Impact of Soil Organic Layer Thickness on Soil-to-Atmosphere GHG Fluxes in Grassland in Latvia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. GHG Sampling Design and Measurements
2.3. Soil Sampling and Analyses
2.4. Measurements of Environmental Parameters
2.5. Sampling and Analyses of Above- and Below-Ground Parts of Vegetation
2.6. Estimation of Soil Annual GHG Emissions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Soil Total Respiration (Instantaneous)
3.2. Soil Heterotrophic Respiration (Instantaneous)
3.3. Soil-to-Atmosphere CH4 and N2O Fluxes (Instantaneous)
3.4. Annual GHG Fluxes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Research Site | Biomass, t DM ha−1 | C Stock, t C ha−1 | Annual C Input, t C ha−1 yr−1 | ||
|---|---|---|---|---|---|
| AGB | BGB | AGB | BGB | Total | |
| RS1 | 3.88 ± 1.13 | 0.69 ± 0.10 | 1.75 ± 0.52 | 0.29 ± 0.04 | 1.89 ± 0.43 |
| RS2 | 6.50 ± 0.92 | 0.98 ± 0.21 | 3.00 ± 0.43 | 0.40 ± 0.09 | 3.20 ± 0.61 |
| RS3 | 2.75 ± 0.38 | 6.26 ± 0.49 | 1.26 ± 0.18 | 2.49 ± 0.18 | 2.50 ± 0.25 |
| All research sites pooled | 4.37 ± 0.55 | 2.65 ± 0.47 | 2.00 ± 0.26 | 1.06 ± 0.18 | 2.53 ± 0.30 |

References
- Joosten, H. What are peatlands? In Assessment on Peatlands, Biodiversity and Climate Change; Parish, F., Sirin, A., Charman, D., Joosten, H., Minayeva, T., Silvius, M., Eds.; Global Environment Centre: Kuala Lumpur, Malaysia; Wetlands International: Wageningen, The Netherlands, 2008; pp. 8–19. [Google Scholar]
- Mitsch, W.J.; Bernal, B.; Hernandez, M.E. Ecosystem services of wetlands. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 1–4. [Google Scholar] [CrossRef]
- An, S.; Verhoeven, J.T.A. Wetland functions and ecosystem services: Implications for wetland restoration and wise use. In Wetlands: Ecosystem Services, Restoration and Wise Use; Verhoeven, J.T.A., Ed.; Springer: Cham, Switzerland, 2019; Volume 238, pp. 1–10. [Google Scholar]
- FAO. Peatlands and Climate Planning—Part 1: Peatlands and Climate Commitments; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; pp. 1–70. [Google Scholar]
- Oleszczuk, R.; Regina, K.; Szajdak, L.; Höper, H.; Maryganova, V. Impact of agricultural utilization of peat soils on the greenhouse gas balance. In Peatlands and Climate Change; Strack, M., Ed.; International Peat Society: Jyväskylä, Finland, 2008; pp. 70–91. [Google Scholar]
- Tubiello, F.N.; Biancalani, R.; Salvatore, M.; Rossi, S.; Conchedda, G. A worldwide assessment of greenhouse gas emissions from drained organic soils. Sustainability 2016, 8, 371. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Don, A.; Fiedler, S.; Bechtold, M.; Amelung, W.; Freibauer, A. Drained organic soils under agriculture—The more degraded the soil the higher the specific basal respiration. Geoderma 2019, 355, 113911. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Dettmann, U.; Don, A. How do sand addition, soil moisture and nutrient status influence greenhouse gas fluxes from drained organic soils? Soil Biol. Biochem. 2019, 135, 71–84. [Google Scholar] [CrossRef]
- Joosten, H.; Couwenberg, J. Peatlands and carbon. In Assessment on Peatlands, Biodiversity and Climate Change; Parish, F., Sirin, A., Charman, D., Joosten, H., Minayeva, T., Silvius, M., Eds.; Global Environment Centre: Kuala Lumpur, Malaysia; Wetlands International: Wageningen, The Netherlands, 2008; pp. 99–117. [Google Scholar]
- Epron, D. Separating autotrophic and heterotrophic components of soil respiration: Lessons learned from trenching and related root-exclusion experiments. In Soil Carbon Flux Measurements: An Integrated Methodology; Kutsch, W.L., Bahn, M., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 157–168. [Google Scholar]
