Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
- Thermi-typical fertilization/typical sowing time (mid-November). With typical fertilization (total N amount of 180 kg ha−1), one-third of which was applied (ammonium phosphate 20-10-0) before sowing and two-thirds (ammonium nitrate 33.5-0-0) at full tillering (Zadok 29). (High-productivity environment);
- Thermi-organic field (no fertilization)/ typical sowing time; low-productivity environment);
- Thermi-typical fertilization/late sowing time, i.e., end of January. All the other agronomic treatments were identically applied to all plots. (Low-productivity environment);
- Thermi-splitting topdressing N fertilization/typical sowing time. Splitting topdressing N fertilization involved splitting one-third (ammonium phosphate 20-10-0) before sowing, one-third (ammonium nitrate 33.5-0-0) at full tillering (Zadok 29), and one-third during the first node (Zadok 31). (High-productivity environment);
- Thermi-splitting topdressing N fertilization/late sowing time (as described above). (High-productivity environment);
- Nea Gonia (typical sowing time) with typical fertilization (total N amount of 150 kg ha−1), one-third of which was applied (ammonium phosphate 20-10-0) before sowing and two-thirds (ammonium nitrate 33.5-0-0) at full tillering (Zadok 29). (High-productivity environment);
- Sindos-typical fertilization/late sowing time. (Low-productivity environment).
2.2. Vitreous Kernel Percentage
2.3. Protein Content
2.4. Free Phenolic Extraction
2.5. Total Phenolic Content (TPC)
2.6. Antioxidant Capacity
2.6.1. Radical Scavenging Activity (ABTS)
2.6.2. Ferric Reducing/Antioxidant Power (FRAP)
2.6.3. Radical Scavenging Capacity Activity (DPPH)
2.7. Statistical Analysis
2.8. Data Analysis
3. Results and Discussion
3.1. Effect of Genotype, Environment, and Genotype by Environment
3.2. Total Phenolic Compounds
3.3. Antioxidant Capacity
3.4. Organic and Late Sowing Environments
3.5. Vitreous Kernel and Protein
3.6. Correlation among Traits
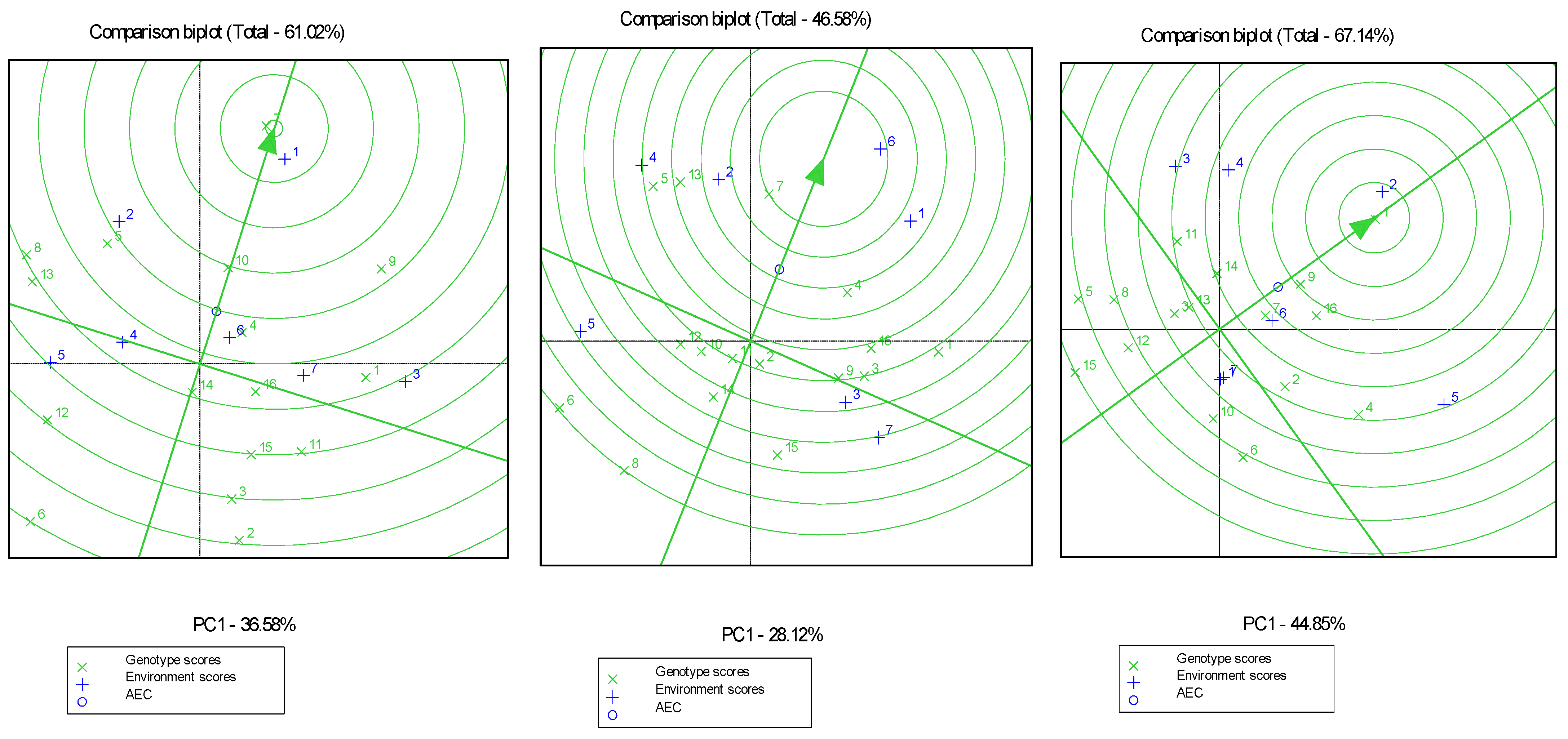
3.7. G × E Interaction Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Royo, C.; Soriano, J.M.; Alvaro, F. Wheat: A Crop in the Bottom of the Mediterranean Diet Pyramid. In Mediterranean Identities—Environment, Society, Culture; Fuerst-Bjeliš, B., Ed.; IntechOpen: London, UK, 2017; pp. 381–399. [Google Scholar]
- Martinez-Moreno, F.; Solis, I.; Noguero, D.; Blanco, A.; Ozberk, I.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum Wheat in the Mediterranean Rim: Historical Evolution and Genetic Resources. Genet. Resour. Crop Evol. 2020, 8, 1415–1436. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Head, L.; Atchison, J.; Gates, A. Wheat Becomes Quality Food: Bread, Pasta and More. In Ingrained: A Human Bio-Geography of Wheat; Head, L., Atchison, J., Gates, A., Eds.; ASHGATE: Farnham, UK, 2012; p. 137. [Google Scholar]
- Arendt, E.K.; Zannini, E. Wheat and Other Triticum Grains. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; WOODHEAD Publishing: Cambridge, UK, 2013; pp. 1–67. [Google Scholar]
- Turnbull, K. Basic Semolina Requirements, Advances in Durum Milling. In Pasta and Semolina Technology; Kill, R., Turnbull, K., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 43–45. [Google Scholar]
