Digital Image Analysis of Low-Temperature Responses in Sweet Corn Hybrid Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Trait Measurements
2.3. Various Saccharide Contents in Seeds
2.4. Field Validation
2.5. Statistical Analyses
3. Results
3.1. Growth Response to Low-Temperature Treatment
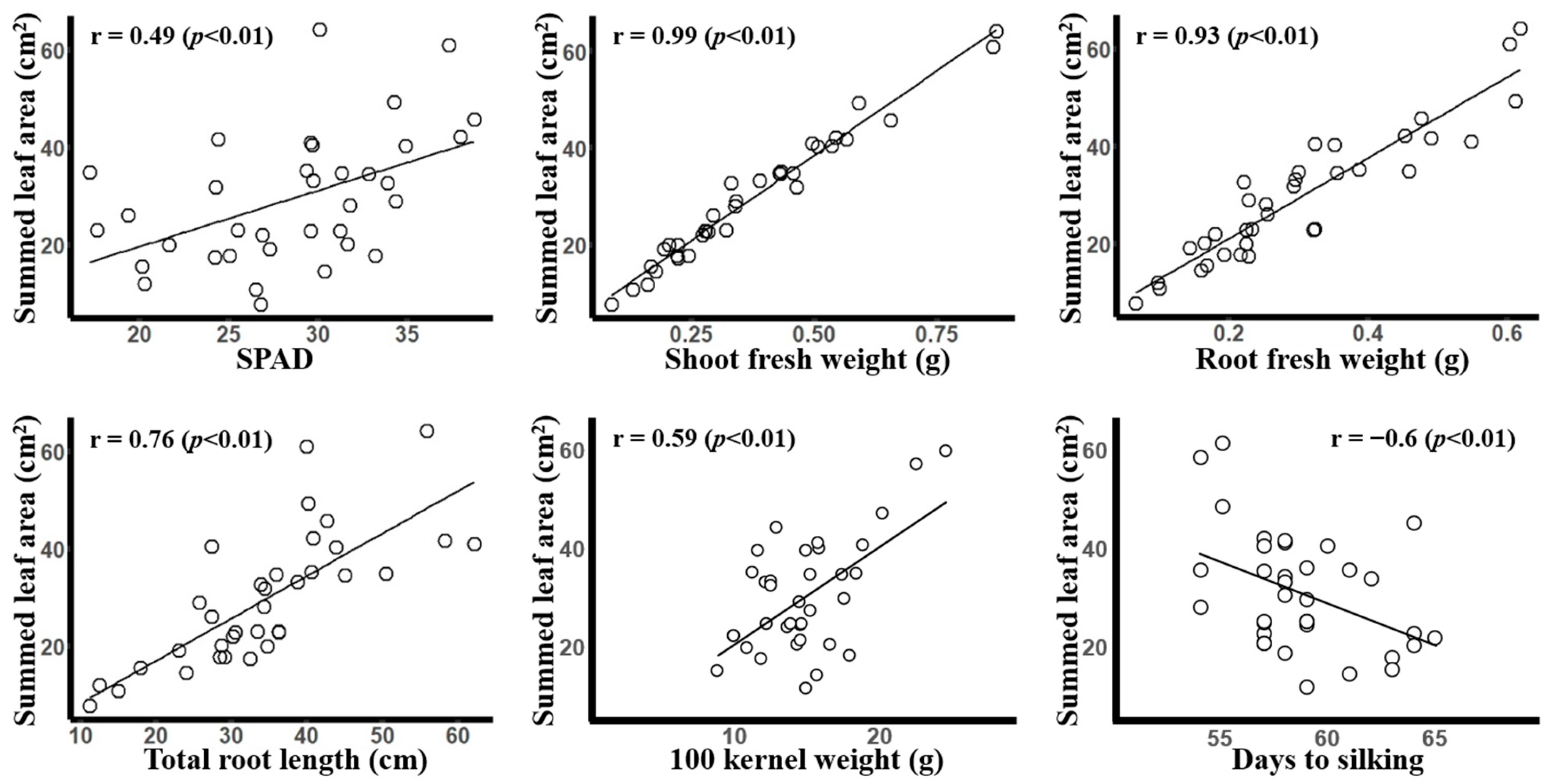