- Tang, X.; Du, J.; Shi, Y.; Lei, N.; Chen, G.; Cao, L.; Pei, X. Global patterns of soil heterotrophic respiration—A meta-analysis of available dataset. Catena 2020, 191, 104574. [Google Scholar] [CrossRef]
- Tang, X.; Pei, X.; Lei, N.; Luo, X.; Liu, L.; Shi, L.; Chen, G.; Liang, J. Global patterns of soil autotrophic respiration and its relation to climate, soil and vegetation characteristics. Geoderma 2020, 369, 114339. [Google Scholar] [CrossRef]
- Tiemeyer, B.; Albiac Borraz, E.; Augustin, J.; Bechtold, M.; Beetz, S.; Beyer, C.; Drösler, M.; Ebli, M.; Eickenscheidt, T.; Fiedler, S.; et al. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Chang. Biol. 2016, 22, 4134–4149. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Sci. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Truu, J.; Espenberg, M.; Mander, Ü.; Smith, P. Emissions of methane from northern peatlands: A review of management impacts and implications for future management options. Ecol. Evol. 2016, 6, 7080–7102. [Google Scholar] [CrossRef] [PubMed]
- Latvia National Inventory Report. 1990–2021. Available online: https://unfccc.int/documents/627724 (accessed on 14 January 2024).
- Eickenscheidt, T.; Heinichen, J.; Drösler, M. The greenhouse gas balance of a drained fen peatland is mainly controlled by land-use rather than soil organic carbon content. Biogeosciences 2015, 12, 5161–5184. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Tokyo, Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/pub-lic/2006gl/index.html (accessed on 14 January 2024).
- Leiber-Sauheitl, K.; Fuß, R.; Voigt, C.; Freibauer, A. High CO2 fluxes from grassland on histic Gleysol along soil carbon and drainage gradients. Biogeosciences 2014, 11, 749–761. [Google Scholar] [CrossRef]
- Leppelt, T.; Dechow, R.; Gebbert, S.; Freibauer, A.; Lohila, A.; Augustin, J.; Drösler, M.; Fiedler, S.; Glatzel, S.; Höper, H.; et al. Nitrous oxide emission hotspots from organic soils in Europe. Biogeosciences 2014, 11, 6595–6612. [Google Scholar] [CrossRef]
- Beyer, C.; Liebersbach, H.; Höper, H. Multiyear greenhouse gas flux measurements on a temperate fen soil used for cropland or grassland. J. Plant. Nutr. Soil Sci. 2015, 178, 99–111. [Google Scholar] [CrossRef]
- Rūsiņa, S. Dabiskie zālāji un mežmalas. In Latvija. Zeme, Daba, Tauta, Valsts; Nikodemus, O., Kļaviņš, M., Krišjāne, Z., Zelčs, V., Eds.; Latvijas Universitātes Akadēmiskais apgāds: Riga, Latvia, 2018; pp. 414–423. [Google Scholar]
- LEGMC. Klimata Portals. Available online: https://klimats.meteo.lv/laika_apstaklu_raksturojums/2022/gads/ (accessed on 14 January 2024).
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Harper, L.A., Mosier, A.R., Duxbury, J.M., Rolston, D.E., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1993; pp. 63–78. [Google Scholar]
- Pumpanen, J.; Kolari, P.; Ilvesniemi, H.; Minkkinen, K.; Vesala, T.; Niinistö, S.; Lohila, A.; Larmola, T.; Morero, M.; Pihlatie, M.; et al. Comparison of different chamber techniques for measuring soil CO2 efflux. Agric. For. Meteorol. 2004, 123, 159–176. [Google Scholar] [CrossRef]
- Purvina, D.; Licite, I.; Butlers, A.; Lazdins, A.; Saule, G.; Turks, A.; Prysiazhniuk, L. Evaluation of peat layer thickness effect on soil GHG fluxes. In Proceedings of the 22nd International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 24–26 May 2023; pp. 454–460. [Google Scholar]
- LVS ISO 11464:2006; Soil Quality. Pretreatment of Samples for Physico-Chemical Analysis. Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2006.