- Di Loreto, A.; Bosi, S.; Montero, L.; Bregola, V.; Marotti, I.; Sferrazza, R.E.; Dinelli, G.; Herrero, M.; Cifuentes, A. Determination of Phenolic Compounds in Ancient and Modern Durum Wheat Genotypes. Electrophoresis 2018, 39, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Nesamony, E.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Hurst, R.D. Review Polyphenolic Phytochemicals—Just Antioxidants or Much More? Cell Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2022, 25, 63. [Google Scholar] [CrossRef]
- Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E. Effect of Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic Plants of Lamiaceae Family in a Traditional Bread Recipe: Effects on Quality and Phytochemical Content. Food Biochem. 2019, 43, e13020. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef]
- Irakli, M.; Tsifodimou, K.; Sarrou, E.; Chatzopoulou, P. Optimization Infusions Conditions for Improving Phenolic Content and Antioxidant Activity in Sideritis Scardica Tea Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2018, 8, 67–74. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of Phenolic Compounds in Greek Plants of Lamiaceae Family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Adamidis, T.; Papageorgiou, M.; Zinoviadou, K.G. Food, Nutrition, and Health in Greece. In Nutritional and Health Aspects of Food in the Balkans; Gostin, A.-I., Bogueva, D., Kakurinov, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 107–124. ISBN 9780128207826. [Google Scholar]
- Marecek, J.; Francakova, H.; Liskova, M.; Mendonça, A.; Ivanisova, E.; Mocko, K. Evaluation of technological and properties of Triticum aestivum L. varieties. J. Microbiol. Biotech. Food Sci. 2014, 3, 253–255. [Google Scholar]
- Ciudad-Mulero, M.; Barros, L.; Fernandes, Â.; Ferreira, I.C.F.R.; Jesus Callejo, M.; Matallana-Gonnzalez, C.M.; Fernandez-Ruiz, V.; Morales, P.; Carrillo, J.M. Potential Health Claims of Durum and Bread Wheat Flours as Functional Ingredients. Nutrients 2020, 12, 504. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant and Free Radical Scavenging Activities of Whole Wheat and Milling Fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E.; Re, C. Is the in Vitro Antioxidant Potential of Whole-Grain Cereals and Cereal Products Well Reflected in Vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Maria, A.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant Activity and Dietary W Bre in Durum Wheat Bran. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. The Antioxidant Potential of Milling Fractions from Breadwheat and Durum. J. Cereal Sci. 2007, 45, 238–247. [Google Scholar] [CrossRef]
- Acquistucci, R.; Melini, V.; Carbonaro, M.; Finotti, E.; Acquistucci, R.; Melini, V.; Carbonaro, M.; Finotti, E.; Acquistucci, R.; Melini, V.; et al. Bioactive Molecules and Antioxidant Activity in Durum Wheat Grains and Related Millstream Fractions and Related Millstream Fractions. Int. J. Food Sci. Nutr. 2013, 64, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic Content and Antioxidant Activity of Pearled Wheat and Roller-Milled Fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Martini, D.; Taddei, F.; Nicoletti, I.; Ciccoritti, R.; Corradini, D.; Egidio, M.G.D. Effects of Genotype and Environment on Phenolic Acids Content and Total Antioxidant Capacity in Durum Wheat. Cereal Chem. 2014, 91, 310–317. [Google Scholar] [CrossRef]
- Di Silvestro, R.; Marotti, I.; Bosi, S.; Bregola, V.; Carretero, A.S.; Sedej, I.; Mandic, A.; Sakac, M.; Benedettelli, S.; Dinelli, G. Health-Promoting Phytochemicals of Italian Common Wheat Varieties Grown under Low-Input Agricultural Management. J. Sci. Food Agric. 2012, 92, 2800–2810. [Google Scholar] [CrossRef]