3.2. Correlation of Summed Leaf Area with Other Agronomic Traits
3.3. Saccharide Contents in Seeds by Endosperm Types
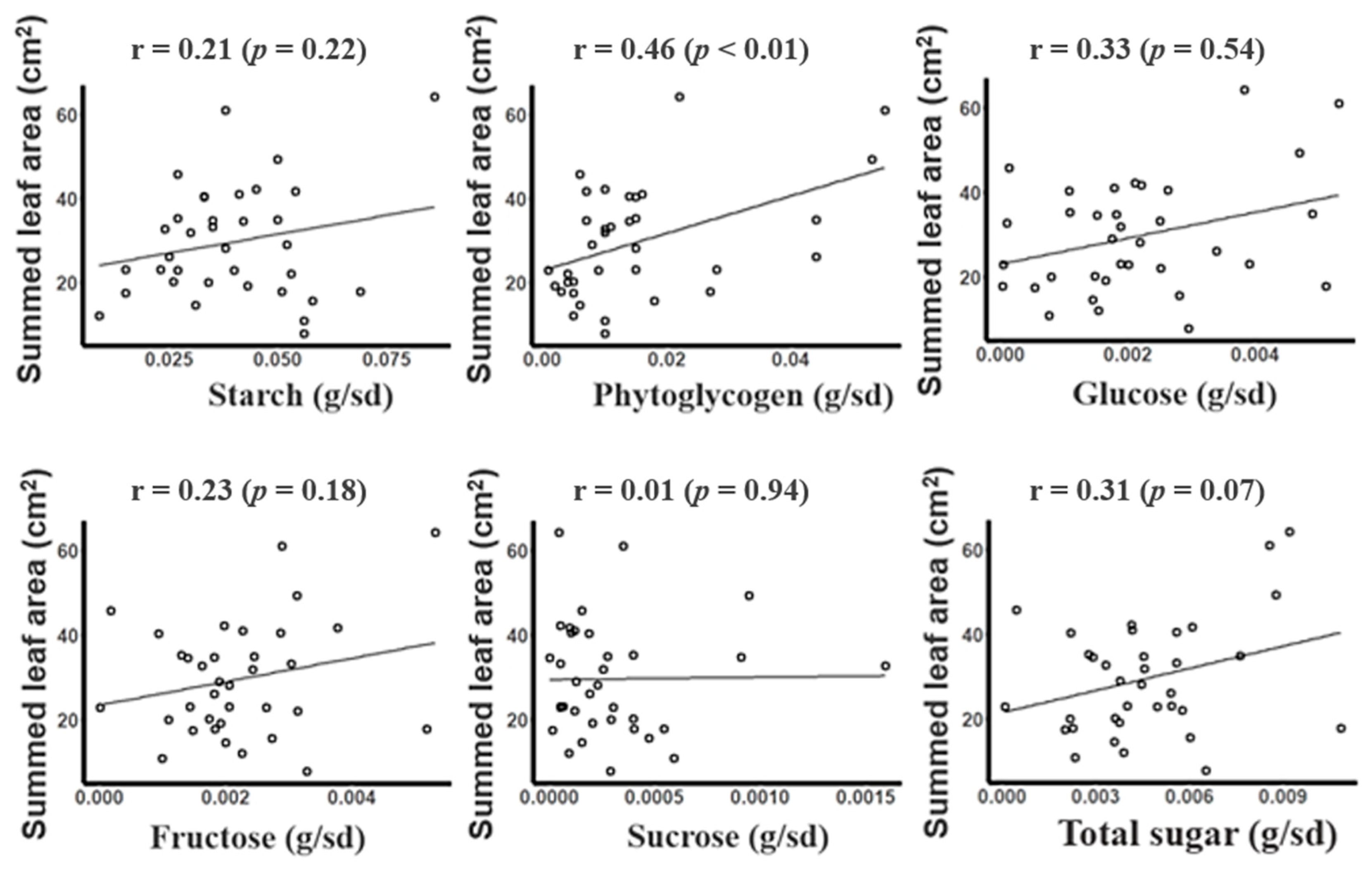
3.4. Relationship between Summed Leaf Area and Saccharide Contents under Low-Temperature Condition
3.5. Comparison of Growth Response in Low-Temperature and Normal-Temperature Conditions
