Abstract
Syrphine hoverflies (Diptera: Syrphinae) are important predators of aphids in agricultural crops. While the use of flowering plants to enhance their efficacy is well established, recent research has developed an artificial diet for adult hoverflies consisting of a sugar solution and pollen in a dispenser. To ensure that the artificial diet is suitable to support hoverfly reproduction, a comparative analysis was conducted between a natural diet of flowering buckwheat plants versus an artificial diet consisting of artificial flowers (including honey solution and pollen), complemented by a sugar solution disperser. The study evaluated the fecundity, fertility, oviposition period, egg hatchability, and overall lifespan of the American hoverfly, Eupeodes americanus (Wiedemann 1830). The results indicate that the artificial diet does not negatively impact the reproductive parameters of E. americanus when compared to the buckwheat-based diet. Consequently, artificial diets emerge as a promising and more convenient alternative to flowering plants to support hoverflies in biological control strategies and for their mass rearing in research facilities and commercial insectaries.
1. Introduction
Worldwide, the larvae of hoverflies (Diptera: Syrphidae) in the Syrphinae subfamily are recognized as effective predators of aphids [1,2,3], and their use in aphid biological control has thus been studied extensively [4]. A recent surge in interest has coincided with the commercialization of several species now accessible to growers in Europe, such as Sphaerophoria rueppellii (Wiedemann, 1820) [5,6], Episyrphus balteatus (De Geer, 1776) [7], and Eupeodes corollae (Fabricius, 1794) [8,9]. Additionally, in 2020, the American hoverfly, Eupeodes americanus (Wiedemann, 1830) became the first commercially available hoverfly species in North America [10].
Adult hoverflies act as pollinators and target aphid colonies for oviposition while requiring pollen for egg development and nectar to power their flight [11,12,13,14]. Indeed, pollen is the main source of protein for hoverflies [15], and nectar provides sugars such as sucrose, glucose, and fructose [16]. According to Pinheiro et al. [14], glucose is the main effective carbohydrate source for hoverflies’ survival and energy supply. Furthermore, Hogg et al. [17] have proven that pollen is essential for the fecundity of hoverfly females and therefore for aphid control, as the addition of an artificial nectar solution (1:4 honey to water (v/v) and 0.25% sodium benzoate) alone was not sufficient and was similar to a control without additional resources. Several studies highlight the importance of additional flower sources when using hoverflies in biological control strategies to enhance their longevity, fecundity, and establishment in greenhouse or field crops [17,18,19]. For example, Pineda et Marcos-García [19] demonstrated that the addition of flowering plants in greenhouses increases the presence of native hoverfly populations in the crop, not only at the immature stages (larvae and pupae) but also at the adult stage, compared to a control greenhouse without flowering plants. This therefore indicates that this conservation biological control strategy is an effective method. In parallel, Hogg et al. [17] also demonstrated that the addition of flowering plants enhances aphid biocontrol compared to a control when hoverflies are introduced into crops. Due to their short mouthparts, it is crucial to select flowers with easily accessible pollen and nectar such as actinomorphic plants with flat corollae (e.g., Apiaceae, Asteraceae, Ranunculaceae, and Rosaceae) [20,21]. While many studies have used sweet alyssum, Lobularia maritima L. (Brassicaceae), as a flowering resource for hoverflies [17,19,22], others investigated buckwheat, Fagopyrum esculentum L. (Polygonaceae) [22,23,24,25]; yarrow, Achillea millefolium (L.) (Asteraceae) [26]; fennel, Foeniculum vulgare (Miller) (Apiaceae) [26]; Korean licorice mint, Agastache rugosa (Fisch. & C.A.Mey.) (Lamiaceae) [26]; field mint, Mentha arvensis L. and field forget-me-not, Myosotis arvensis (L.) Hill (Boraginaceae) [18]; basil, Ocimum basilicum L. (Lamiaceae) [23]; and coriander, Coriandrum sativum L. (Apiaceae) [18,19,25,26].
Concurrently, the use of artificial diets or supplemental food sources, which include sugar solutions and commercially collected pollen, for adult hoverflies is increasingly applied in mass rearing facilities. Since 2014, a method using artificial flowers (including honey solution and pollen) combined with a sugar solution disperser as an artificial adult diet has been employed in the rearing of E. americanus at the biological control laboratory of Université du Québec à Montréal (UQAM). This type of artificial diet has also been successfully used in several research experiments both in the laboratory and commercial greenhouses [10,24,27,28,29]. Unfortunately, there is currently no commercial version of such a system for greenhouse producers, who primarily rely on the installation of flowering plants. Considering that a commercial supplemental food source is available for bumblebees in the form of a sugar solution container, Biogluc® (Biobest) [30], exploring the development of a similar solution with added pollen for hoverflies could improve their efficiency in greenhouses, saving time and labor for both producers and insect mass rearing facilities. In order to achieve this, it is crucial to ensure that the use of an artificial diet does not negatively impact the reproductive and predatory behaviors of hoverflies compared to a regular flowering plant diet. These factors are essential for an effective biological control program and for the successful mass-rearing of hoverflies [31,32]. Two studies have already demonstrated that the predation parameters and the effectiveness of hoverflies in greenhouses align with the artificial diet for adults [10,24] but have not directly compared this to fresh pollen using flowering plants. However, Arcaya et al. [33] and Sadeghi and Gilbert [34] imply that fresh pollen may significantly improve hoverfly reproduction and longevity compared to collected pollen.
The aim of the present study is therefore to compare, for the first time, the effect of a diet based on flowering plants versus an artificial adult diet on the reproductive parameters of the hoverfly, E. americanus. For this purpose, buckwheat, known for its rapid growth [35] and great potential as a hoverfly insectary plant [22], will be compared with artificial flowers (including honey solution and pollen), complemented by a sugar solution disperser.