- Yan, W. Singular-Value Partitioning in Biplot Analysis of Multienvironment Trial Data. Agron. J. 2002, 94, 990–996. [Google Scholar] [CrossRef]
- Crossa, J.; Fox, P.N.; Pfeiffer, W.H.; Rajaram, S.; Gauch, H.G. AMMI Adjustment for Statistical Analysis of an International Wheat Yield Trial. Theor. Appl. Genet. 1991, 81, 27–37. [Google Scholar] [CrossRef]
- Smutná, P.; Mylonas, I.; Tokatlidis, I.S. The Use of Stability Statistics to Analyze Genotype × Environments Interaction in Rainfed Wheat Under Diverse Agroecosystems. Int. J. Plant Prod. 2021, 15, 261–271. [Google Scholar] [CrossRef]
- Purchase, J.L.; Hatting, H.; van Deventer, C.S. Genotype × Environment Interaction of Winter Wheat (Triticum aestivum L.) in South Africa: II. Stability Analysis of Yield Performance. South. Afr. J. Plant Soil. 2000, 17, 101–107. [Google Scholar] [CrossRef]
- Gauch, H.G. Model Selection and Validation for Yield Trials with Interaction. Biometrics 1988, 44, 705–715. [Google Scholar] [CrossRef]
- Nassar, R.; Hühn, M. Studies on Estimation of Phenotypic Stability: Tests of Significance for Nonparametric Measures of Phenotypic Stability. Biometrics 1987, 43, 45–53. [Google Scholar] [CrossRef]
- Shukla, G.K. Some Statistical Aspects of Partitioning Genotype-Environmental Components of Variability. Heredity 1972, 29, 237–245. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability Parameters for Comparing Varieties1. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Kang, M.S. A Rank-Sum Method for Selecting High-Yielding, Stable Corn Genotypes. Cereal Res. Commun. 1988, 16, 113–115. [Google Scholar]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE Biplot vs. AMMI Analysis of Genotype-by-Environment Data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L. McMahon Updated World Map of the K¨oppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 16–1644. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-ciocalteu Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Kozak, M.; Piepho, H.-P. What’s Normal Anyway? Residual Plots Are More Telling than Significance Tests When Checking ANOVA Assumptions. J. Agron. Crop Sci. 2018, 204, 86–98. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A Method of Analyzing Cultivar x Location x Year Experiments: A New Stability Parameter. Theor. Appl. Genet. 1988, 76, 425–430. [Google Scholar] [CrossRef]
- Kang, M.S.; Pham, H.N. Simultaneous Selection for High Yielding and Stable Crop Genotypes. Agron. J. 1991, 83, 161–165. [Google Scholar] [CrossRef]
- Šukalović, V.H.-T.; Dodig, D.; Žilić, S.; Basic, Z.; Kandic, V.; Delic, N.; Miritescu, M. Genotypic and Environmental Variation of Bread and Durum Wheat Proteins and Antioxidant Compounds. Romanian Agric. Res. 2013, 30, 125–134. [Google Scholar]
- Pandino, G.; Mattiolo, E.; Lombardo, S.; Lombardo, G.M.; Mauromicale, G. Organic Cropping System Affects Grain Chemical Composition, Rheological and Agronomic Performance of Durum Wheat. Agriculture 2020, 10, 46. [Google Scholar] [CrossRef]
- Martini, D.; Taddei, F.; Ciccoritti, R.; Pasquini, M.; Nicoletti, I.; Corradini, D.; Grazia, M.; Egidio, D. Variation of Total Antioxidant Activity and of Phenolic Acid, Total Phenolics and Yellow Coloured Pigments in Durum Wheat (Triticum turgidum L. var. Durum) as a Function of Genotype, Crop Year and Growing Area. J. Cereal Sci. 2015, 65, 175–185. [Google Scholar] [CrossRef]