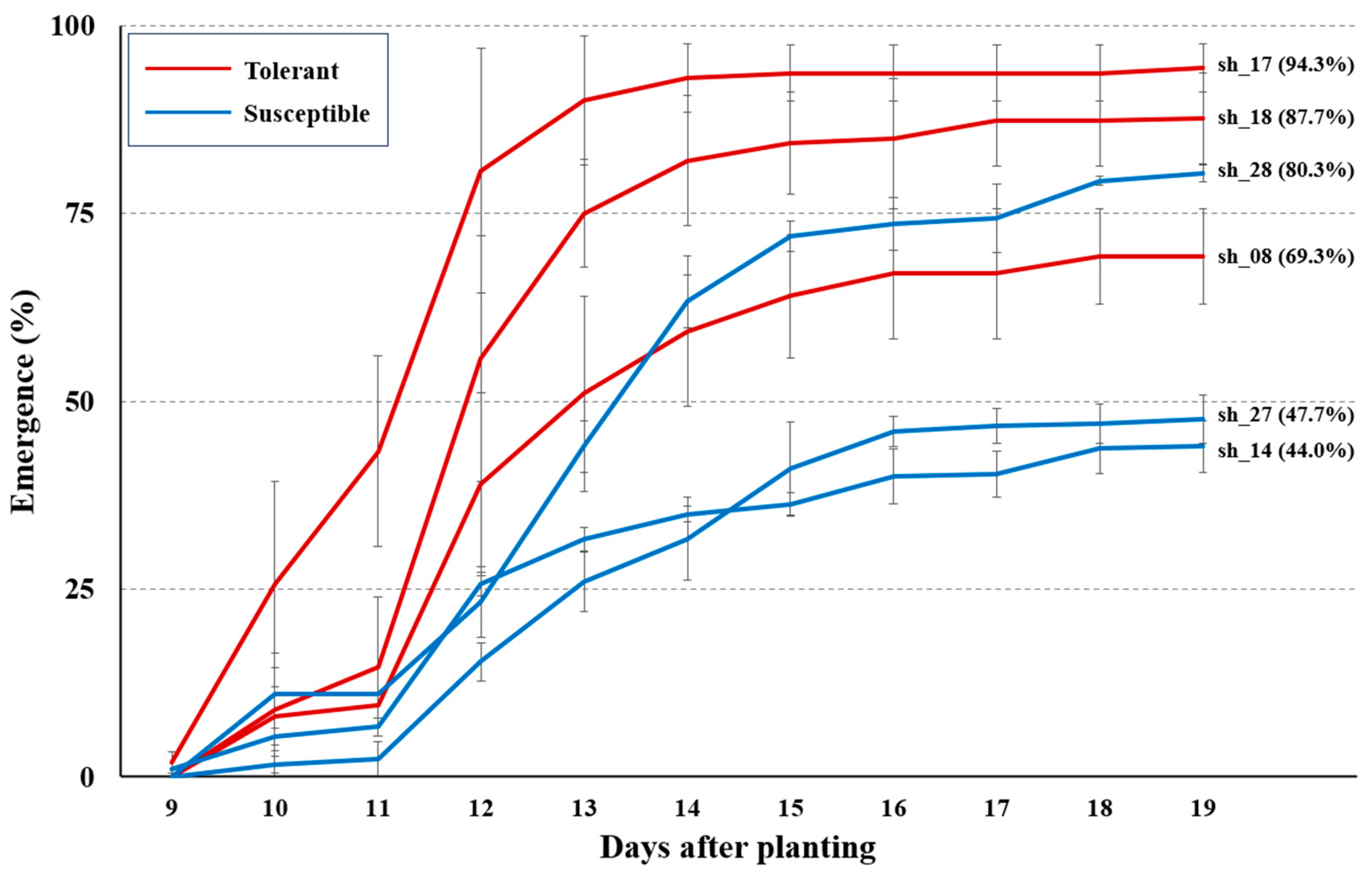
3.6. Validation of Selected Sweet Corn Hybrids in Field Condition
4. Discussion
5. Conclusions
- (1)
- There was a substantial difference among sweet corn hybrids on summed leaf area, SPAD, shoot and root fresh weight, and total root length at the seedling stage germinated and grown under low-temperature conditions.