2. Materials and Methods
2.1. Plants
All plants were cultivated in the experimental greenhouses of Université du Québec à Montréal (UQAM). The environmental conditions were maintained at 25 °C during the day, 19 °C at night, and 60% RH and 16:8 (L:D) facilitated by high-pressure sodium lamps (High-Pressure Sodium HID Light Bulbs, Model LucaloxTM PSL750 W, GE®, Cleveland, OH, USA). Buckwheat (Semences du Portage®, Montréal, QC, Canada); broad bean plants, Vicia faba L. (Fabaceae); (Norseco, Laval, QC, Canada) along with cucumber seedlings, Cucumis sativus L., 1753 (Cucurbitaceae) (cv. hybrid Speedway, Norseco, Laval, QC, Canada), were individually transplanted in plastic pots (9 × 9 cm) (Kord Products®, Burlington, ON, Canada). In parallel, barley, Hordeum vulgare L. (Poaceae) (Sollio Agriculture, Montréal, QC, Canada), was sown in 13 cm × 13 cm plastic pots. The substrate used was a humus-containing potting mix enriched with compost (Garden soil, Scotts Fafard, Saint-Bonaventure, QC, Canada). The plants were watered as needed and provided weekly with a balanced fertilizer (20–20–20 NPK). Throughout the growth period, no chemical insecticides were applied to the plants. The germination rate (seeds germinated/total seeds × 100) and the time taken for buckwheat to flower (days from sowing to first appearance of open flowers) were calculated across five different batches consisting of 14–21 seeds each, sown between March and May 2022.
2.2. Insect Rearing
All insect colonies were maintained at the UQAM in the biocontrol laboratory. Melon aphids (Aphis gossypii, Glover) were reared on cucumber in a 35 × 35 × 35 cm cage kept in a growth chamber (Conviron™, Model E15, Winnipeg, MB, Canada) at 24 °C, with a 16:8 (L:D) photoperiod and 70% relative humidity (RH).
Eupeodes americanus were collected in 2014 on Phlox sp. in Sainte-Agathe-de-Lotbinière (N 46°23′726″, W 71°21′446″), QC, Canada. Based on around 100 individuals, a standardized rearing protocol was established in the UQAM in the biocontrol laboratory. This rearing approach of E. americanus has already been described by Bellefeuille et al. [27], but it has since been improved. Adults were kept in an 81 × 53 × 60 cm rearing cage covered in muslin which was kept in a controlled environment room at 22 °C with a 16:8 (L:D) photoperiod and at 60% RH. Adults were fed the artificial diet, the composition of which is detailed in Section 2.3. For oviposition purposes, four broad bean plants infested with pea aphids (Acyrthosiphon pisum (Harris), Hemiptera: Aphididae) were placed within the adult rearing cage. These infested plants were systematically replaced twice weekly to ensure continuous availability for oviposition. Plants bearing eggs were subsequently relocated to a smaller cage measuring 35 × 35 × 35 cm and were kept in the same controlled room for three days to facilitate egg hatching. Following this period, the larvae were collected using a fine brush and transferred to barley plants, which were infested with bird cherry-oat aphids (Rhopalosiphum padi L., Hemiptera: Aphididae), to support their development. Larvae rearing was conducted within cages of identical dimensions to those used for egg incubation, situated in a growth chamber at 24 °C, with a 16L:8D photoperiod (800 Series 32 Watt fluorescent bulbs, F32T8/TL841, Philips, Amsterdam, Netherlands) and 70% relative humidity (RH). In each larval rearing cage, six plastic pots containing barley were inoculated with 30 to 60 larvae of E. americanus maximally. Barley plants were watered as needed to maintain soil moisture, which is crucial as it serves as the pupation site for E. americanus larvae. Upon completion of the pupal stage, newly emerged adults were collected and reintroduced into the previously described adult rearing cage. This rearing protocol was designed to be continuous throughout the year, with an annual refreshment of the rearing population with new wild individuals, when feasible, to preserve adequate genetic diversity within the population of E. americanus.
The adults of E. americanus used in this experiment were newly emerged individuals, collected on their first day after pupal emergence.
2.3. Experimental Design
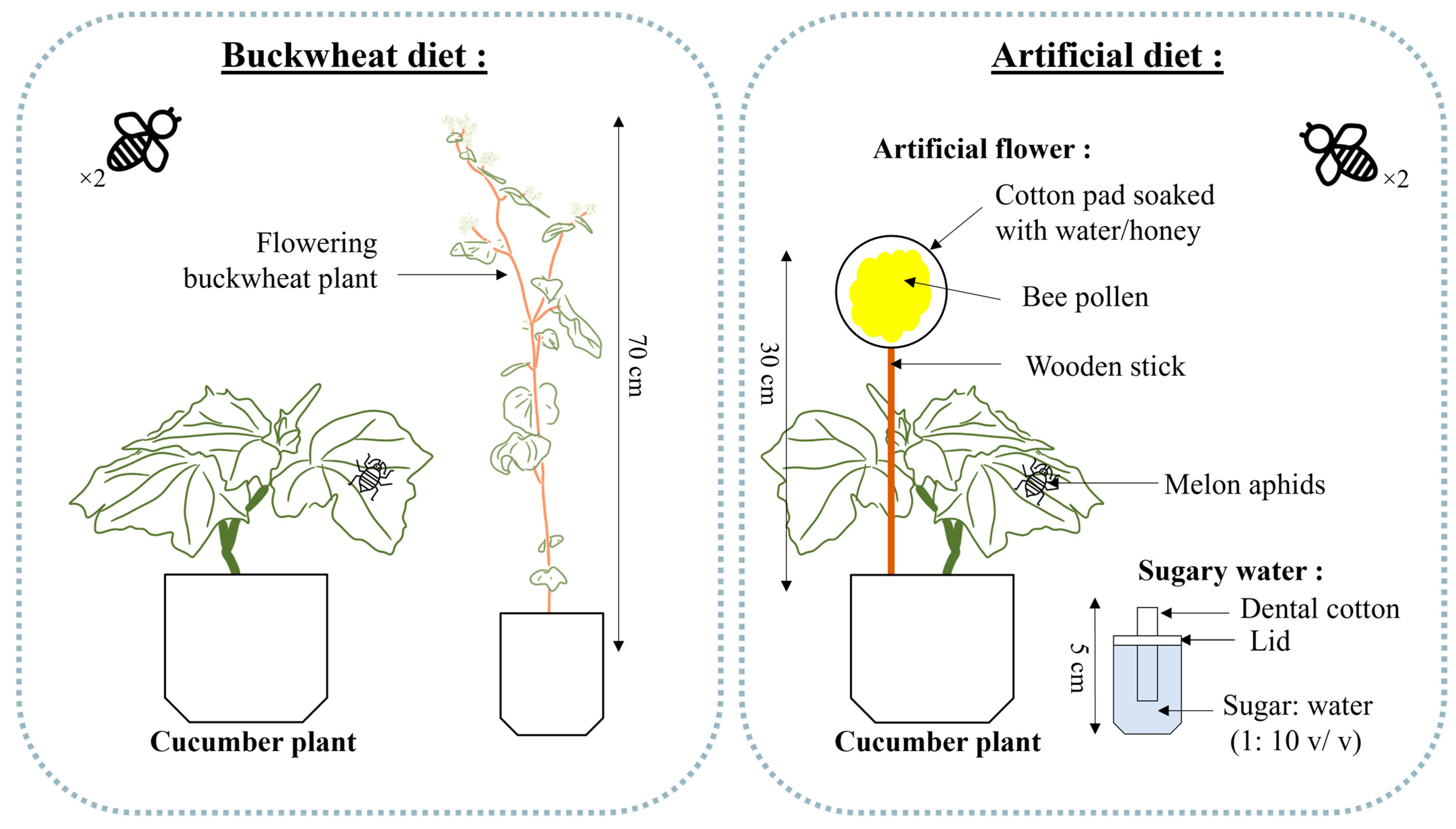