- Irakli, M.; Kargiotidou, A.; Tigka, E.; Beslemes, D.; Fournomiti, M.; Pankou, C.; Stavroula, K.; Tsivelika, N.; Vlachostergios, D.N. Genotypic and Environmental Effect on the Concentration of Phytochemical Contents of Lentil (Lens culinaris L.). Agronomy 2021, 11, 1154. [Google Scholar] [CrossRef]
- Lv, J.; Lu, Y.; Niu, Y.; Whent, M.; Ramadan, M.F.; Costa, J.; Yu, L. Effect of Genotype, Environment, and Their Interaction on Phytochemical Compositions and Antioxidant Properties of Soft Winter Wheat Flour. Food Chem. 2013, 138, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, F.; Chen, Y.; Huang, Y.; Beta, T.; Bao, J. Analysis of Genotype, Environment, and Their Interaction Effects on the Phytochemicals and Antioxidant Capacities of Red Rice (Oryza sativa L.). Cereal Chem. 2015, 92, 204–210. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant Properties of Bran Extracts from “Akron” Wheat Grown at Different Locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef]
- Dupont, F.; Hurkman, W.; Vensel, W.; Tanaka, C.; Kothari, K.; Chung, O.; Altenbach, S. Protein Accumulation and Composition in Wheat Grains: Effects of Mineral Nutrients and High Temperature. Eur. J. Agron. 2006, 25, 96–107. [Google Scholar] [CrossRef]
- Tian, W.; Jaenisch, B.; Gui, Y.; Hu, R.; Chen, G.; Lollato, R.P.; Li, Y. Effect of Environment and Field Management Strategies on Phenolic Acid Profiles of Hard Red Winter Wheat Genotypes. J. Sci. Food Agric. 2022, 102, 2424–2431. [Google Scholar] [CrossRef]
- Di Silvestro, R.; Di Loreto, A.; Bosi, S.; Bregola, V.; Marotti, I.; Benedettelli, S.; Segura-Carretero, A.; Dinelli, G. Environment and Genotype Effects on Antioxidant Properties of Organically Grown Wheat Varieties: A 3-Year Study. J. Sci. Food Agric. 2017, 97, 641–649. [Google Scholar] [CrossRef]
- Beleggia, R.; Platani, C.; Nigro, F.; De Vita, P.; Cattivelli, L.; Papa, R. Effect of Genotype, Environment and Genotype-by-Environment Interaction on Metabolite Profiling in Durum Wheat (Triticum durum Desf.) Grain. J. Cereal Sci. 2013, 57, 183–192. [Google Scholar] [CrossRef]
- Carter, J.W.; Madl, R.; Padula, F. Wheat Antioxidants Suppress Intestinal Tumor Activity in Min Mice. Nutr. Res. 2006, 26, 33–38. [Google Scholar] [CrossRef]
- Fardet, A. New Hypotheses for the Health-Protective Mechanisms of Whole-Grain Cereals: What Is beyond Fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Laus, M.N.; Tozzi, D.; Soccio, M.; Fratianni, A.; Panfili, G.; Pastore, D. Dissection of Antioxidant Activity of Durum Wheat (Triticum durum Desf.) Grains as Evaluated by the New LOX/RNO Method. J. Cereal Sci. 2012, 56, 214–222. [Google Scholar] [CrossRef]
- Acquistucci, R.; Melini, V.; Garaguso, I.; Nobili, F. Effect of Bread Making Process on Bioactive Molecules in Durum Wheat Bread and Assessment of Antioxidant Properties by Caco-2 cell Culture Model. J. Cereal Sci. 2018, 83, 188–195. [Google Scholar] [CrossRef]
- Boukid, F.; Dall’Asta, M.; Bresciani, L.; Mena, P.; Del Rio, D.; Calani, L.; Sayar, R.; Yong Seo, W.; Yacoubi, I.; Mejri, M. Phenolic Profile and Antioxidant Capacity of Landraces, Old and Modern Tunisian Durum Wheat. Eur. Food Res. Technol. 2018, 245, 73–82. [Google Scholar] [CrossRef]
- Truzzi, F.; Dinelli, G.; Spisni, E.; Simonetti, E.; Trebbi, G.; Marotti, I. Phenolic Acids of Modern and Ancient Grains: Effect on in Vitro Cell Model. J. Sci. Food Agric. 2019, 100, 4075–4082. [Google Scholar] [CrossRef] [PubMed]