- (2)
- The group mean of the sugary endosperm type was significantly higher than that of the shrunken endosperm type for all traits but SPAD.
- (3)
- For the summed leaf area, the top three ranked hybrids were all from the sugary endosperm type, but the area for the following three hybrids (4th~6th rank) from the shrunken endosperm type did not differ significantly from that for the first three hybrids.
- (4)
- The summed leaf area was positively correlated with SPAD, shoot and root fresh weight, and total root length.
- (5)
- The phytoglycogen content in seeds of sweet corn hybrids was the only saccharide that had a significant correlation with the summed leaf area.
- (6)
- When low-temperature stress was compared to normal-temperature growing condition for the same growing degree unit, the summed leaf area of 34 out of 35 hybrids examined did not differ between the two temperature conditions.
- (7)
- The level of low-temperature response based on the summed leaf area was reflected in the low-temperature field condition, with a few exceptions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lertrat, K.; Pulam, T. Breeding for Increased Sweetness in Sweet Corn. Int. J. Plant Breed. 2007, 1, 27–30. [Google Scholar]
- Lee, J.-S.; Jung, T.-W.; Son, B.-Y.; Kim, J.-T.; Jung, G.-H.; Shin, S.-H.; Kim, S.-K.; Seo, J.-H.; Lee, J.-E.; Baek, S.-B. A New Sweet Corn Hybrid with Good Eating Quality and High Sugar Content, ‘Guseulok’. Korean J. Breed. Sci. 2013, 45, 416–419. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jung, T.-W.; Son, B.-Y.; Shin, S.-H.; Kim, J.-T.; Bae, H.-H.; Baek, S.-B.; Ku, J.-H.; Hwang, J.-J.; Kim, S.-L. A Yellow Sweet Corn Hybrid with High Sugar Content and Lodging Tolerance, ‘Godangok’. Korean J. Breed. Sci. 2014, 46, 476–480. [Google Scholar] [CrossRef]
- Enders, T.A.; St Dennis, S.; Oakland, J.; Callen, S.T.; Gehan, M.A.; Miller, N.D.; Spalding, E.P.; Springer, N.M.; Hirsch, C.D. Classifying Cold-Stress Responses of Inbred Maize Seedlings Using RGB Imaging. Plant Direct 2019, 3, 104. [Google Scholar] [CrossRef]
- Sellschop, J.P.F.; Salmon, S.C. The Influence of Chilling, above the Freezing Point, on Certain Crop Plants. J. Agric. Res. 1928, 37, 315–338. [Google Scholar]
- Revilla, P.; Hotchkiss, J.R.; Tracy, W.F. Cold Tolerance Evaluation in a Diallel among Open-Pollinated Sweet Corn Cultivars. HortScience 2003, 38, 88–91. [Google Scholar] [CrossRef]
- Azanza, F.; Bar-Zur, A.; Juvik, J.A. Variation in Sweet Corn Kernel Characteristics Associated with Stand Establishment and Eating Quality. Euphytica 1996, 87, 7–18. [Google Scholar] [CrossRef]
- Hassell, R.L.; Dufault, R.J.; Phillips, T.L. Low-Temperature Germination Response of Su, Se, and Sh2 Sweet Corn Cultivars. HortTechnology 2003, 13, 136–141. [Google Scholar] [CrossRef]
- Simon, E.W. Plant Membranes under Dry Conditions. Pestic. Sci. 1978, 9, 169–172. [Google Scholar] [CrossRef]