All experiments were conducted in a growth chamber (Conviron™, Model E15, Winnipeg, MB, Canada) at 24 °C, with a 16:8 (L:D) photoperiod and 70% RH. The adult diet treatments included one with buckwheat as the flowering plant and one with an artificial flower and sugar water (sucrose, e.g., granulated sugar) (1:10 v/v) as an artificial diet. Buckwheat plants were approximately 70 cm tall and bore around fifty flowers (Figure 1). The artificial flowers consisted of a wooden stick inserted inside a round cotton pad, soaked in a mixture of water and honey (3:1 v/v), and covered with 1.5 g of ground commercially collected pollen (Miel Gauvin Inc., Saint-Hyacinthe, QC, Canada). Honey was chosen due to its high fructose and glucose content [36], essential for meeting the glucose requirements of hoverflies [13,14]. Moreover, the composition of the pollen used came from a field in Manitoba, Canada, consisting of a mixture of canola and alfalfa, clovers, and other wild plants and trees found in the vicinity of the beehives. The sugar water was separated from the artificial flower and placed in a solo cup with a dental cotton roll protruding from the lid (Figure 1) [29].
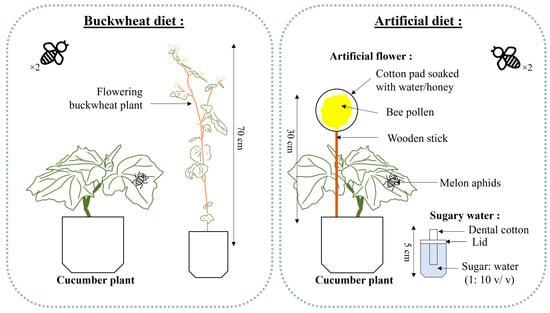
Figure 1.
Buckwheat diet treatment (left) and artificial diet treatment composed of one artificial flower and one sugar water disperser (right) for feeding a pair of the American hoverfly, E. americanus (modified from Gonzalez et al. [29]).
Experimental units consisted of wooden cages (91 × 91 × 61 cm) covered with an insect-proof screen and equipped with a corrugated plastic panel base (Coroplast). A door fastened by a hook and loop tape provided access to plants. In each cage, a cucumber plant with 4 leaves was manually inoculated with 100 A. gossypii of mixed developmental stages, using a brush for precise transfer. Depending on the treatment, one buckwheat plant was added per cage or one artificial flower was inserted into the cucumber pot in the cage, and one cup of sugar water was placed beside it on the ground (Figure 1). The buckwheat was changed as needed when the flowers wilted, and the artificial flower was replaced when it dried out, about twice a week. The sugar water cup was changed at the same time as the artificial flower, even though it did not dry out or empty as quickly. In the experimental units, the plants were watered twice a week. To facilitate watering while preventing hoverflies from drowning, a common occurrence when they encounter open water, a piece of muslin cloth was placed in each water-receiving pot.
After emergence, one male and one female of E. americanus (less than 24 h old) were immediately introduced in each experimental unit, as described above. Observations were made twice a week and the experiment continued until the death of both hoverfly adults. The cucumber plant was changed with a new infested plant twice a week for oviposition. The old cucumber plants were maintained in the laboratory for an additional two days prior to the egg count corresponding to the time necessary for the hatching of the eggs [37]. The lifetime fecundity of E. americanus was determined as the total of all the eggs laid per female. To achieve this, all aerial parts of cucumber plants were inspected under a stereo microscope, and the eggs were classified into three distinct categories: hatched eggs, unhatched eggs, and cannibalized eggs [28]. The proportion of eggs within each category was determined by dividing the number of successfully hatched, unhatched, or cannibalized eggs by the total number of eggs laid by females. Given the unreliability of larval counts due to their mobility and ability to hide, the fertility per female was determined as the number of hatched eggs which corresponded to the number of larvae produced per female (with each egg yielding exactly one larva). The date of the last oviposition was noted, and the oviposition period (the period between the first and the last oviposition) was determined in days. Then, the daily fecundity of E. americanus females was determined by dividing the lifetime fecundity by the oviposition period. Finally, male and female longevity was determined as the period between the adults’ emergence and their death. Fifteen replicates were performed per adult diet treatment (15 replicates for the buckwheat diet and 15 for the artificial diet).