- Nocente, F.; De Stefanis, E.; Ciccoritti, R.; Pucciarmati, S.; Taddei, F.; Campiglia, E.; Radicetti, E.; Mancinelli, R. How Do Conventional and Organic Management a Ff Ect the Healthy Potential of Durum Wheat Grain and Semolina Pasta Traits? Food Chem. 2019, 297, 124884. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Menga, V.; Codianni, P.; Russo, M.; Perrone, D.; Suriano, S.; Rascio, A. Phenolic Acids Variability and Grain Quality of Organically and Conventionally Fertilised Old Wheats under a Warm Climate. J. Sci. Food Agric. 2019, 99, 4615–4623. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Li, Y.; Wang, C.; Xie, Y.; Guo, T. Effect of Nitrogen Fertilisation and Irrigation on Phenolic Content, Phenolic Acid Composition, and Antioxidant Activity of Winter Wheat Grain. J. Sci. Food Agric. 2014, 95, 1039–1046. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Ma, Y.; Wen, Z.; Li, J.; Zhang, H.; Wu, Y.; Lei, C.; Wang, S.; Wang, J.; et al. Ecophysiological Factors on Phytic Acid Concentration in Soybean Seed. Crop Sci. 2013, 53, 2195–2201. [Google Scholar] [CrossRef]
- Kishore, G.; Ranjan, S.; Pandey, A.; Gupta, S. Influence of Altitudinal Variation on the Antioxidant Potential of Tartar Buckwheat of Western Himalaya. Food Sci. Biotechnol. 2010, 19, 1355–1363. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Kaur, N. Influence of Drought and Sowing Time on Protein Composition, Antinutrients, and Mineral Contents of Wheat. Sci. World J. 2012, 2012, 485751. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of Baking on Free and Bound Phenolic Acids in Wholegrain Bakery Products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Isolani, L.; Arfaioli, P.; Ghiselli, L.; Romani, A. Polyphenol Content of Modern and Old Varieties of Triticum aestivum L. and T. durum Desf. Grains in Two Years of Production. J. Agric. Food Chem. 2010, 58, 7329–7334. [Google Scholar] [CrossRef]
- Dixon, R.; Paiva, N. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Vicas, S.I.; Teusdea, A.C.; Carbunar, M.; Socaci, S.A.; Socaciu, C. Glucosinolates Profile and Antioxidant Capacity of Romanian Brassica Vegetables Obtained by Organic and Conventional Agricultural Practices. Plant Foods Hum. Nutr. 2013, 68, 313–321. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, F.M.; Anjos, R. Influence of Cultivar and of Conventional and Organic Agricultural Practices on Phenolic and Sensory Profile of Blackberries (Rubus fruticosus). J. Sci. Food Agric. 2018, 98, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of Processing and Cooking on Phenolic Acid Profile and Antioxidant Capacity of Durum Wheat Pasta Enriched with Debranning Fractions of Wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chem. 2005, 4, 713–718. [Google Scholar] [CrossRef]
- Žilić, S.; Dodig, D.; Šukalović, V.; Maksimovic, M.; Saratlić, G.; Skrbic, B. Bread and Durum Wheat Compared for Antioxidants Contents, and Lipoxygenase and Peroxidase Activities. Int. J. Food Sci. Technol. 2010, 45, 1360–1367. [Google Scholar] [CrossRef]
- Graziano, S.; Marmiroli, N.; Visioli, G.; Gullì, M. Proteins and Metabolites as Indicators of Flours Quality and Nutritional Properties of Two Durum Wheat Varieties Grown in Different Italian Locations. Foods 2020, 9, 315. [Google Scholar] [CrossRef]