- LVS EN ISO 10390:2022; Soil, Treated Biowaste and Sludge–Determination of pH (ISO 10390:2021). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2022.
- LVS ISO 10694:2006; Soil Quality–Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2006.
- LVS ISO 13878:1998; Soil Quality. Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 1999.
- LVS ISO 10693:2014; Soil Quality–Determination of Carbonate Content—Volumetric Method (ISO 10693:1995). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2014.
- LVS ISO 10523:2012; Water Quality–Determination of pH (ISO 10523:2008). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2012.
- LVS EN 27888:1993; Water Quality–Determination of Electrical Conductivity. Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 1999.
- LVS EN ISO 20236:2022; Water Quality–Determination of Total Organic Carbon (TOC), Dissolved Organic Carbon (DOC), Total Bound Nitrogen (TNb) and Dissolved Bound Nitrogen (DNb) after High Temperature Catalytic Oxidative Combustion (ISO 20236:2018). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2022.
- LVS EN 1484:2000; Water Analysis–Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 2000.
- LVS ISO 9964-3:2000; Water Quality–Determination of Sodium and Potassium—Part 3: Determination of Sodium and Potassium by Flame Emission Spectrometry. Ltd. “Latvijas standarts” (LVS): Riga, Latvia, 1999.
- Gill, R.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.R-project.org (accessed on 14 January 2024).
- Pumpanen, J.; Longdoz, B.; Kutsch, W.L. Field measurements of soil respiration: Principles and constraints, potentials and limitations of different methods. In Soil Carbon Dynamics. An Integrated Methodology; Kutsch, W.L., Bahn, M., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 16–33. [Google Scholar]
- Yli-Halla, M.; Lötjönen, T.; Kekkonen, J.; Virtanen, S.; Marttila, H.; Liimatainen, M.; Saari, M.; Mikkola, J.; Suomela, R.; Joki-Tokola, E. Thickness of peat influences the leaching of substances and greenhouse gas emissions from a cultivated organic soil. Sci. Total Environ. 2022, 806, 150499. [Google Scholar] [CrossRef] [PubMed]
- Licite, I.; Lupikis, A. Impact of land use practices on greenhouse gas emissions from agriculture land on organic soils. In Proceedings of the 19th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 20–22 May 2020; pp. 1823–1830. [Google Scholar]
- Elsgaard, L.; Görres, C.-M.; Hoffmann, C.C.; Blicher-Mathiesen, G.; Schelde, K.; Petersen, S.O. Net ecosystem exchange of CO2 and carbon balance for eight temperate organic soils under agricultural management. Agric. Ecosyst. Environ. 2012, 162, 52–67. [Google Scholar] [CrossRef]
- Nieveen, J.P.; Campbell, D.I.; Schipper, L.A.; Blair, I.J. Carbon exchange of grazed pasture on a drained peat soil. Glob. Chang. Biol. 2005, 11, 607–618. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K.; Klemedtsson, L. Plant-derived CO2 flux from cultivated peat soils. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 508–513. [Google Scholar]
- Berglund, Ö.; Kätterer, T.; Meurer, K.H.E. Emissions of CO2, N2O and CH4 from cultivated and set aside drained peatland in Central Sweden. Front. Environ. Sci. 2021, 9, 630721. [Google Scholar] [CrossRef]
- Norberg, L.; Berglund, Ö.; Berglund, K. Seasonal CO2 emission under different cropping systems on Histosols in southern Sweden. Geoderma Regional. 2016, 7, 338–345. [Google Scholar] [CrossRef]
- Van den Pol-van Dasselaar, A. Methane Emissions from Grasslands. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1998. [Google Scholar]
- Lai, D.Y.F. Methane dynamics in Northern peatlands: A review. Pedosphere 2009, 19, 409–421. [Google Scholar] [CrossRef]
- Butlers, A.; Lazdiņš, A.; Kalēja, S.; Purviņa, D.; Spalva, G.; Saule, G.; Bārdule, A. CH4 and N2O emissions of undrained and drained nutrient-rich organic forest soil. Forests 2023, 14, 1390. [Google Scholar] [CrossRef]
- Petersen, S.O.; Hoffmann, C.C.; Schäfer, C.-M.; Blicher-Mathiesen, G.; Elsgaard, L.; Kristensen, K.; Larsen, S.E.; Torp, S.B.; Greve, M.H. Annual emissions of CH4 and N2O, and ecosystem respiration, from eight organic soils in Western Denmark managed by agriculture. Biogeosciences 2012, 9, 403–422. [Google Scholar] [CrossRef]
- Pärn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Q.; Tian, Y.; Sun, W.; Scheer, C.; Li, T.; Zhang, W. Global variations and drivers of nitrous oxide emissions from forests and grasslands. Front. Soil Sci. 2022, 2, 1–10. [Google Scholar] [CrossRef]
- IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014; Available online: https://www.ipcc-nggip.iges.or.jp/public/wetlands/index.html (accessed on 14 January 2024).