2.4. Data Analysis
Statistical analyses were carried out using the statistical software R, version 4.0.5 [38]. The daily and lifetime fecundity and the fertility of E. americanus were tested with a nonparametric Wilcoxon test. Subsequently, we used a Z-test to analyze differences in egg hatchability between treatments. Separate analyses were performed for each of the three distinct categories: the proportion of hatched, unhatched, and cannibalized eggs. Prior to conducting these analyses, assumptions for parametric analyses (normality and homoscedasticity) were checked following a Shapiro–Wilk test of normality (p > 0.05) and with the inspection of diagnostic plots (residuals vs. fitted, normal QQ plot, scale location, constant leverage). To assess E. americanus adult longevity, Kaplan–Meier survival curves were generated, and a Log-Rank test was employed to assess the differences among four groups categorized by adult diet treatments (either buckwheat or artificial diet) and gender (male or female). The statistical analysis involved multiple comparisons, facilitated by R packages specifically designed for survival analysis such as ‘survival’ [39] and ‘survminer’ [40]. For each test, the significance level was set at alpha = 0.05.
3. Results
Buckwheat showed a mean germination rate of 71.43 ± 11.17%. Additionally, the mean duration required for a buckwheat plant to transition from the sowing stage to the initial day of flowering was 33.20 ± 1.20 days.
3.1. Effect of Diets on Female Fecundity, Fertility, and Oviposition Period
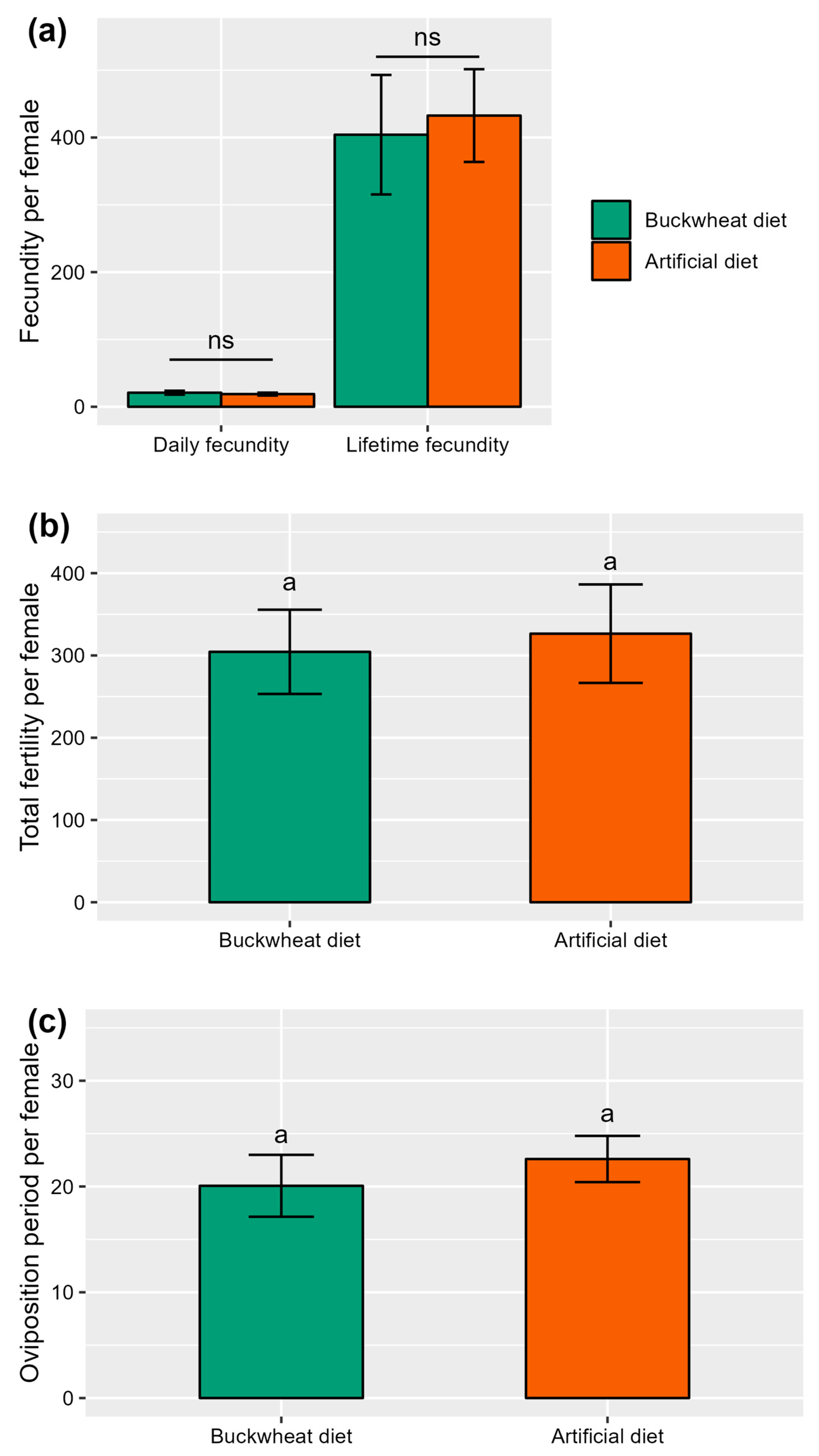
Throughout the duration of the study, it was observed that all females of the American hoverfly, E. americanus, across both diet treatment groups successfully laid eggs. The majority of females continued laying eggs until death. The daily fecundity of female hoverflies was observed to be 18.83 ± 2.71 eggs for those on the artificial diet and 20.92 ± 2.59 eggs for those fed with the buckwheat diet. Similarly, the lifetime fecundity of female hoverflies reached 432.60 ± 88.76 eggs for the artificial diet and 404.20 ± 68.89 eggs for the buckwheat diet. These differences in both daily and lifetime fecundity between the diet treatments were not statistically significant (respectively, W = 89, p = 0.35 and W = 114, p = 0.97) (Figure 2a). The fertility (i.e., total number of hatched eggs) of female hoverflies also showed no significant variation between the diet treatments, with 326.47 ± 59.85 hatched eggs for the artificial diet and 304.40 ± 51.19 for the buckwheat diet (W = 114, p = 0.97) (Figure 2b). Finally, there was no difference between the treatments in the oviposition period of female hoverflies, with averages of 22.60 ± 2.19 days for the artificial diet and 20.07 ± 2.93 days for the buckwheat diet (W = 135.5, p = 0.35) (Figure 2c).