- Park, E.Y.; Morimae, M.; Matsumura, Y.; Nakamura, Y.; Sato, K. Antioxidant Activity of Some Protein Hydrolysates and Their Fractions with Different Isoelectric Points. J. Agric. Food Chem. 2008, 56, 9246–9251. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic Acids Composition, Total Polyphenols Content and Antioxidant Activity of Triticum Monococcum, Triticum Turgidum and Triticum Aestivum: A Two-Years Evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative Stress and Antioxidants in Health and Disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet Activating Factor—A Molecular Link between Atherosclerosis Theories. Eur. J. Lipid Sci. Technol. 2003, 105, 705–716. Available online: https://https://onlinelibrary.wiley.co (accessed on 7 January 2024). [CrossRef]
- Mohammadi, R.; Amri, A. Comparison of Parametric and Non-Parametric Methods for Selecting Stable and Adapted Durum Wheat Genotypes in Variable Environments. Euphytica 2008, 159, 419–432. [Google Scholar] [CrossRef]
- Kebede, A.; Getahun, A. Adaptability and Stability Analysis of Groundnut Genotypes Using AMMI Model and GGE-Biplot. J. Crop Sci. Biotechnol. 2017, 20, 343–349. [Google Scholar] [CrossRef]
| Genotype Code | Name of Genotype | Country of Origin | Genealogy | Year Released |
|---|---|---|---|---|
| G1 | Pigreco | Italy | Not available (NA) | NA |
| G2 | Canavaro | Italy | Coloseo/Simeto | 2008 |
| G3 | Maestrale | Italy | Iride/Svevo | 2004 |
| G4 | M. Aurelio | Italy | D95241/Arcobaleno/Svevo | NA |
| G5 | Meridiano | Italy | Simeto/WB881/Duilio/F21 | 1999 |
| G6 | Mexicali-81 | Greece | Selection from Mexicali 75 | 1981 |
| G7 | Monastir | France | Not Available (NA) | NA |
| G8 | Simeto | Italy | Capeiti 8/Valnova | 1988 |
| G9 | Svevo | Italy | Linea Cimmyt/Zenith | 1996 |
| G10 | Vendeta | Italy | Creso/Ofanto | 2003 |
| G11 | Egeo | Italy | Claudio/v80 | NA |
| G12 | Elpida | Greece | Sifnos/Mexicali-81 | 2010 |
| G13 | Zoi | Greece | Simeto /Mexicali-81 | 2011 |
| G14 | Secolo | Italy | NA | NA |
| G15 | Grecale | Italy | NA | NA |
| G16 | Zeta E. | Greece | NA | NA |
| Code | Location | Latitude/ Longitude | Climate Type | PrA 2 (mm) | PrA-M 3 (mm) | T 4 (°C) | Prod. 5 | Fertilization 6 | Planting Date | Soil Texture 7 | pH (1:1) | EC 8 | SOM 9 % | POlsen mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | Thermi/typical fertilization/typical sowing date | 40°54′ N/ 23°00′ E | BSk 1 | 343.6 | 45.4 | 12.8 | HP | Typical | Typical | L | 7.89 | 0.516 | 1.8 | 12.28 |
| E2 | Thermi-Organic/typical sowing date | 40°54′ N/ 23°00′ E | BSk | 343.6 | 45.4 | 12.8 | LP | Organic | Typical | L | 7.67 | 0.585 | 2.5 | 22.47 |
| E3 | Thermi-typical fertilization/late sowing | 40°54′ N/ 23°00′ E | BSk | 343.6 | 45.4 | 12.8 | LP | Typical | Late sowing | L | 7.84 | 0.681 | 1.8 | 18.29 |
| E4 | Thermi-splitting fertilization/typical sowing date | 40°54′ N/ 23°00′ E | BSk | 343.6 | 45.4 | 12.8 | HP | Splitting topdressing application | Typical | L | 8.14 | 0.564 | 1.7 | 20.63 |
| E5 | Thermi-late splitting fertilization/late sowing date | 40°54′ N/ 23°00′ E | BSk | 343.6 | 45.4 | 12.8 | LP | Splitting topdressing application | Late sowing | L | 7.99 | 0.497 | 1.8 | 23.21 |
| E6 | Nea Gonia/typical fertilization/typical sowing date | 40°35′ N/ 23°08′ E | BSk | 335.5 | 57.6 | 12.4 | HP | Typical | Mid-November | CL | 7.05 | 0.525 | 1.8 | 30.10 |
| E7 | Sindos/typical fertilization/late sowing date | 40°68′ N/ 22°80′ E | BSk | 376.2 | 41.6 | 12.7 | LP | Typical | Late sowing | SL | 7.85 | 0.485 | 1.7 | 28.50 |
| TPC | ABTS | DPPH | FRAP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | SS | SS% | MS | SS | SS% | MS | SS | SS% | MS | SS | SS% | |
