS on the Characteristic Parameters of Root Growth of Maize Seedlings under Salt Stress
3.3. Effect of Exogenous CTS on Salt Concentration of Maize Seedlings under Salt Stress
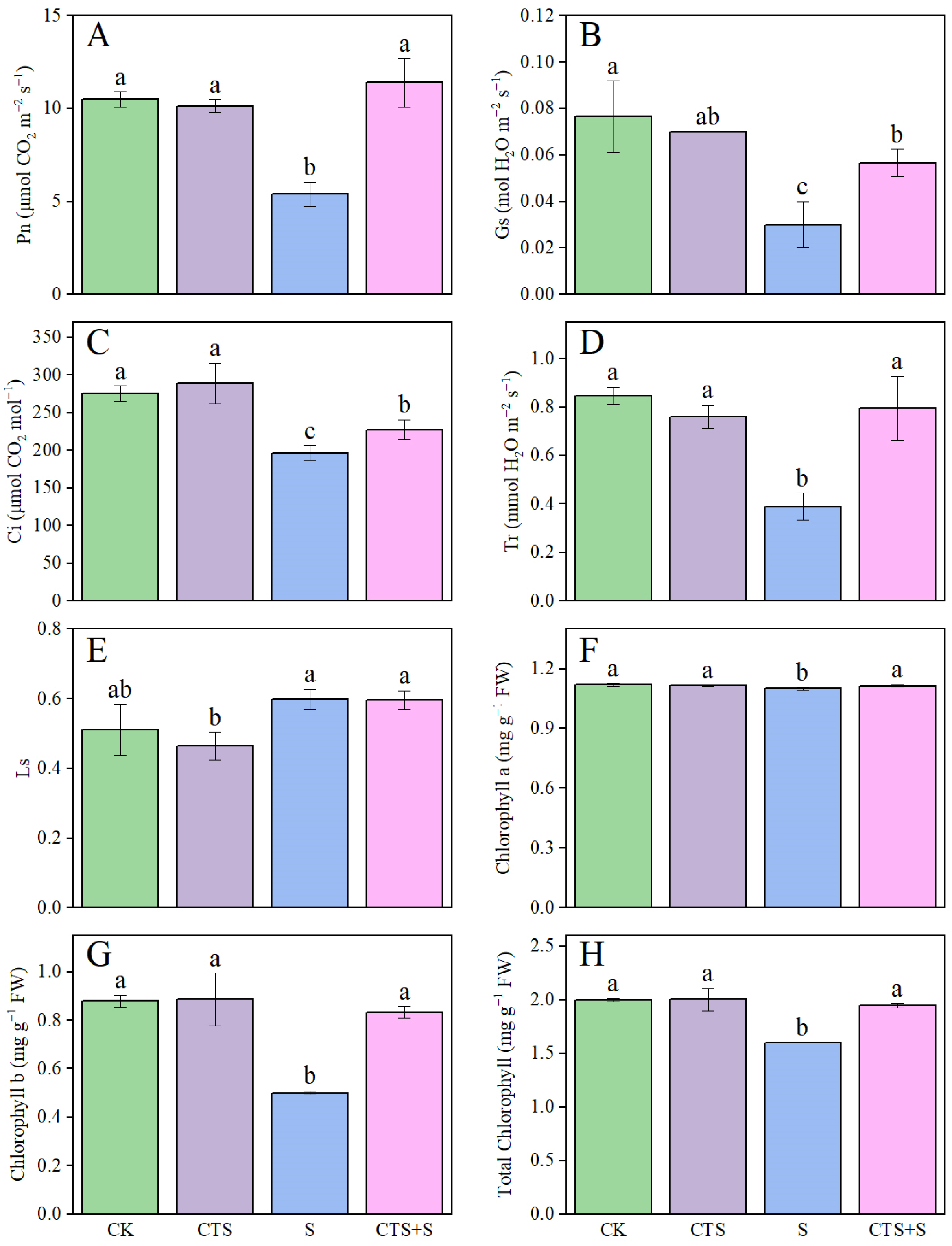
3.4. Effect of Exogenous CTS on Photosynthetic Characteristics and Pigment Contents of Maize Seedlings under Salt Stress
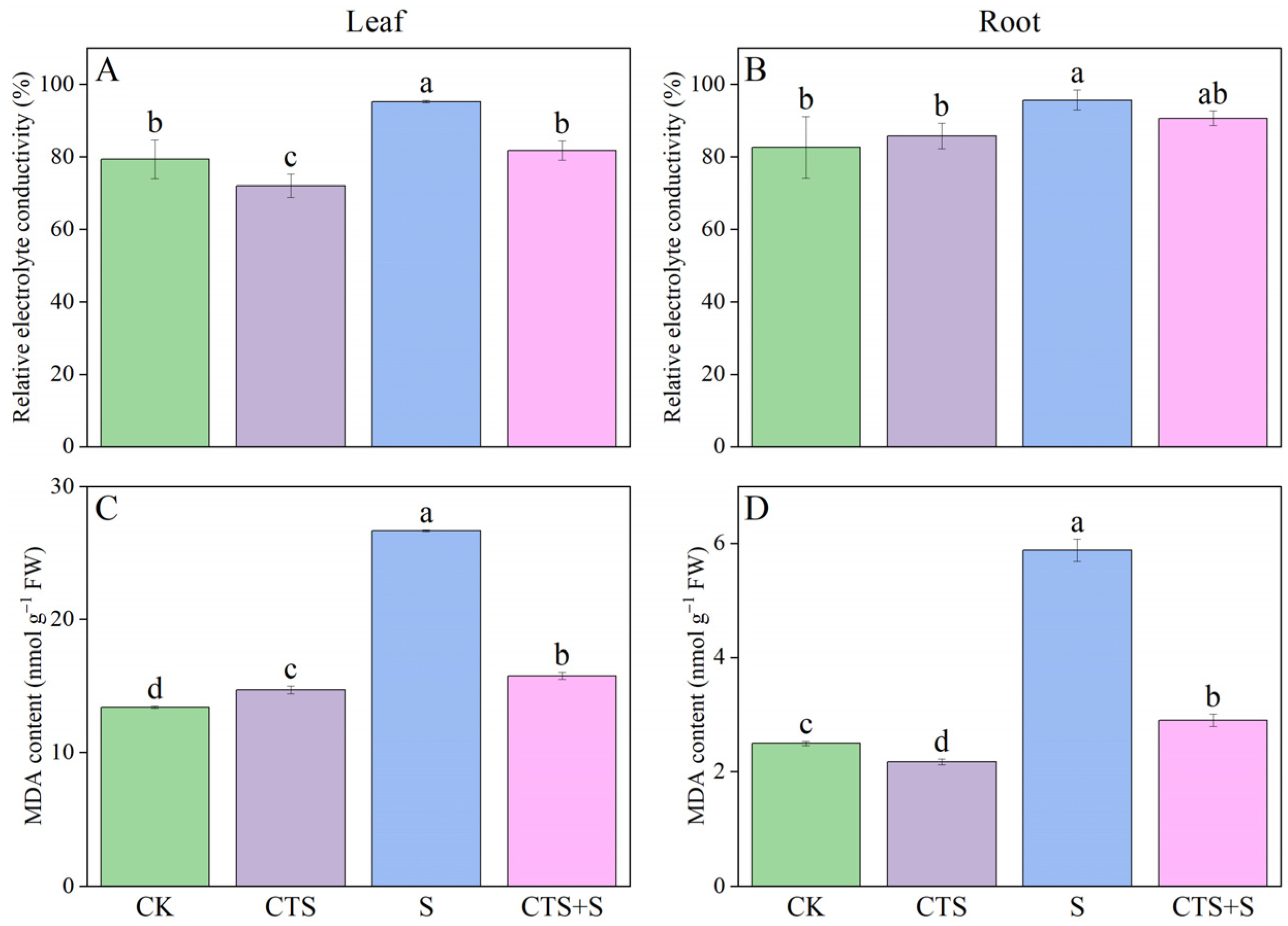
3.5. Effect of Exogenous CTS on Relative Electrolyte Conductivity, Malondialdehyde and Ascorbate Content of Maize Seedlings under Salt Stress
3.6. Effect of Exogenous CTS on Soluble Protein and the Activity of Antioxidant Enzymes in Maize Seedlings under Salt Stress
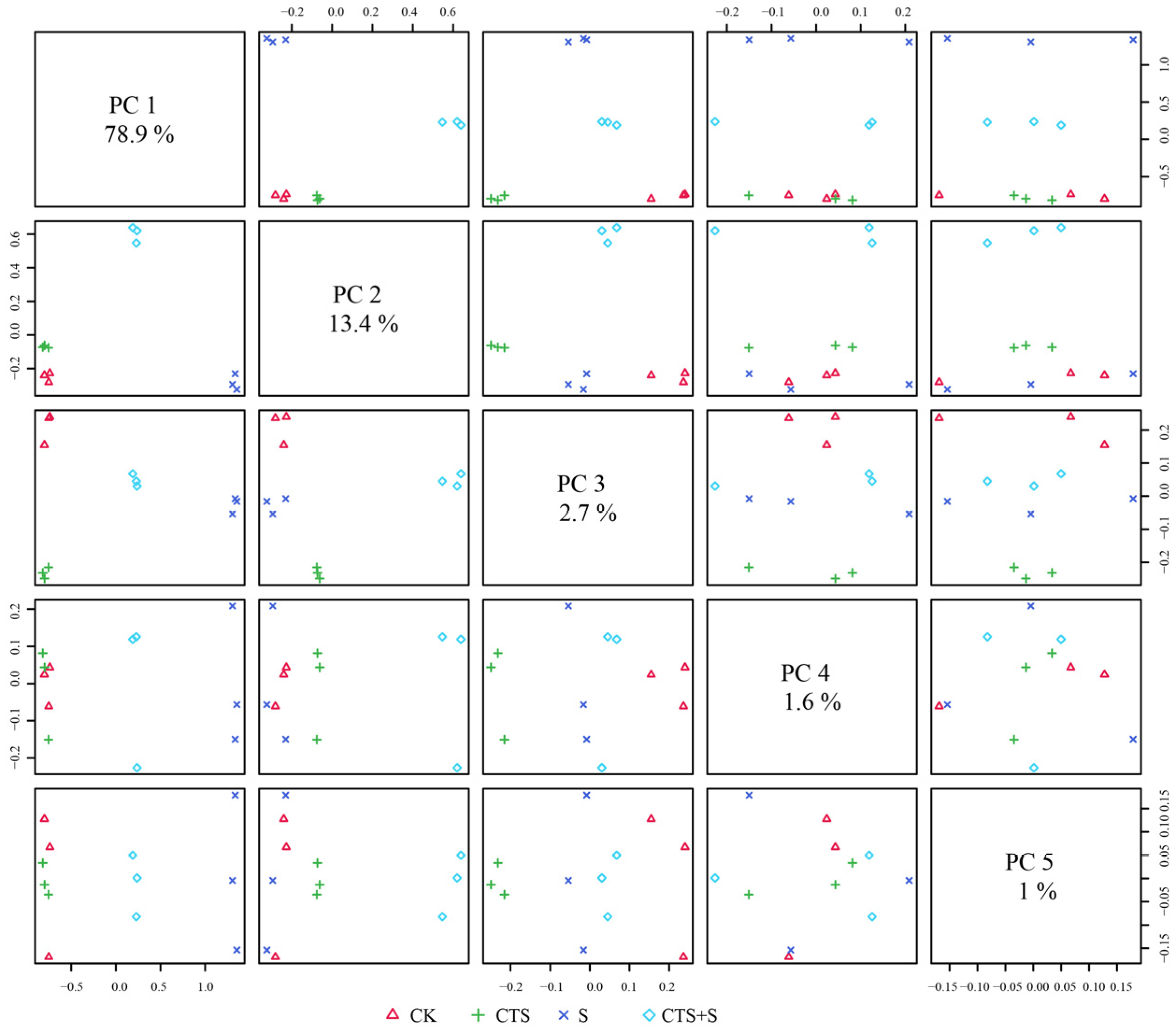
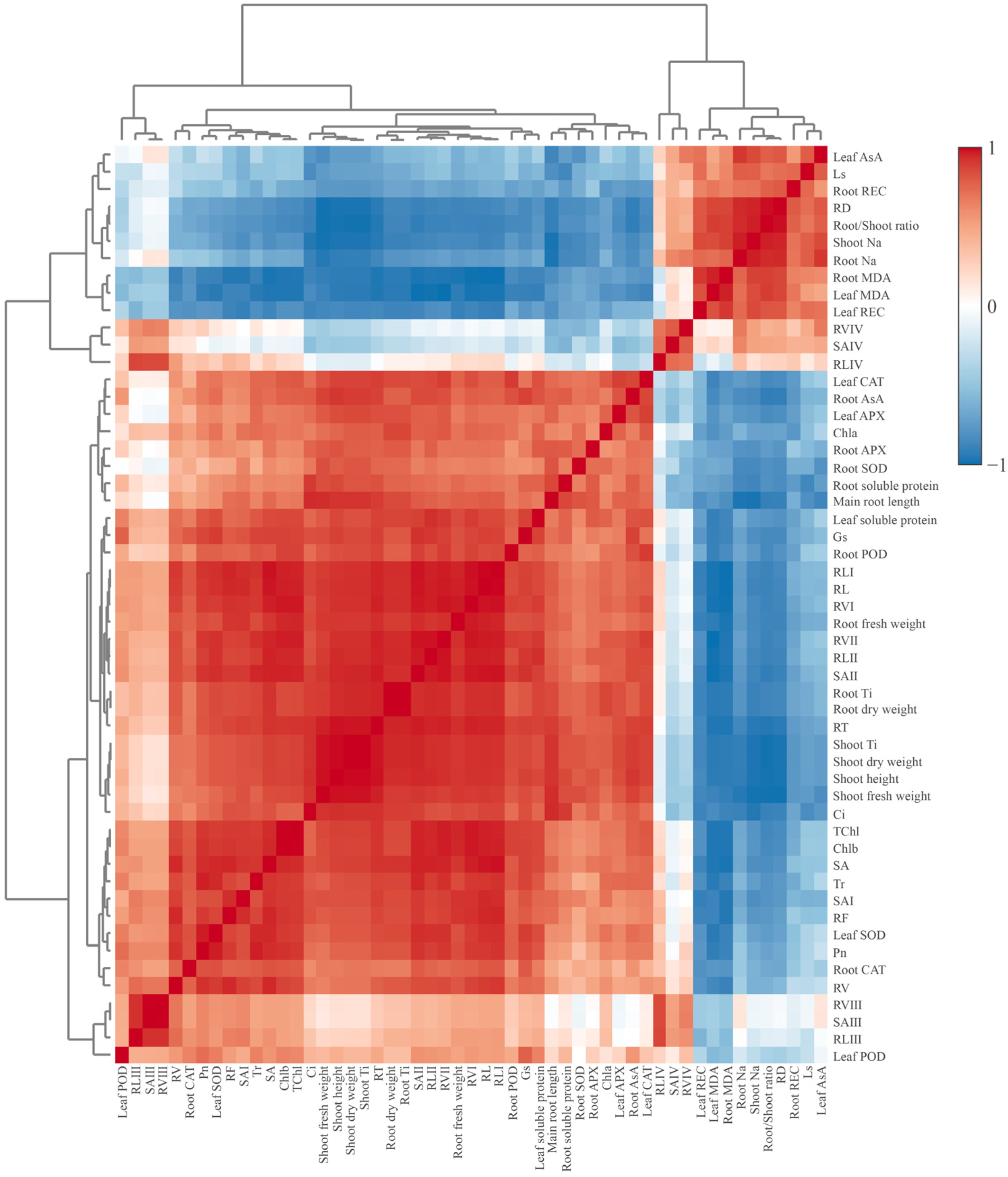
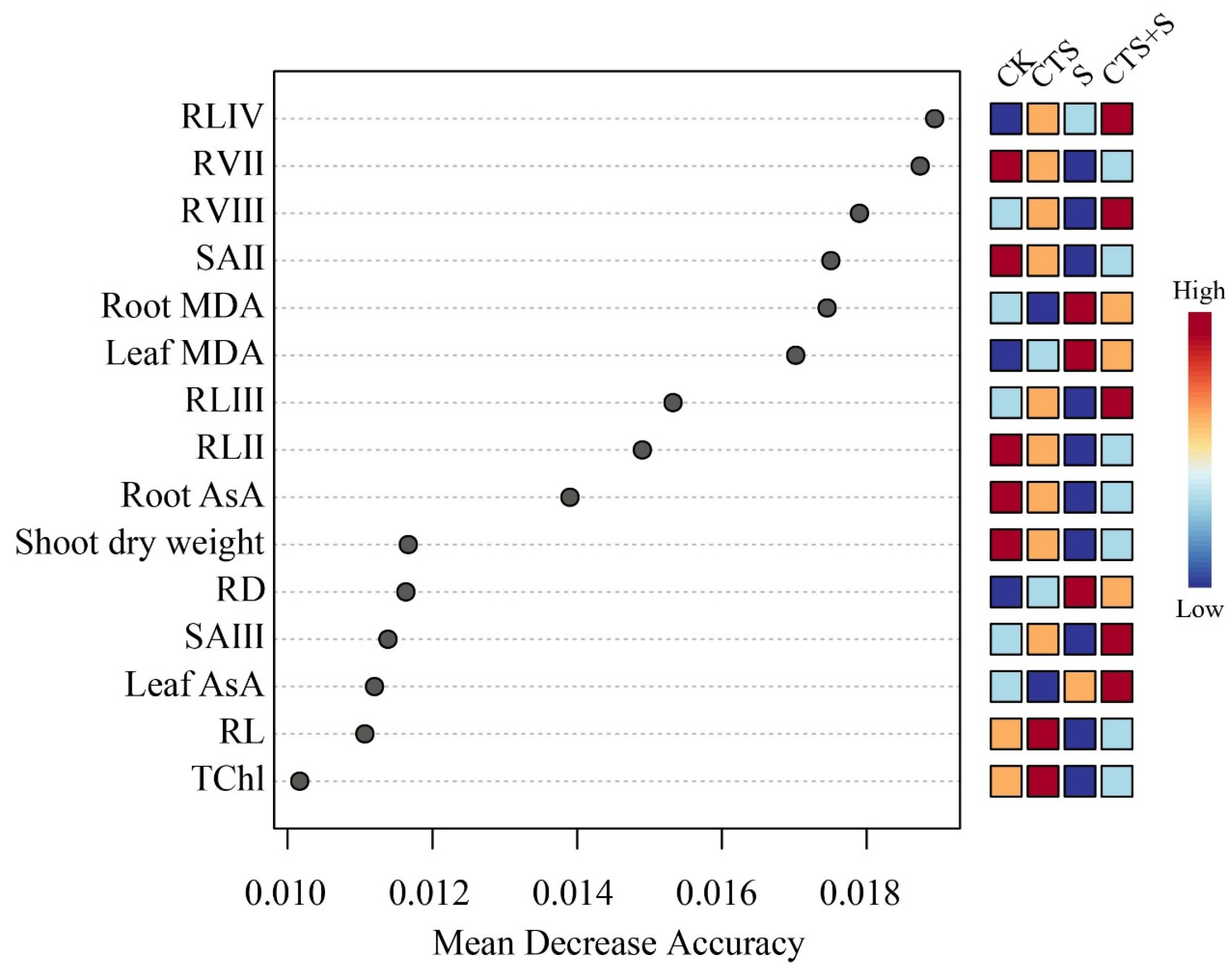
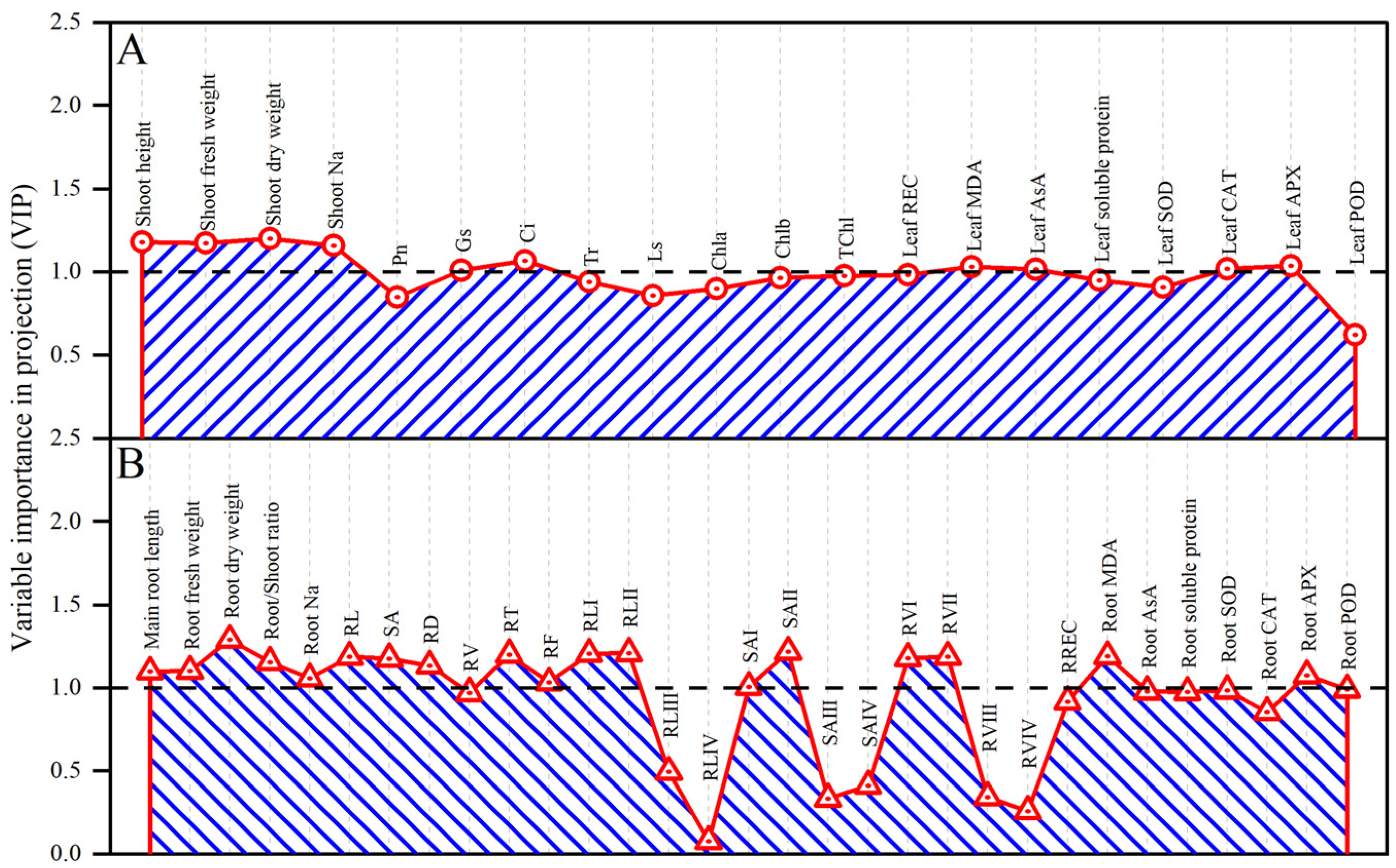
3.7. Multiple Analysis of Influence of Exogenous CTS on Maize Seedlings under Salt Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Behera, L.M.; Hembram, P. Advances on plant salinity stress responses in the post-genomic era: A review. J. Crop Sci. Biotechnol. 2021, 24, 117–126. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, S.; Singh, M.; Srivastava, P.K.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Transcriptional regulation of salinity stress in plants: A short review. Plant Gene 2017, 11, 160–169. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, J.G.; Giri, B. Augmentative Role of Arbuscular Mycorrhizal Fungi, Piriformospora indica, and Plant Growth-Promoting Bacteria in Mitigating Salinity Stress in Maize (Zea mays L.). J. Plant Growth Regul. 2023, 1–21. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Turk, H. Chitosan-induced enhanced expression and activation of alternative oxidase confer tolerance to salt stress in maize seedlings. Plant Physiol. Biochem. 2019, 141, 415–422. [Google Scholar] [CrossRef]