- Maljanen, M.; Komulainen, V.M.; Hytonen, J.; Martikainen, P.; Laine, J. Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics. Soil Biol. Biochem. 2004, 36, 1801–1808. [Google Scholar] [CrossRef]
- Schäfer, C.M.; Elsgaard, L.; Hoffmann, C.C.; Petersen, S.O. Seasonal methane dynamics in three temperate grasslands on peat. Plant Soil 2012, 357, 339–353. [Google Scholar] [CrossRef]
- Maljanen, M.; Sigurdsson, B.D.; Guðmundsson, J.; Óskarsson, H.; Huttunen, J.T.; Martikainen, P.J. Greenhouse gas balances of managed peatlands in the Nordic countries—Present knowledge and gaps. Biogeosciences 2010, 7, 2711–2738. [Google Scholar] [CrossRef]
- van Beek, C.L.; Pleijter, M.; Jacobs, C.M.J.; Velthof, G.L.; van Groenigen, J.W.; Kuikman, P.J. Emissions of N2O from fertilized and grazed grassland on organic soil in relation to groundwater level. Nutr. Cycl. Agroecosyst. 2010, 86, 331–340. [Google Scholar] [CrossRef]




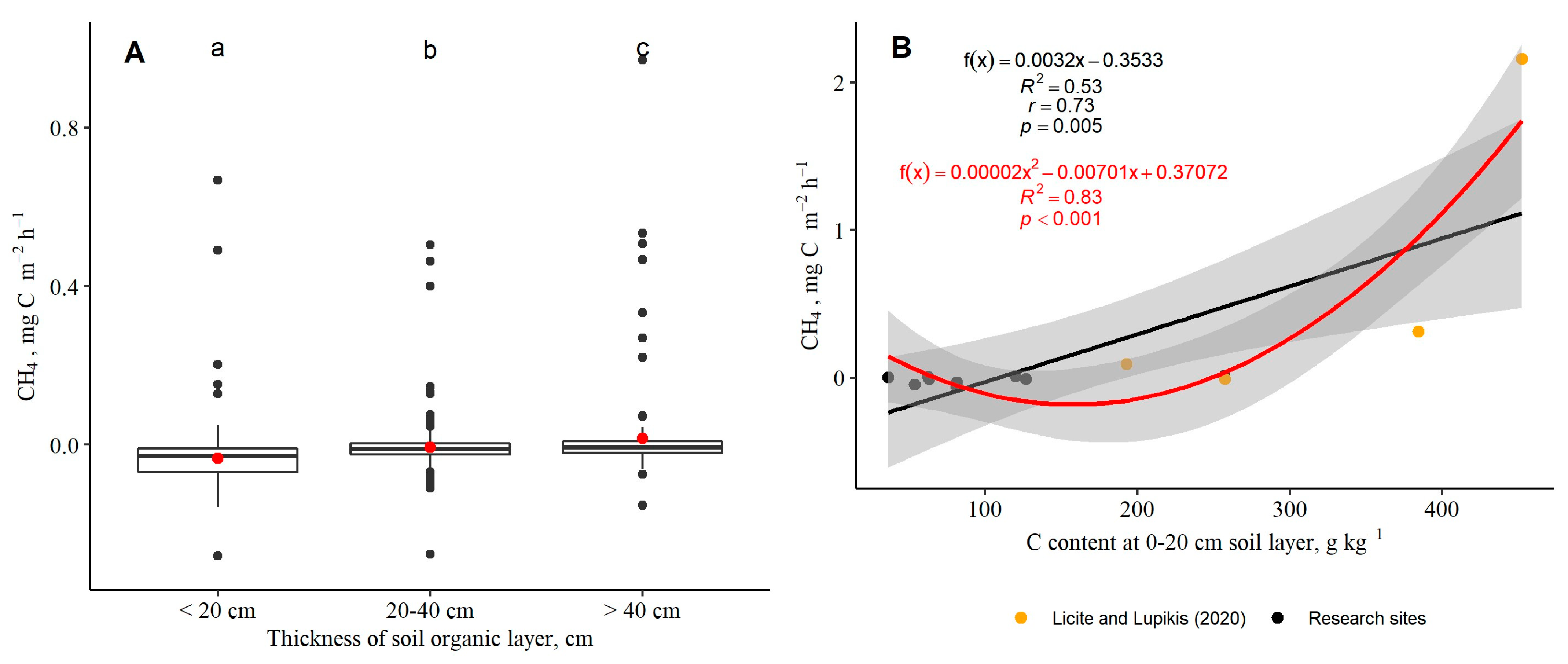
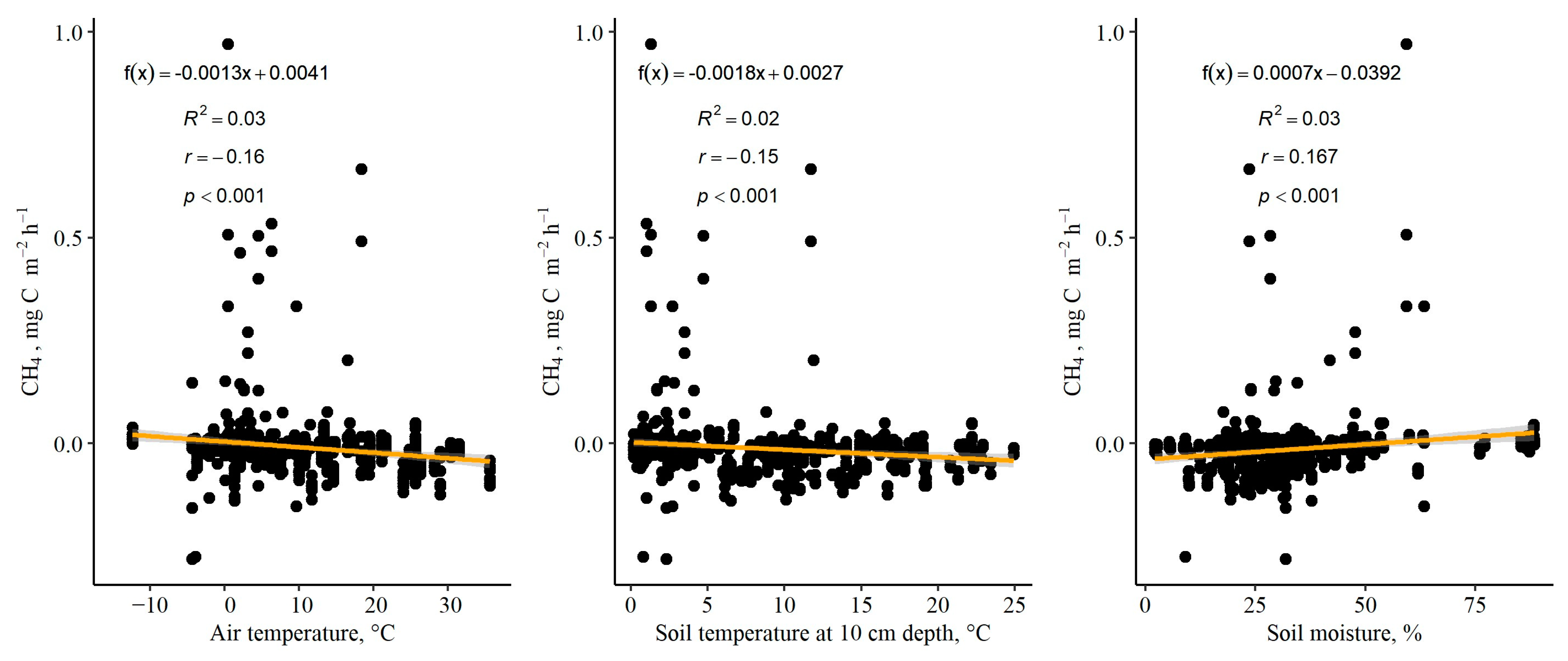
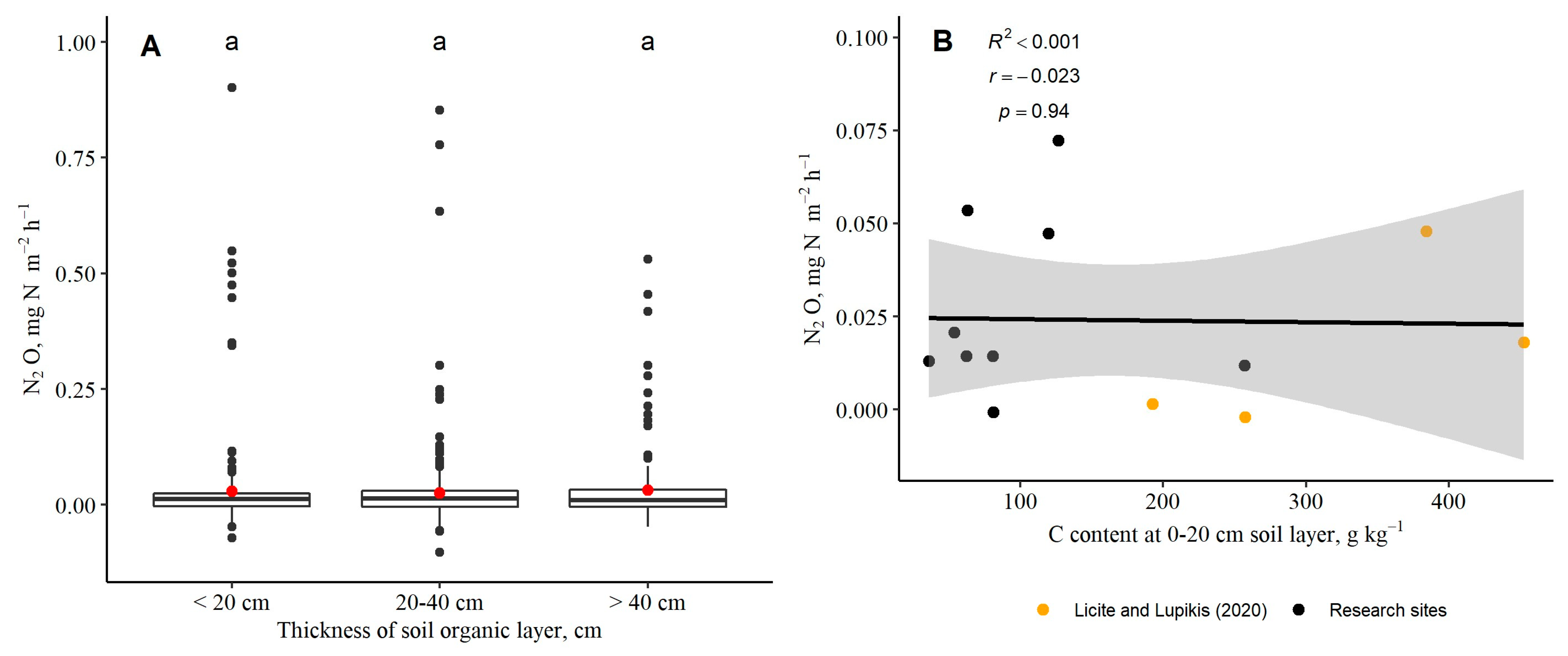

| Research Site (RS), the Dominant Plant Functional Group | Subplot | Thickness of Soil Organic Layer, cm | Mean Groundwater Level ± S.E. (Range), cm | Coordinates of Subplot (WGS84) | |