| Environment | 6 | 1787.6 *** | 10,725.5 | 42.4 | 7348.9 *** | 44,093.6 | 33.1 | 2664.9 *** | 3992.1 | 38.3 | 25,886.9 *** | 155,321.4 | 20.7 |
| Genotype | 15 | 154.7 *** | 2320.2 | 9.2 | 594.0 *** | 8910.0 | 6.7 | 266.1 *** | 3992.1 | 9.6 | 6325.1 *** | 94,876.7 | 12.7 |
| G × E | 90 | 118.6 *** | 10,670.6 | 42.2 | 671.0 *** | 60,389.3 | 45.3 | 231.6 *** | 20,844.8 | 49.9 | 5403.5 *** | 486,314.2 | 64.9 |
| Error | 224 | 6.9 | 1551.4 | 6.2 | 89.4 | 20,016.5 | 15.0 | 4.1 | 926.3 | 2.2 | 56.9 | 12,746.9 | 1.7 |
| TPC * | ABTS | DPPH | FRAP | |
|---|---|---|---|---|
| Environment | ||||
| Ε1 | 48.8 ± 1.1 c ** | 118.3 ± 2.2 c | 14.4 ± 1.7 g | 75.7 ± 4.6 d |
| Ε2 | 49.5 ± 1.1 c,d | 122.2 ± 2.2 c | 18.7 ± 1.8 e | 91.2 ± 9.6 c |
| Ε3 | 57.3 ± 1.2 a | 142.6 ± 2.8 a | 21.5 ± 2.3 d | 106.7 ± 10.0 b |
| Ε4 | 42.0 ± 1.0 d | 110.8 ± 2.6 d | 16.6 ± 0.9 f | 88.7 ± 2.5 c |
| Ε5 | 39.8 ± 0.7 e | 104.8 ± 2.3 e | 34.4 ± 0.6 a | 52.4 ± 3.0 e |
| Ε6 | 51.7 ± 0.7 b | 128.4 ± 2.4 b | 24.5 ± 0.5 c | 79.2 ± 1.6 d |
| Ε7 | 52.8 ± 0.8 b | 122.4 ± 2.1 c | 31.1 ± 0.8 b | 125.1 ± 5.7 a |
| Average | 48.8 ± 0.5 | 121.3 ± 1.1 | 23.0 ± 0.6 | 88.4 ± 2.6 |
| Genotype | ||||
| G1 | 49.9 ± 2.4 b,c ** | 122.8 ± 5.1 a,b | 26.1 ± 2.1 b | 106.6 ± 9.7 b |
| G2 | 43.8 ± 1.9 f | 112.8 ± 3.4 c,d | 20.0 ± 2.3 e | 77.1 ± 8.3 e,f |
| G3 | 46.5 ± 2.1 d,e | 124.7 ± 5.0 a,b | 22.7 ± 1.6 c,d | 80.0 ± 5.6 d–f |
| G4 | 49.4 ± 1.7 c | 124.0 ± 4.0 a,b | 23.6 ± 1.8 c | 109.2 ± 12.3 b |
| G5 | 52.4 ± 1.0 a,b | 129.0 ± 5.1 a | 22.7 ± 1.5 c,d | 72.9 ± 4.9 f,g |
| G6 | 49.0 ± 2.2 c,d | 121.1 ± 5.3 a–c | 27.0 ± 4.2 b | 112.0 ± 19.7 b |
| G7 | 52.4 ± 1.7 a,b | 125.5 ± 3.2 a,b | 23.9 ± 2.0 c | 108.0 ± 8.6 b |
| G8 | 46.9 ± 1.2 d,e | 107.0 ± 3.4 d | 20.3 ± 2.1 e | 69.9 ± 5.5 g |
| G9 | 53.1 ± 2.6 a | 122.4 ± 5.0 a,b | 31.7 ± 4.1 a | 90.4 ± 9.8 c |
| G10 | 50.4 ± 1.7 b,c | 120.2 ± 3.4 a–c | 20.9 ± 2.0 d,e | 86.3 ± 6.0 c,d |
| G11 | 47.0 ± 2.0 d,e | 121.3 ± 3.5 a–c | 18.9 ± 2.0 e,f | 81.0 ± 5.3 d,e |
| G12 | 46.9 ± 1.2 d,e | 116.7 ± 3.1 b,c | 17.8 ± 2.0 f | 72.9 ± 4.3 f,g |
| G13 | 49.6 ± 1.4 c | 125.4 ± 2.9 a,b | 22.9 ± 1.5 c | 65.8 ± 2.6 g |
| G14 | 45.7 ± 1.7 e,f | 120.1 ± 3.7 a–c | 20.1 ± 1.3 e | 80.0 ± 4.5 d–f |
| G15 | 52.0 ± 2.3 a–c | 124.2 ± 6.1 a–c | 27.2 ± 2.9 b | 122.1 ± 21.1 a |
| G16 | 46.5 ± 1.7 d,e | 124.2 ± 5.7 a,b | 22.4 ± 1.7 c,d | 80.5 ± 7.1 d,e |
| Average | 48.8 ± 0.5 | 121.2 ± 1.1 | 23.0 ± 0.6 | 88.4 ± 2.6 |
| Mean Protein % | Vitreous % | |
|---|---|---|
| Environment | ||
| E1 | 13.3 ± 0.1 c * | Not Availiable (NA) |
| E2 | 10.7 ± 0.3 d,e | NA |
| E3 | 11.6 ± 0.2 d | NA |
| E4 | 15.1 ± 0.2 a | NA |
| E5 | 13.1 ± 0.2 c | NA |
| E6 | 10.3 ± 0.1 e | NA |
| E7 | 14.1 ± 0.1 a,b | NA |
| Genotype | ||
| G1 | 13.9 ± 0.5 a | 62.25 |
| G2 | 12.9 ± 0.4 a | 87.5 |
| G3 | 12.2 ± 0.5 a | 74.8 |
| G4 | 13.1 ± 0.4 a | 86.7 |
| G5 | 12.2 ± 0.4 a | 69.3 |
| G6 | 11.8 ± 0.3 a | 66.6 |
| G7 | 12.7 ± 0.4 a | 55.1 |
| G8 | 12.1 ± 0.5 a | 73.1 |
| G9 | 13.0 ± 0.5 a | 83.5 |
| G10 | 12.6 ± 0.5 a | 92.2 |
| G11 | 12.7 ± 0.5 a | 63.6 |
| G12 | 11,9 ± 0.4 a | 56.6 |
| G13 | 12.5 ± 0.3 a | 77.4 |
| G14 | 12.5 ± 0.4 a | 45.6 |
| G15 | 12.1 ± 0.4 a | 72.7 |
| G16 | 13.3 ± 0.4 a | 76.9 |
| Average | 12.6 ± 0.1 | 71.5 |
| TPC | ABTS | DPPH | FRAP | Protein | Vitreous % | |
|---|---|---|---|---|---|---|
| TPC | 1 | 0.676 ** | 0.273 ** | 0.525 ** | −0.333 ** | −0.052 |
| ABTS | 1 | 0.110 | 0.452 ** | −0.313 ** | 0.097 | |
| DPPH | 1 | 0.443 ** | 0.077 | −0.112 | ||
| FRAP | 1 | 0.113 | −0.351 * | |||
| Protein | 1 | 0.706 ** | ||||
| Vitreous | 1 |