- Mulaudzi, T.; Nkuna, M.; Sias, G.; Doumbia, I.Z.; Njomo, N.; Iwuoha, E. Antioxidant Capacity of Chitosan on Sorghum Plants under Salinity Stress. Agriculture 2022, 12, 1544. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and Sodium Nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.L.; Zhang, C.L. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A.; et al. Strigolactone (GR24) Application Positively Regulates Photosynthetic Attributes, Stress-Related Metabolites and Antioxidant Enzymatic Activities of Ornamental Sunflower (Helianthus annuus cv. Vincent’s Choice) under Salinity Stress. Agriculture 2023, 13, 50. [Google Scholar] [CrossRef]
- Singh, D.; Sillu, D.; Kumar, A.; Agnihotri, S. Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environ. Sci. Nano 2021, 8, 1308–1325. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc Oxide Nanoparticles Improve Salt Tolerance in Rice Seedlings by Improving Physiological and Biochemical Indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Gaber, A.; Fetouh, M.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.J.; Wei, G.Q.; Li, J.; Qian, Q.Q.; Yu, J.Q. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Al Murad, M.; Muneer, S. Physiological and Molecular Analysis Revealed the Role of Silicon in Modulating Salinity Stress in Mung Bean. Agriculture 2023, 13, 1493. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Pavithra, G.-S.; Malarvizhi, P.; Murugan, P.; Deva-Andrews, A.; Janaki, M.; Sivanesh, H.; Ramasubramanian, R.; Stanley-Raja, V.; et al. Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry. Agriculture 2022, 12, 2049. [Google Scholar] [CrossRef]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Ullah, N.; Basit, A.; Ahmad, I.; Ullah, I.; Shah, S.T.; Mohamed, H.I.; Javed, S. Mitigation the adverse effect of salinity stress on the performance of the tomato crop by exogenous application of chitosan. Bull. Natl. Res. Cent. 2020, 44, 181. [Google Scholar] [CrossRef]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2019, 145, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, J.J.; Li, J.L.; Wu, X.L.; Qian, Y.R. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.B.; Li, J.Y.; AL-Huqail, A.A.; AL-Harbi, M.S.; Ali, E.F.; Wang, J.S.; Ding, Z.L.; Rekaby, S.A.; Ghoneim, A.M.; Eissa, M.A. Mechanisms of Chitosan Nanoparticles in the Regulation of Cold Stress Resistance in Banana Plants. Nanomaterials 2021, 11, 2670. [Google Scholar] [CrossRef]
- Akanksha, T.S.; James, E.R.; Sagar, S.A. Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings. Polysaccharides 2023, 4, 129–141. [Google Scholar]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Geng, W.; Li, Z.; Hassan, M.J.; Peng, Y. Chitosan regulates metabolic balance, polyamine accumulation, and Na+ transport contributing to salt tolerance in creeping bentgrass. BMC Plant Biol. 2020, 20, 506. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jiao, Q.J.; Xu, Z.Y.; Fan, L.N.; Jiang, Y.; Song, J.; Hua, D.L.; Li, G.Z.; Lin, D.; Liu, H.T. Efeect of Exogenous Chitosan on Physiological Property, Antioxidant Activity, and Cadmium Uptake of Wheat (Triticum Aestivum L.) Seedlings under Cadmium Stress. Environ. Sci. 2023, 1–16. (In Chinese) [Google Scholar]
- Shams Peykani, L.; Farzami Sepehr, M. Effect of chitosan on antioxidant enzyme activity, proline, and malondialdehyde content in Triticum aestivum L. and Zea maize L. under salt stress condition. Iran. J. Plant Physiol. 2018, 9, 2661–2670. [Google Scholar]
- Liu, H.T.; Yuan, Q.S.; Wei, C.; Jiao, Q.J.; Xu, Z.Y.; Zhang, J.J.; Li, G.Z.; Zhang, X.H.; Zhang, B.B.; Jiang, Y. Effect of exogenous chitosan on maize root architecture classification and physiological parameters under drought stress. J. Henan Agric. Univ. 2023, 57, 646–656. (In Chinese) [Google Scholar]