|---|---|---|---|---|---|
| X | Y | ||||
| RS1, graminoid | A | 15 | 87.6 ± 2.4 (47–118) | 21.18826 | 56.21136 |
| B | 20 | 96.1 ± 2.5 (60–126) | 21.18817 | 56.21148 | |
| C | 30 | 98.8 ± 2.6 (58–127) | 21.18812 | 56.21168 | |
| RS2, graminoid | A | 20 | 55.4 ± 3.1 (0–121) | 22.84421 | 56.55879 |
| B | 40 | 54.8 ± 3.3 (8–125) | 22.84415 | 56.55887 | |
| C | 70 | 27.2 ± 3.8 (0–123) | 22.84395 | 56.55900 | |
| RS3, forbs and graminoid | A | 10 | 89.2 ± 4.0 (0–146) | 24.75648 | 56.77243 |
| B | 15 | 84.2 ± 4.0 (0–144) | 24.75663 | 56.77254 | |
| C | 25 | 75.6 ± 8.8 (16–124) | 24.75687 | 56.77279 | |
| Values | Soil Physico-Chemical Variables (0–20, 20–40 and 40–100 cm Soil Layer) | |||||
|---|---|---|---|---|---|---|
| OC Stock, t ha−1 | TN Stock, t ha−1 | P Stock, t ha−1 | K Stock, t ha−1 | C/N Ratio | pH (KCl) | |
| 0–20 cm soil layer (nsubplots = 9, nsoil samples = 18) | ||||||
| Mean ± S.E. | 132.9 ± 12.2 | 10.7 ± 1.1 | 1.04 ± 0.12 | 3.04 ± 0.69 | 12.8 ± 0.8 | 5.9 ± 0.3 |
| Minimum | 68.5 | 5.5 | 0.65 | 0.98 | 10.6 | 5.0 |
| Maximum | 174.7 | 14.9 | 1.89 | 6.73 | 18.8 | 7.5 |