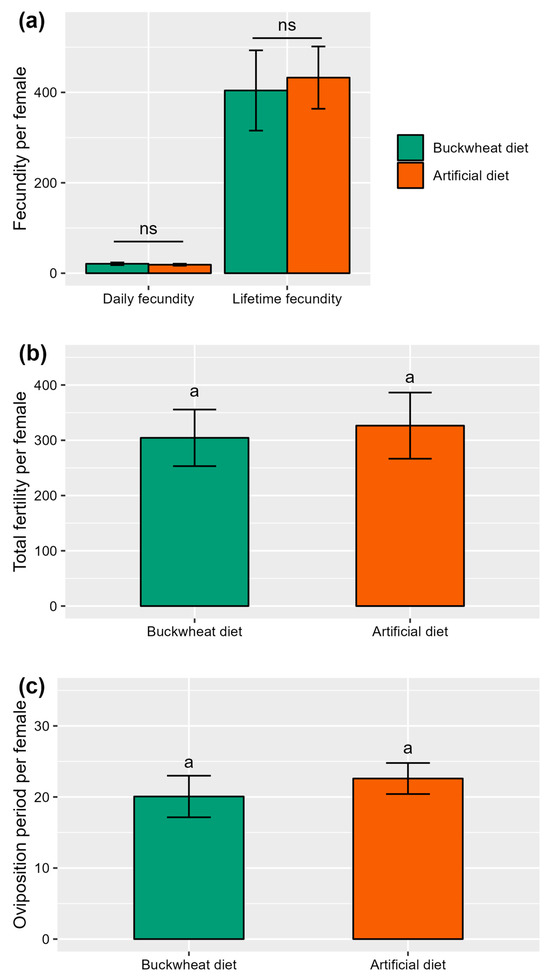
Figure 2.
Oviposition of E. americanus females under different adult diets (i.e., buckwheat or artificial diet), (a) daily and lifetime fecundity (i.e., number of eggs laid), (b) total fertility (i.e., number of hatched eggs), and (c) oviposition period in days (n = 15). The letters indicate the significant differences with an alpha = 0.05 (Wilcoxon test), “ns” indicate non-significant differences. The lines represent the mean ± SE.
The relative percentage of E. americanus eggs that successfully hatched was similar between the diet treatments (z = −0.20, p = 0.84) (Table 1). Conversely, the percentage of unhatched eggs was significantly lower in the group fed with a buckwheat diet compared to those on an artificial diet (z = −2.88, p = 0.0039). Finally, the percentage of cannibalized eggs was significantly lower with an artificial diet than a buckwheat diet (z = 4.01, p < 0.001).

Table 1.
Fate of eggs depending on E. americanus adult diets used (buckwheat or artificial diet): hatched, unhatched, or cannibalized eggs. The letters indicate the significant differences between adult diets with an alpha = 0.05 (Z-test).
3.2. Effect of Diets on Adults’ Longevity
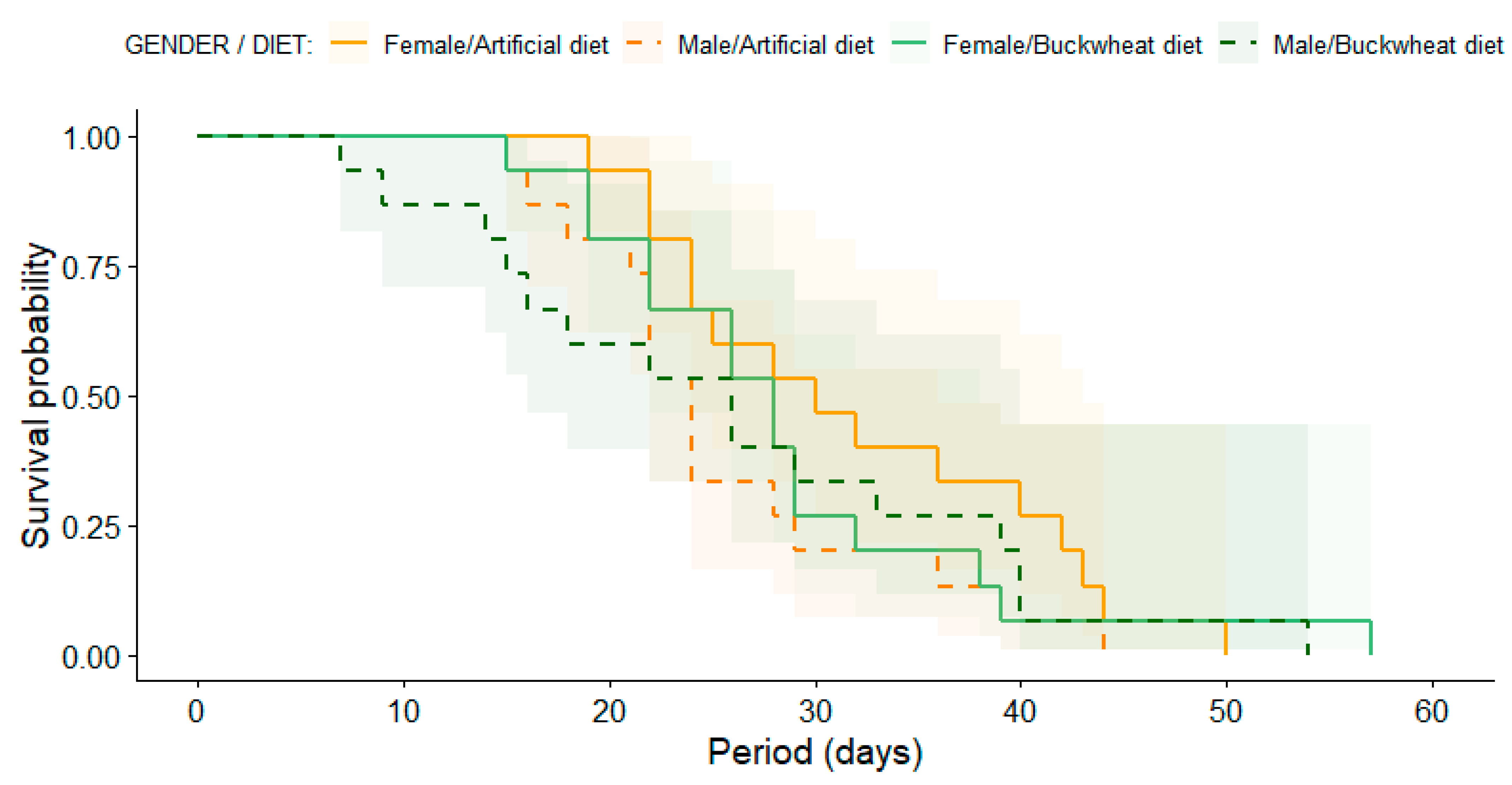
The analysis of E. americanus adult longevity, employing Kaplan–Meier survival curves, revealed no statistically significant differences between treatment (diets) and gender (male and female) (Log-Rank test: χ2 = 2.6, df = 3, p = 0.50) (Figure 3). Indeed, male hoverflies had a mean lifetime longevity of 25.9 days, with the shortest lifespan recorded at 7 days and the longest at 54 days, when fed with a buckwheat diet. Conversely, when provided with an artificial diet, the mean male longevity was 25.6 days, with lifespans ranging from a minimum of 15 days to a maximum of 44 days. Female E. americanus hoverflies had a mean lifetime longevity of 28.6 days (min: 15 and max: 57 days) with a buckwheat diet and 32.1 days (min: 19 and max: 50 days) with an artificial diet.