- Qu, D.Y.; Gu, W.R.; Li, L.J.; Li, J.; Li, C.F.; Wei, S. Regulation of chitosan on the ascorbate-gluthione cycle in Zea mays seedlings leaves under cadmium stress. Plant Sci. J. 2018, 36, 291–299. (In Chinese) [Google Scholar]
- Qu, D.Y.; Gu, W.R.; Zhang, L.G.; Chen, X.C.; Li, J.; Li, C.F.; Wei, S. Effect of Exogenous Chitosan on Antioxidant Enzyme Activities and Endogenous Hormones in Maize Seedlings Root under Cadmium Stress. Acta Bot. Boreal.-Occident. Sin. 2017, 37, 719–727. (In Chinese) [Google Scholar]
- Wei, C.; Jiao, Q.J.; Agathokleous, E.; Liu, H.T.; Li, G.Z.; Zhang, J.J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Jiao, Q.J.; Fan, L.N.; Jiang, Y.; Alyemeni, M.N.; Ahmad, P.; Chen, Y.L.; Zhu, M.; Liu, H.P.; Zhao, Y.; et al. Integrated physio-biochemical and transcriptomic analysis revealed mechanism underlying of Si-mediated alleviation to cadmium toxicity in wheat. J. Hazard. Mater. 2023, 452, 131366. [Google Scholar] [CrossRef]
- Shi, H.T. Experimental Guidance in Plant Stress Physiology, 1st ed.; Science Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Draper, H.; Squires, E.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Tejera, N.; Soussi, M.; Lluch, C. Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ. Exp. Bot. 2006, 58, 17–24. [Google Scholar] [CrossRef]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzym. 2007, 428, 419–438. [Google Scholar]
- Sun, C.X.; Gao, X.X.; Fu, J.Q.; Zhou, J.H.; Wu, X.F. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 2015, 388, 99–117. [Google Scholar] [CrossRef]
- Miao, Y.X.; Luo, X.Y.; Gao, X.X.; Wang, W.J.; Li, B.; Hou, L.P. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Tabassum, M.; Noreen, Z.; Aslam, M.; Shah, A.N.; Usman, S.; Waqas, A.; Alsherif, E.A.; Korany, S.M.; Nazim, M. Chitosan modulated antioxidant activity, inorganic ions homeostasis and endogenous melatonin to improve yield of Pisum sativum L. accessions under salt stress. Sci. Hortic. 