| 20–40 cm soil layer (nsubplots = 9, nsoil samples = 18) | ||||||
| Mean ± S.E. | 67.7 ± 19.0 | 4.7 ± 1.5 | 0.68 ± 0.09 | 2.91 ± 0.47 | 12.3 ± 1.2 | 6.0 ± 0.3 |
| Minimum | 2.3 | 0.4 | 0.33 | 1.63 | 6.5 | 5.1 |
| Maximum | 167.5 | 11.2 | 1.09 | 5.22 | 18.8 | 8.3 |
| 40–100 cm soil layer (nsubplots = 9, nsoil samples = 27) | ||||||
| Mean ± S.E. | 143.7 ± 37.9 | 3.9 ± 1.7 | 2.14 ± 0.32 | 25.1 ± 11.6 | - | 6.6 ± 0.3 |
| Minimum | 9.9 | 1.1 | 0.62 | 4.58 | - | 5.6 |
| Maximum | 317.9 | 17.4 | 3.50 | 93.5 | - | 8.2 |
| Values | Groundwater Physico-Chemical Variables | |||||
|---|---|---|---|---|---|---|
| pH | TN, mg L−1 | DOC, mg L−1 | K, mg L−1 | Cond., μS cm−1 | DO, mg L−1 | |
| Mean ± S.E. | 7.3 ± 0.1 | 4.48 ± 1.51 | 19.8 ± 2.1 | 2.25 ± 0.74 | 357.6 ± 83.8 | 7.75 ± 0.93 |
| Minimum | 6.9 | 1.54 | 9.4 | 0.41 | 99.5 | 5.40 |
| Maximum | 7.7 | 15.16 | 27.0 | 7.10 | 723.0 | 14.4 |
| Thickness of Organic Soil Layer, cm | Research Site (RS), Subplot | CH4, kg C ha−1 yr−1 | N2O, kg N ha−1 yr−1 | Rtot, t C ha−1 yr−1 | Rhet *, t C ha−1 yr−1 | Cinput **, t C ha−1 yr−1 | Rhet − Cinput, t C ha−1 yr−1 |
|---|---|---|---|---|---|---|---|