- Huner, N.; Elfman, B.; Król, M.; Mcintosh, A. Growth and Development at Cold-Hardening Temperatures. Chloroplast Ultrastructure, Pigment Content, and Composition. Can. J. Bot. 1984, 62, 53–60. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Hock, M.W.; Reddy, K.R. Growth and Physiological Trait Variation among Corn Hybrids for Cold Tolerance. Can. J. Plant Sci. 2016, 96, 639–656. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How Plants Sense Temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Stamp, P. Cold Tolerance of Maize Seedlings as Determined by Root Morphology and Photosynthetic Traits. Eur. J. Agron. 2008, 28, 178–185. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Römheld, V.; Neumann, G. Nutrient Seed Priming Improves Seedling Development of Maize Exposed to Low Root Zone Temperatures during Early Growth. Eur. J. Agron. 2013, 49, 141–148. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold Stress Tolerance Mechanisms in Plants BT—Sustainable Agriculture Volume 2; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 605–620. ISBN 978-94-007-0394-0. [Google Scholar]
- Kubien, D.S.; von Caemmerer, S.; Furbank, R.T.; Sage, R.F. C4 Photosynthesis at Low Temperature. A Study Using Transgenic Plants with Reduced Amounts of Rubisco. Plant Physiol. 2003, 132, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Thomashow, M.F. Pant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Marocco, A.; Lorenzoni, C.; Fracheboud, Y. Chilling Stress in Maize. Maydica 2005, 50, 571–580. [Google Scholar]
- Hartmann, A.; Czauderna, T.; Hoffmann, R.; Stein, N.; Schreiber, F. HTPheno: An Image Analysis Pipeline for High-Throughput Plant Phenotyping. BMC Bioinform. 2011, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, N.; Feldman, M.; Gehan, M.A.; Wilson, M.S.; Shyu, C.; Bryant, D.W.; Hill, S.T.; McEntee, C.J.; Warnasooriya, S.N.; Kumar, I. A Versatile Phenotyping System and Analytics Platform Reveals Diverse Temporal Responses to Water Availability in Setaria. Mol. Plant 2015, 8, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Lobet, G. Image Analysis in Plant Sciences: Publish Then Perish. Trends Plant Sci. 2017, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hong*, S.-T.; Jeong, Y.-S.; Kim, J.-W.; Lee, E.-S.; Han, Y.-Y.; Gil, N.-Y.; Lee, M.-J.; Lee, G.-H. Studies on Physico-Chemical Characterization of Starch Extracted from Domestic Barley Cultivars. Food Eng. Prog. 2013, 17, 203–211. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening Corn Hybrids for Cold Tolerance Using Morphological Traits for Early-Season Seeding. Crop Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef]
- Wu, Q.; Pagès, L.; Wu, J. Relationships between Root Diameter, Root Length and Root Branching along Lateral Roots in Adult, Field-Grown Maize. Ann. Bot. 2016, 117, 379–390. [Google Scholar] [CrossRef]
- Sun, J.; Wu, D.; Xu, J.; Rasmussen, S.K.; Shu, X. Characterisation of Starch during Germination and Seedling Development of a Rice Mutant with a High Content of Resistant Starch. J. Cereal Sci. 2015, 62, 94–101. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and Role of Starch, Protein, and Fat Reserves during Seed Germination of Six Wild Grassland Species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef]
- Andriotis, V.M.E.; Rejzek, M.; Barclay, E.; Rugen, M.D.; Field, R.A.; Smith, A.M. Cell Wall Degradation Is Required for Normal Starch Mobilisation in Barley Endosperm. Sci. Rep. 2016, 6, 33215. [Google Scholar] [CrossRef]