2024, 323, 112509. [Google Scholar] [CrossRef]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving Regulation of Enzymatic and Non-Enzymatic Antioxidants and Stress-Related Gene Stimulation in Cucumber mosaic cucumovirus-Infected Cucumber Plants Treated with Glycine Betaine, Chitosan and Combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef]
- van Zelm, E.; Bugallo-Alfageme, S.; Behrouzi, P.; Meyer, A.J.; Testerink, C.; Gommers, C.M.M. Natural variation in salt-induced root growth phases and their contribution to root architecture plasticity. Plant Cell Environ. 2023, 46, 2174–2186. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Hoefsloot, H.C.; Mol, S.; Feron, R.; de Boer, G.-J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis Root Architecture Dynamics with root-fit Reveals Diversity in Responses to Salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Fan, L.; Dalpé, Y.; Fang, C.Q.; Dubé, C.; Khanizadeh, S. Influence of arbuscular mycorrhizae on biomass and root morphology of selected strawberry cultivars under salt stress. Botany 2011, 89, 397–403. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 2021, 285, 110145. [Google Scholar] [CrossRef]
- Zong, H.Y.; Liu, S.; Xing, R.E.; Chen, X.L.; Li, P.C. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.A.; Nawaz, F.; Ahmad, F.; Ahmad, N.; Masood, S. Protective effect of potassium and chitosan supply on growth, physiological processes and antioxidative machinery in sunflower (Helianthus annuus L.) under drought stress. Ecotoxicol. Environ. Saf. 2020, 187, 109841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.H.; Wu, K.; Zhang, Q.; Feng, Y.N.; Miao, Y.; Yan, Z.M. Exogenous Application of Chitosan Alleviate Salinity Stress in Lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 342. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants 2021, 10, 1365. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Liu, T.; Liang, L.L.; Li, Z.; Hassan, M.J.; Peng, Y.; Wang, D. Enhanced photosynthesis, carbohydrates, and energy metabolism associated with chitosan-induced drought tolerance in creeping bentgrass. Crop Sci. 2020, 60, 1064–1076. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Adabavazeh, F.; Komatsu, S. Chitosan effects on the elevation of essential oils and antioxidant activity of Carum copticum L. seedlings and callus cultures under in vitro salt stress. J. Plant Biochem. Biotechnol. 2020, 29, 473–483. [Google Scholar] [CrossRef]
- Ávila, R.G.; Magalhães, P.C.; Vitorino, L.C.; Bessa, L.A.; de Souza, K.R.D.; Queiroz, R.B.; Jakelaitis, A.; Teixeira, M.B. Chitosan Induces Sorghum Tolerance to Water Deficits by Positively Regulating Photosynthesis and the Production of Primary Metabolites, Osmoregulators, and Antioxidants. J. Soil Sci. Plant Nutr. 2022, 23, 1156–1172. [Google Scholar] [CrossRef]
- Jiao, Z.L.; Li, Y.; Li, J.J.; Xu, X.Y.; Li, H.; Lu, D.Q.; Wang, J.Y. Effects of Exogenous Chitosan on Physiological Characteristics of Potato Seedlings Under Drought Stress and Rehydration. Potato Res. 2012, 55, 293–301. [Google Scholar] [CrossRef]
- Younas, H.S.; Abid, M.; Ashraf, M. Ameliorating detrimental effects of water deficit stress in maize by foliarally applied silicon and chitosan. Pak. J. Bot. 2022, 54, 393–400. [Google Scholar] [CrossRef]
- Younas, H.S.; Abid, M.; Ashraf, M.; Shaaban, M. Seed priming with silicon and chitosan for alleviating water stress effects in maize (ZEA MAYS L.) by improving antioxidant enzyme activities, water status and photosynthesis. J. Plant Nutr. 2022, 45, 2263–2276. [Google Scholar] [CrossRef]
- Attia, M.S.; Osman, M.S.; Mohamed, A.S.; Mahgoub, H.A.; Garada, M.O.; Abdelmouty, E.S.; Latef, A.A.H.A. Impact of Foliar Application of Chitosan Dissolved in Different Organic Acids on Isozymes, Protein Patterns and Physio-Biochemical Characteristics of Tomato Grown under Salinity Stress. Plants 2021, 10, 388. [Google Scholar] [CrossRef]