| TPC | ABTS | Protein | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Mean | GGE | ASVi | Pi | σ2i | s2di | 𝘒R | Mean | GGE | ASVi | Pi | σ2i | s2di | 𝘒R | Mean | GGE | ASVi | Pi | σ2i | s2di | 𝘒R |
| Top five | G9 | G7 | G14 | G5 | G16 | G3 | G10 | G53 | G7 | G23 | G5 | G2 | G3 | G3 | G13 | G1 | G134 | G1 | G13 | G13 | G16 |
| G54 * | G10 | G4 | G33 | G10 | G16 | G5 | G74 | G43 | G7 | G3 | G11 | G2 | G4 | G165 | G94 | G34 | G16 | G16 | G3 | G9 | |
| G73 | G9 | G10 | G13 | G4 | G10 | G4 | G134 | G13 | G113 | G13 | G12 | G12 | G11 | G4 | G16 | G7 | G4 | G3 | G16 | G2 | |
| G15 | G5 | G163 | G4 | G3 | G5 | G15 | G34 | G5 | G14 | G4 | G10 | G11 | G13 | G94 | G72 | G123 | G9 | G12 | G12 | G13 | |
| G105 | G46 | G2 | G7 | G14 | G4 | G9 | G16 | G16 | G10 | G7 | G4 | G13 | G7 | G2 | G14 | G14 | G101 | G2 | G9 | G3 | |
| Bottom five | G85 | G152 | G1 | G6 | G9 | G15 | G11 | G10 | G12 | G6 | G6 | G15 | G16 | G14 | G55 | G6 | G6 | G14 | G6 | G10 | G10 |
| G3 | G12 | G73 | G16 | G13 | G13 | G2 | G14 | G14 | G16 | G9 | G16 | G1 | G15 | G84 | G8 | G13 | G3 | G5 | G5 | G8 | |
| G16 | G3 | G134 | G13 | G7 | G11 | G12 | G123 | G153 | G1 | G12 | G9 | G5 | G9 | G155 | G12 | G5 | G8 | G1 | G1 | G5 | |
| G14 | G2 | G8 | G8 | G8 | G7 | G6 | G2 | G66 | G6 | G2 | G5 | G9 | G8 | G123 | G5 | G15 | G12 | G4 | G4 | G6 | |
| G23 | G66 | G6 | G2 | G6 | G6 | G8 | G83 | G8 | G95 | G8 | G6 | G6 | G6 | G66 | G15 | G43 | G6 | G15 | G15 | G15 | |
| TPC | ABTS | Protein | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GGE | ASVi | Pi | σ2i | s2di | 𝘒R | GGE | ASVi | Pi | σ2i | s2di | 𝘒R | GGE | ASVi | Pi | σ2i | s2di | 𝘒R | |
| Mean | 0.609 * | ns+ | ns | ns | ns | ns | 0.712 ** | ns | 0.785 *** | −0.506 * | ns | ns | 0.865 *** | ns | 0.915 *** | ns | ns | 0.703 ** |
| GGE | 1 | ns | ns | ns | ns | ns | 1 | ns | 0.559 * | ns | ns | 0.714 ** | 1 | ns | 0.659 ** | ns | ns | 0.773 ** |
| ASVi | 1 | ns | ns | ns | ns | 1 | ns | ns | ns | ns | 1 | ns | 0.903 *** | 0.888 *** | 0.582 * | |||
| Pi | 1 | ns | ns | ns | 1 | ns | ns | 0.677 ** | 1 | ns | ns | ns | ||||||
| σ2i | 1 | 0.815 ** | 0.599 * | 1 | 0.865 ** | ns | 1 | 0.988 ** | 0.718 ** | |||||||||
| s2di | 1 | 0.587 * | 1 | 0.685 ** | 1 | 0.696 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melios, S.; Ninou, E.; Irakli, M.; Tsivelika, N.; Sistanis, I.; Papathanasiou, F.; Didos, S.; Zinoviadou, K.; Karantonis, H.C.; Argiriou, A.; et al. Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time. Agriculture 2024, 14, 328. https://doi.org/10.3390/agriculture14020328
Melios S, Ninou E, Irakli M, Tsivelika N, Sistanis I, Papathanasiou F, Didos S, Zinoviadou K, Karantonis HC, Argiriou A, et al. Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time. Agriculture. 2024; 14(2):328. https://doi.org/10.3390/agriculture14020328
Chicago/Turabian StyleMelios, Stergios, Elissavet Ninou, Maria Irakli, Nektaria Tsivelika, Iosif Sistanis, Fokion Papathanasiou, Spyros Didos, Kyriaki Zinoviadou, Haralabos Christos Karantonis, Anagnostis Argiriou, and et al. 2024. "Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time" Agriculture 14, no. 2: 328. https://doi.org/10.3390/agriculture14020328
APA StyleMelios, S., Ninou, E., Irakli, M., Tsivelika, N., Sistanis, I., Papathanasiou, F., Didos, S., Zinoviadou, K., Karantonis, H. C., Argiriou, A., & Mylonas, I. (2024). Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time. Agriculture, 14(2), 328. https://doi.org/10.3390/agriculture14020328