| <20 cm | RS1, A | −0.66 | 4.63 | 8.78 | 6.17 | 1.28 | 4.90 |
| RS3, A | −5.03 | 1.27 | 10.09 | 7.09 | 2.01 | 5.08 | |
| RS3, B | −4.09 | 1.81 | 8.85 | 6.22 | 2.62 | 3.60 | |
| 20–40 cm | RS1, B | 0.59 | 1.23 | 7.76 | 5.46 | 1.67 | 3.78 |
| RS1, C | 0.26 | 1.15 | 8.47 | 5.95 | 2.72 | 3.23 | |
| RS2, A | −0.82 | 6.29 | 9.52 | 6.69 | 4.01 | 2.69 | |
| RS3, C | −2.73 | −0.06 | 8.04 | 5.65 | 2.87 | 2.78 | |
| >40 cm | RS2, B | 0.86 | 4.12 | 8.03 | 5.64 | 3.58 | 2.06 |
| RS2, C | 1.07 | 1.03 | 7.42 | 5.21 | 2.01 | 3.21 | |
| <20 cm | Mean | −3.26 ± 1.33 | 2.39 ± 0.70 | 8.55 ± 0.29 | 6.01 ± 0.20 | 2.53 ± 0.30 | 3.48 ± 0.33 |
| 20–40 cm | Mean | −0.68 ± 0.75 | |||||
| >40 cm | Mean | 0.96 ± 0.10 | |||||
| Results from previous study in Latvia conducted in drained grasslands with deep peat (>40 cm) soils *** [41] | 57.8 ± 44.3 | 0.26 ± 0.25 | - | - | - | 4.39 ± 0.87 | |
| IPCC default emission factors for deep-drained, nutrient-rich organic soils in grassland in temperate zone [53] | 12.0 (95% confidence interval 1.8–21.7) | 8.2 (95% confidence interval 4.9–11) | - | - | - | 6.1 (95% confidence interval 5.0–7.