| Treatment | CK | CTS | S | CTS + S |
|---|---|---|---|---|
| Shoot height (cm) | 51.03 ± 1.70 a | 46.6 ± 0.26 b | 21.43 ± 0.49 d | 31.67 ± 1.00 c |
| Main root length (cm) | 30.63 ± 1.21 a | 32.8 ± 3.30 a | 18.57 ± 1.29 b | 20.23 ± 0.25 b |
| Shoot FW (g plant−1) | 4.93 ± 0.37 a | 5.01 ± 0.45 a | 1.28 ± 0.05 c | 2.16 ± 0.05 b |
| Root FW (g plant−1) | 1.62 ± 0.12 ab | 1.78 ± 0.13 a | 1.00 ± 0.03 c | 1.48 ± 0.11 b |
| Shoot DW (g plant−1) | 0.34 ± 0.02 a | 0.30 ± 0.01 b | 0.12 ± 0.00 d | 0.19 ± 0.01 c |
| Root DW (g plant−1) | 0.08 ± 0.00 a | 0.08 ± 0.01 a | 0.06 ± 0.00 c | 0.07 ± 0.00 b |
| Root/shoot ratio | 0.24 ± 0.01 c | 0.26 ± 0.02 c | 0.48 ± 0.03 a | 0.37 ± 0.02 b |
| Shoot tolerance index | 100.00 ± 7.21 a | 90.03 ± 2.10 b | 35.61 ± 1.24 d | 57.28 ± 2.35 c |
| Root tolerance index | 100.00 ± 2.98 a | 97.74 ± 6.84 a | 72.04 ± 4.70 c | 89.10 ± 1.21 b |
| Treatment | CK | CTS | S | CTS + S |
|---|---|---|---|---|
| Total root length (cm) | 794.67 ± 41.94 b | 864.74 ± 50.20 a | 337.73 ± 5.63 d | 684.86 ± 9.78 c |
| I | 616.05 ± 44.13 a | 665.65 ± 31.47 a | 248.48 ± 5.62 c | 533.89 ± 12.54 b |
| II | 146.77 ± 4.42 a | 132.71 ± 0.58 b | 66.23 ± 8.23 d | 117.82 ± 2.24 c |
| III | 13.53 ± 2.18 b | 21.33 ± 3.08 a | 12.54 ± 0.28 b | 23.39 ± 0.70 a |
| IV | 7.57 ± 0.18 c | 10.75 ± 0.67 b | 8.79 ± 0.89 c | 12.34 ± 0.95 a |
| Root surface area (cm2) | 93.22 ± 8.08 a | 98.81 ± 7.39 a | 49.01 ± 2.08 b | 88.68 ± 0.54 a |
| I | 28.89 ± 1.64 b | 39.76 ± 2.44 a | 15.79 ± 0.54 c | 29.78 ± 3.46 b |
| II | 31.86 ± 0.66 a | 29.87 ± 0.75 b | 13.25 ± 0.85 d | 25.29 ± 0.87 c |
| III | 5.08 ± 0.80 c | 6.95 ± 0.67 b | 4.71 ± 0.13 c | 8.73 ± 0.20 a |
| IV | 6.32 ± 0.29 b | 7.72 ± 1.25 b | 7.90 ± 0.89 ab | 9.54 ± 0.97 a |