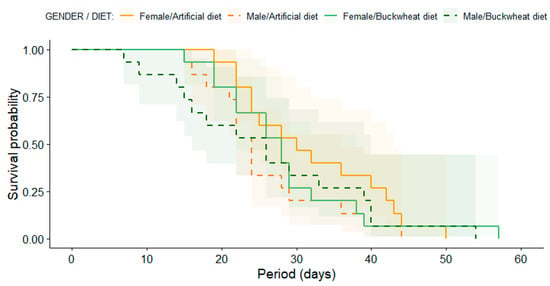
Figure 3.
Kaplan–Meier survival curves of females (solid lines) and males (dashed lines) E. americanus reared under artificial diet (orange colors) and buckwheat diet (green colors). Shade-colored areas indicate a 95% confidence interval computed from the medians of Kaplan–Meier survival curves. There were no significant differences among genders or treatments.
4. Discussion
Some studies have suggested that fresh pollen from flowering plants might play a significant role in hoverfly reproduction and longevity compared to commercially collected pollen [33,34], but as yet, nobody had tested this. Our study is therefore the first to directly compare the effect of an artificial diet versus a flowering plant on hoverfly performance. It is also the first to study different diets for the American hoverfly, E. americanus, a Nearctic hoverfly species which is poorly studied as a biocontrol agent.
The results showed that the use of an artificial diet does not affect their reproductive parameters. Indeed, the fecundity, fertility, oviposition period, and longevity of adult E. americanus were similar between the artificial adult diet and a diet of flowering buckwheat plants. Moreover, egg hatchability also did not appear to be negatively affected by the artificial diet, as indicated by the very slight differences recorded (unhatched and cannibalized eggs), coupled with a consistently high hatching rate of approximately 75% across both diet treatments. The artificial diet for adult hoverflies therefore holds important potential for use in greenhouses by producers or in laboratory and commercial rearing facilities. This greenhouse application was already confirmed in Canada by Bellefeuille et al. [10]. In their study, they tested the effectiveness of the American hoverfly, E. americanus, against the foxglove aphid, Aulacorthum solani (Kaltenbach 1843) (Hemiptera: Aphididae), in a commercial spring greenhouse and introduced the hoverflies via banker plants in association with the same artificial diet tested in our study. They achieved very encouraging results, with the control of the aphids within six weeks thanks to the hoverflies. Similarly, in Europe, Leman et al. [24] also demonstrated that hoverflies were effective in experimental greenhouses against aphids even when nourished with the addition of sugars and commercially collected pollen. These studies collectively reinforce our findings, highlighting the significant potential of this artificial diet.
The use of flowering plants as a food source for hoverflies in biological control strategies and in mass rearing facilities is effective but requires time (planting, time before flowering), labor (setup and watering), resources (compost, pots, fertilizer, grow lights), and space in the greenhouse. The buckwheat plant is considered a fast-growing species, with a short time from sowing to flowering [35]. In our study, the duration from sowing to its first day of flowering still took 33.2 days, which is in agreement with previous research (30–33 days; [22]). Moreover, flowering plants can not only be a habitat for beneficial insects but also for pests [41]. Specifically, buckwheat is even used as a trap crop for thrips [42], and A. gossypii has been frequently found on it [43]. Thus, selecting a flowering plant with fewer disadvantages, like sweet alyssum, already widely used in greenhouses and orchards [19,22], is advisable. Consequently, an artificial alternative to these plants seems compelling. Indeed, Leman et al. [24] suggest that it has the potential to decrease the likelihood of promoting flower thrips compared to flowering plants, although this hypothesis needs to be tested.
In our study, the artificial flowers used still required regular replacement (two times per week) as they dried out. Hence, it is crucial to develop a commercially viable version that is easy to use and long-lasting (requiring minimal labor). Furthermore, the inclusion of glucose in our sugar water mixture is potentially crucial for replacing honey water in our system. Indeed, van Rijn et al. [13] found that the longevity of hoverflies was comparable when fed a diet of either honey or glucose, but sucrose alone significantly decreases the longevity of females. In Europe, the version proposed by Leman et al. [24] seems promising and addresses the issue we encountered. It involves a plastic bottle with a wick on the lid, filled with the same sugar solution that is used as an artificial diet for bumblebees (Biogluc®, containing different sugars: fructose (37.5%), glucose (34.5%), sucrose (25%), maltose (2%), and oligosaccharides (1%) [44]). Around the bottle, a yellow plastic hollow ring filled with commercially collected pollen is inserted [24].
5. Conclusions
This study confirms the viability of using an artificial diet for E. americanus adult feeding without diminishing their reproductive parameters and acts similarly to a flowering buckwheat plant. These findings emphasize the potential for artificial diets to not only simplify and enhance the implementation of hoverflies in biocontrol strategies but also to address some of the challenges associated with the often lengthy and costly process of hoverfly laboratory or commercial mass rearing. They also highlight the need to develop a marketable version in Canada, similar to the one studied in Europe. Additionally, further studies should optimize the nutritional composition of artificial diets to cater to specific hoverfly species. The impact of adding floral resources, whether through flowering plants or artificial diets, must also be studied in the future to verify whether their use will hinder the utilization of hoverflies for pollination and yield enhancement purposes, as observed in several studies [8,45].