- Dirk, L.M.A.; van der Krol, A.R.; Vreugdenhil, D.; Hilhors, H.W.M.; Bewley, J.D. Galactomannan, Soluble Sugar and Starch Mobilization Following Germination of Trigonella Foenum-Graecum Seeds. Plant Physiol. Biochem. 1999, 37, 41–50. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars-Metabolism, Sensing and Abiotic Stress a Complex Network in the Life of Plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, S.; Teng, S.; Arru, L.; Smeekens, S.C.M.; Perata, P. Sugar Effects on Early Seedling Development in Arabidopsis. Plant Growth Regul. 2007, 52, 217–228. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm During Seed Germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Van den Ende, W. Cold Tolerance Triggered by Soluble Sugars: A Multifaceted Countermeasure. Front. Plant Sci. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Koster, K.L.; Leopold, A.C. Sugars and Desiccation Tolerance in Seeds 1. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef]
- Nickels, J.D.; Atkinson, J.; Papp-Szabo, E.; Stanley, C.; Diallo, S.O.; Perticaroli, S.; Baylis, B.; Mahon, P.; Ehlers, G.; Katsaras, J. Structure and Hydration of Highly-Branched, Monodisperse Phytoglycogen Nanoparticles. Biomacromolecules 2016, 17, 735–743. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Kuo, T.M.; Juvik, J.A.; Beers, E.P.; Duke, S.H. Characteristics of Carbohydrate Metabolism in Sweet Corn (Sugary-1) Endosperms. J. Am. Soc. Hortic. Sci. 2019, 118, 661–666. [Google Scholar] [CrossRef]
- Zhou, X.; Muhammad, I.; Lan, H.; Xia, C. Recent Advances in the Analysis of Cold Tolerance in Maize. Front. Plant Sci. 2022, 13, 866034. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, S.; Wei, Y.; Xu, X.; Ding, D.; Liu, M. Analysis of root physiology and related gene expression in maize (Zea mays) under low temperature stress. J. Agric. Biotechnol. 2020, 28, 32–41. [Google Scholar]





| n = 35 | Summed Leaf Area (cm2) | SPAD | Shoot Weight (g plant−1) | Root Weight (g plant−1) | Total Root Length (cm) |
|---|---|---|---|---|---|
| AVG ± STD | 29.5 ± 13.29 | 28.5 ± 5.61 | 0.37 ± 0.19 | 0.3 ± 0.15 | 34.3 ± 11.61 |
| Max | 64.2 | 45.7 | 1.22 | 0.97 | 103.6 |
| Min | 7.6 | 0.4 | 0.04 | 0.04 | 0.36 |
| C.V. | 45.1% | 19.7% | 51.4% | 50.0% | 33.8% |
| By endosperm type (AVG ± STD) | |||||
| Shrunken (n = 29) | 26.7 ± 10.83 a | 29.1 ± 4.81 a | 0.33 ± 0.15 a | 0.27 ± 0.12 a | 32.8 ± 11.66 a |
| Sugary (n = 6) | 43.1 ± 17.67 b | 26.0 ± 9.01 a | 0.57 ± 0.26 b | 0.48 ± 0.16 b | 41.7 ± 10.18 b |
| Source of Variation | df | Summed Leaf Area (cm2) | SPAD | Shoot Weight (g plant−1) | Root Weight (g plant−1) | Total Root Length (cm) |
|---|---|---|---|---|---|---|
| Temperature (T) | 1 | ns | *** | ns | * | ns |
| Replication | 5 | |||||
| Cultivar (C) | 34 | *** | *** | *** | *** | *** |
| T × C | 34 | *** | *** | *** | *** | ns |
| Experiment error | 345 | |||||
| Total | 419 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.-C.; Wang, S.; Kang, J.; Kang, M.; Chung, J.-W.; So, Y.-S. Digital Image Analysis of Low-Temperature Responses in Sweet Corn Hybrid Seedlings. Agriculture 2024, 14, 360. https://doi.org/10.3390/agriculture14030360
Park T-C, Wang S, Kang J, Kang M, Chung J-W, So Y-S. Digital Image Analysis of Low-Temperature Responses in Sweet Corn Hybrid Seedlings. Agriculture. 2024; 14(3):360. https://doi.org/10.3390/agriculture14030360
Chicago/Turabian StylePark, Tae-Chun, Seunghyun Wang, Jongwon Kang, Minjeong Kang, Jong-Wook Chung, and Yoon-Sup So. 2024. "Digital Image Analysis of Low-Temperature Responses in Sweet Corn Hybrid Seedlings" Agriculture 14, no. 3: 360. https://doi.org/10.3390/agriculture14030360