| Root volume (cm3) | 0.82 ± 0.08 b | 0.92 ± 0.06 ab | 0.59 ± 0.01 c | 0.93 ± 0.02 a |
| I | 0.25 ± 0.00 a | 0.26 ± 0.02 a | 0.09 ± 0.01 c | 0.21 ± 0.01 b |
| II | 0.61 ± 0.01 a | 0.53 ± 0.02 b | 0.22 ± 0.01 d | 0.48 ± 0.01 c |
| III | 0.15 ± 0.02 c | 0.21 ± 0.02 b | 0.14 ± 0.01 c | 0.26 ± 0.01 a |
| IV | 0.54 ± 0.02 b | 0.59 ± 0.07 b | 0.63 ± 0.04 b | 0.92 ± 0.07 a |
| Root diameter (mm) | 0.35 ± 0.01 c | 0.36 ± 0.01 c | 0.46 ± 0.01 a | 0.42 ± 0.00 b |
| Root tips | 1644.33 ± 128.10 a | 1551.67 ± 26.27 a | 577 ± 46.68 c | 1052.33 ± 13.80 b |
| Root Forks | 2544.33 ± 288.27 b | 3232 ± 181.36 a | 1453.67 ± 109.86 c | 2676.67 ± 274.80 b |
| Item | S Treatment (mmol L−1) | CTS Treatment (mg L−1) | Average Value | Rank |
|---|---|---|---|---|
| CK | 0 | 0 | 0.80 | 1 |
| CTS | 0 | 75 | 0.79 | 2 |
| S | 150 | 0 | 0.12 | 4 |
| CTS + S | 150 | 75 | 0.53 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Q.; Shen, F.; Fan, L.; Song, Z.; Zhang, J.; Song, J.; Fahad, S.; Liu, F.; Zhao, Y.; Tian, Z.; et al. Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling. Agriculture 2024, 14, 304. https://doi.org/10.3390/agriculture14020304
Jiao Q, Shen F, Fan L, Song Z, Zhang J, Song J, Fahad S, Liu F, Zhao Y, Tian Z, et al. Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling. Agriculture. 2024; 14(2):304. https://doi.org/10.3390/agriculture14020304
Chicago/Turabian StyleJiao, Qiujuan, Fengmin Shen, Lina Fan, Zihao Song, Jingjing Zhang, Jia Song, Shah Fahad, Fang Liu, Ying Zhao, Zhiqiang Tian, and et al. 2024. "Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling" Agriculture 14, no. 2: 304. https://doi.org/10.3390/agriculture14020304
APA StyleJiao, Q., Shen, F., Fan, L., Song, Z., Zhang, J., Song, J., Fahad, S., Liu, F., Zhao, Y., Tian, Z., & Liu, H. (2024). Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling. Agriculture, 14(2), 304. https://doi.org/10.3390/agriculture14020304