Author Contributions
Conceptualization, methodology, E.L., R.B., M.F. and N.G.; investigation, formal analysis, writing—original draft preparation, N.G.; writing—review and editing, N.G., E.L., R.B. and M.F; supervision and funding acquisition, E.L. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Applied Bio-nomics Ltd., and by the Government of Canada under the Canadian Agricultural Partnership’s AgriScience Program, a federal, provincial, and territorial initiative.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
We thank Maxime Lamonde-Dubé for her help conducting the experiment and Dylan Hodgkiss for his help, especially for English corrections. We also thank the entire team from the ‘Laboratoire de Lutte Biologique’ (UQAM).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gilbert, F.S. Hoverflies, 2nd ed.; Richmond Publishing Company: Slough, UK, 1993; ISBN 978-0-85546-255-0. [Google Scholar]
- Rotheray, G.E. Colour Guide to Hoverfly Larvae, Diptera, Syrphidae in Britain and Europe; Whiteley: Derek, UK, 1993. [Google Scholar]
- Rojo, S. A World Review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and Their Prey = Revisión Mundial de Los Sírfidos Depredadores (Diptera, Syrphidae:Syrphinae) y Sus Presas; Centro Iberoamericano de la Biodiversidad (CIBIO): Alicante, Spain, 2003; ISBN 978-84-600-9854-6. [Google Scholar]
- Rodríguez-Gasol, N.; Alins, G.; Veronesi, E.R.; Wratten, S. The Ecology of Predatory Hoverflies as Ecosystem-Service Providers in Agricultural Systems. Biol. Control 2020, 151, 104405. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Prey Availability and Abiotic Requirements of Immature Stages of the Aphid Predator Sphaerophoria rueppellii. Biol. Control 2012, 63, 17–24. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Feeding Preferences of the Aphidophagous Hoverfly Sphaerophoria rueppellii Affect the Performance of Its Offspring. BioControl 2014, 59, 427–435. [Google Scholar] [CrossRef]
- Pineda, A.; Marcos-García, M.A. Evaluation of Several Strategies to Increase the Residence Time of Episyrphus balteatus (Diptera, Syrphidae) Releases in Sweet Pepper Greenhouses. Ann. Appl. Biol. 2008, 152, 271–276. [Google Scholar] [CrossRef]
- Pekas, A.; De Craecker, I.; Boonen, S.; Wäckers, F.L.; Moerkens, R. One Stone; Two Birds: Concurrent Pest Control and Pollination Services Provided by Aphidophagous Hoverflies. Biol. Control 2020, 149, 104328. [Google Scholar] [CrossRef]
- Moerkens, R.; Boonen, S.; Wäckers, F.L.; Pekas, A. Aphidophagous Hoverflies Reduce Foxglove Aphid Infestations and Improve Seed Set and Fruit Yield in Sweet Pepper. Pest Manag. Sci. 2021, 77, 2690–2696. [Google Scholar] [CrossRef]
- Bellefeuille, Y.; Fournier, M.; Lucas, E. Biological Control of the Foxglove Aphid Using a Banker Plant with Eupeodes americanus (Diptera: Syrphidae) in Experimental and Commercial Greenhouses. Biol. Control 2021, 155, 104541. [Google Scholar] [CrossRef]
- Schneider, F. Beitrag Zur Kenntnis Der Generationsverhältnisse Und Diapause Räuberischer Schwebfliegen (Syrphidae, Dipt.). Mitt. Schweiz. Entomol. Ges. 1948, 21, 249–285. [Google Scholar]
- Haslett, J.R. A Photographic Account of Pollen Digestion by Adult Hoverflies. Physiol. Entomol. 1983, 8, 167–171. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Kooijman, J.; Wäckers, F. The Impact of Floral Resources on Syrphid Performance and Cabbage Aphid Biological Control. IOBCwprs Bull. 2006, 29, 149–152. [Google Scholar]
- Pinheiro, L.A.; Torres, L.M.; Raimundo, J.; Santos, S.A.P. Effects of Pollen, Sugars and Honeydew on Lifespan and Nutrient Levels of Episyrphus balteatus. BioControl 2015, 60, 47–57. [Google Scholar] [CrossRef]
- Haslett, J.R. Interpreting Patterns of Resource Utilization: Randomness and Selectivity in Pollen Feeding by Adult Hoverflies. Oecologia 1989, 78, 433–442. [Google Scholar] [CrossRef]
- Lenaerts, M.; Pozo, M.I.; Wäckers, F.; Van den Ende, W.; Jacquemyn, H.; Lievens, B. Impact of Microbial Communities on Floral Nectar Chemistry: Potential Implications for Biological Control of Pest Insects. Basic Appl. Ecol. 2016, 17, 189–198. [Google Scholar] [CrossRef]
- Hogg, B.N.; Nelson, E.H.; Mills, N.J.; Daane, K.M. Floral Resources Enhance Aphid Suppression by a Hoverfly. Entomol. Exp. Appl. 2011, 141, 138–144. [Google Scholar] [CrossRef]
- Hodgkiss, D.; Brown, M.J.F.; Fountain, M.T. The Effect of Within-Crop Floral Resources on Pollination, Aphid Control and Fruit Quality in Commercial Strawberry. Agric. Ecosyst. Environ. 2019, 275, 112–122. [Google Scholar] [CrossRef]
- Pineda, A.; Marcos-García, M.Á. Use of Selected Flowering Plants in Greenhouses to Enhance Aphidophagous Hoverfly Populations (Diptera: Syrphidae). Ann. Société Entomol. Fr. NS 2008, 44, 487–492. [Google Scholar] [CrossRef]
- Branquart, E.; Hemptinne, J.-L. Selectivity in the Exploitation of Floral Resources by Hoverflies (Diptera: Syrphinae). Ecography 2000, 23, 732–742. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Wäckers, F.L. Nectar Accessibility Determines Fitness, Flower Choice and Abundance of Hoverflies That Provide Natural Pest Control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Irvin, N.A.; Pierce, C.; Hoddle, M.S. Evaluating the Potential of Flowering Plants for Enhancing Predatory Hoverflies (Syrphidae) for Biological Control of Diaphorina citri (Liviidae) in California. Biol. Control 2021, 157, 104574. [Google Scholar] [CrossRef]