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purviņa, D.; Zvaigzne, Z.A.; Skranda, I.; Meļņiks, R.N.; Petaja, G.; Līcīte, I.; Butlers, A.; Bārdule, A. Impact of Soil Organic Layer Thickness on Soil-to-Atmosphere GHG Fluxes in Grassland in Latvia. Agriculture 2024, 14, 387. https://doi.org/10.3390/agriculture14030387
Purviņa D, Zvaigzne ZA, Skranda I, Meļņiks RN, Petaja G, Līcīte I, Butlers A, Bārdule A. Impact of Soil Organic Layer Thickness on Soil-to-Atmosphere GHG Fluxes in Grassland in Latvia. Agriculture. 2024; 14(3):387. https://doi.org/10.3390/agriculture14030387
Chicago/Turabian StylePurviņa, Dana, Zaiga Anna Zvaigzne, Ilona Skranda, Raitis Normunds Meļņiks, Guna Petaja, Ieva Līcīte, Aldis Butlers, and Arta Bārdule. 2024. "Impact of Soil Organic Layer Thickness on Soil-to-Atmosphere GHG Fluxes in Grassland in Latvia" Agriculture 14, no. 3: 387. https://doi.org/10.3390/agriculture14030387
APA StylePurviņa, D., Zvaigzne, Z. A., Skranda, I., Meļņiks, R. N., Petaja, G., Līcīte, I., Butlers, A., & Bārdule, A. (2024). Impact of Soil Organic Layer Thickness on Soil-to-Atmosphere GHG Fluxes in Grassland in Latvia. Agriculture, 14(3), 387. https://doi.org/10.3390/agriculture14030387