- Kakimoto, K.; Matsuhira, K.; Inoue, H.; Nakasima, A.; Ito, Y.; Abe, J.; Ohta, I.; Mizutani, N.; Ohno, K. Effectiveness of conservation biological control against the cotton aphid Aphis gossypii Glover in okra fields I. Attractiveness of beneficial hoverflies by some varieties or species of basil Ocimum basilicum L. Annu. Rep. Kansai Plant Prot. Soc. 2016, 58, 41–44. [Google Scholar] [CrossRef][Green Version]
- Leman, A.; Mouratidis, A.; Pijnakker, J.; Vervoorn, K.; Wäckers, F.; Messelink, G.J. Sugar and Pollen Supply Enhances Aphid Control by Hoverflies in Strawberry. Biol. Control 2023, 186, 105347. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wratten, S.D.; Hemptinne, J.-L. The Contribution of Potential Beneficial Insectary Plant Species to Adult Hoverfly (Diptera: Syrphidae) Fitness. Biol. Control 2012, 61, 1–6. [Google Scholar] [CrossRef]
- Colley, M.R.; Luna, J.M. Relative Attractiveness of Potential Beneficial Insectary Plants to Aphidophagous Hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Bellefeuille, Y.; Fournier, M.; Lucas, E. Evaluation of Two Potential Biological Control Agents Against the Foxglove Aphid at Low Temperatures. J. Insect Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Gonzalez, N.; Buitenhuis, R.; Lucas, E. Spotlight on Eupeodes americanus: Oviposition and Fertility under HPS- and Full Spectrum LED-Extended Photoperiod in Northern Greenhouses. Biol. Control 2023, 187, 105382. [Google Scholar] [CrossRef]
- Gonzalez, N.; Fauteux, A.; Louis, J.-C.; Buitenhuis, R.; Lucas, E. Oviposition Preference of the American Hoverfly, Eupeodes americanus, between Banker Plants and Target Crops. Insects 2023, 14, 295. [Google Scholar] [CrossRef]
- Billiet, A.; Meeus, I.; Van Nieuwerburgh, F.; Deforce, D.; Wäckers, F.; Smagghe, G. Impact of Sugar Syrup and Pollen Diet on the Bacterial Diversity in the Gut of Indoor-Reared Bumblebees (Bombus terrestris). Apidologie 2016, 47, 548–560. [Google Scholar] [CrossRef]
- Soleyman-Nezhadiyan, E.; Laughlin, R. Voracity of Larvae, Rate of Development in Eggs, Larvae and Pupae, and Flight Seasons of Adults of the Hoverflies Melangyna viridiceps Macquart and Symosyrphus grandicornis Macquart (Diptera: Syrphidae). Aust. J. Entomol. 1998, 37, 243–248. [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. What Makes a Successful Biocontrol Agent? A Meta-Analysis of Biological Control Agent Performance. Biol. Control 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Arcaya, E.; Pérez-Bañón, C.; Mengual, X.; Zubcoff-Vallejo, J.J.; Rojo, S. Life Table and Predation Rates of the Syrphid Fly Allograpta exotica, a Control Agent of the Cowpea Aphid Aphis craccivora. Biol. Control 2017, 115, 74–84. [Google Scholar] [CrossRef]
- Sadeghi, H.; Gilbert, F. The Effect of Egg Load and Host Deprivation on Oviposition Behaviour in Aphidophagous Hoverflies. Ecol. Entomol. 2000, 25, 101–108. [Google Scholar] [CrossRef]
- Radics, L.; Mikóházi, D. Principles of Common Buckwheat Production. Eur. J. Plant Sci. Biotechnol. 2010, 4, 57–63. [Google Scholar]
- Doner, L.W. The Sugars of Honey—A Review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef]
- Ouattara, T.Y.; Fournier, M.; Rojo, S.; Lucas, E. Development Cycle of a Potential Biocontrol Agent: The American Hoverfly, Eupeodes americanus, and Comparison with the Commercial Biocontrol Agent Aphidoletes aphidimyza. Entomol. Exp. Appl. 2022, 170, 394–401. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria, 2017. Available online: https://www.Rproject.org/ (accessed on 30 August 2023).
- Therneau, T.A. A Package for Survival Analysis in r. r Package Version 3.4-0. 2024. Available online: https://CRAN.R-project.org/package=survival (accessed on 30 August 2023).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘Ggplot2’. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 30 August 2023).
- Köneke, A.; Uesugi, R.; Herz, A.; Tabuchi, K.; Yoshimura, H.; Shimoda, T.; Nagasaka, K.; Böckmann, E. Effects of Wheat Undersowing and Sweet Alyssum Intercropping on Aphid and Flea Beetle Infestation in White Cabbage in Germany and Japan. J. Plant Dis. Prot. 2023, 130, 619–631. [Google Scholar] [CrossRef]
- Buckland, K.R.; Alston, D.G.; Reeve, J.R.; Nischwitz, C.; Drost, D. Trap Crops in Onion to Reduce Onion Thrips1 and Iris Yellow Spot Virus. Southwest. Entomol. 2017, 42, 73–90. [Google Scholar] [CrossRef]
- Tahir, I.; Farooq, S.; Bhat, M.R. Insect Pollinators and Pests Associated with Cultivated Buckwheat in Kashmir (India). Fagopyrum 1985, 5, 3–5. [Google Scholar]
- Wäckers, F.L.; Alberola, J.S.; Garcia-Marí, F.; Pekas, A. Attract and Distract: Manipulation of a Food-Mediated Protective Mutualism Enhances Natural Pest Control. Agric. Ecosyst. Environ. 2017, 246, 168–174. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual Ecosystem Services of Syrphid Flies (Diptera: Syrphidae): Pollinators and Biological Control Agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).