Multiple Soil Health Assessment Methods for Evaluating Effects of Organic Fertilization in Farmland Soil of Agro-Pastoral Ecotone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experiment Design
2.2. Soil Sampling and Laboratory Determination
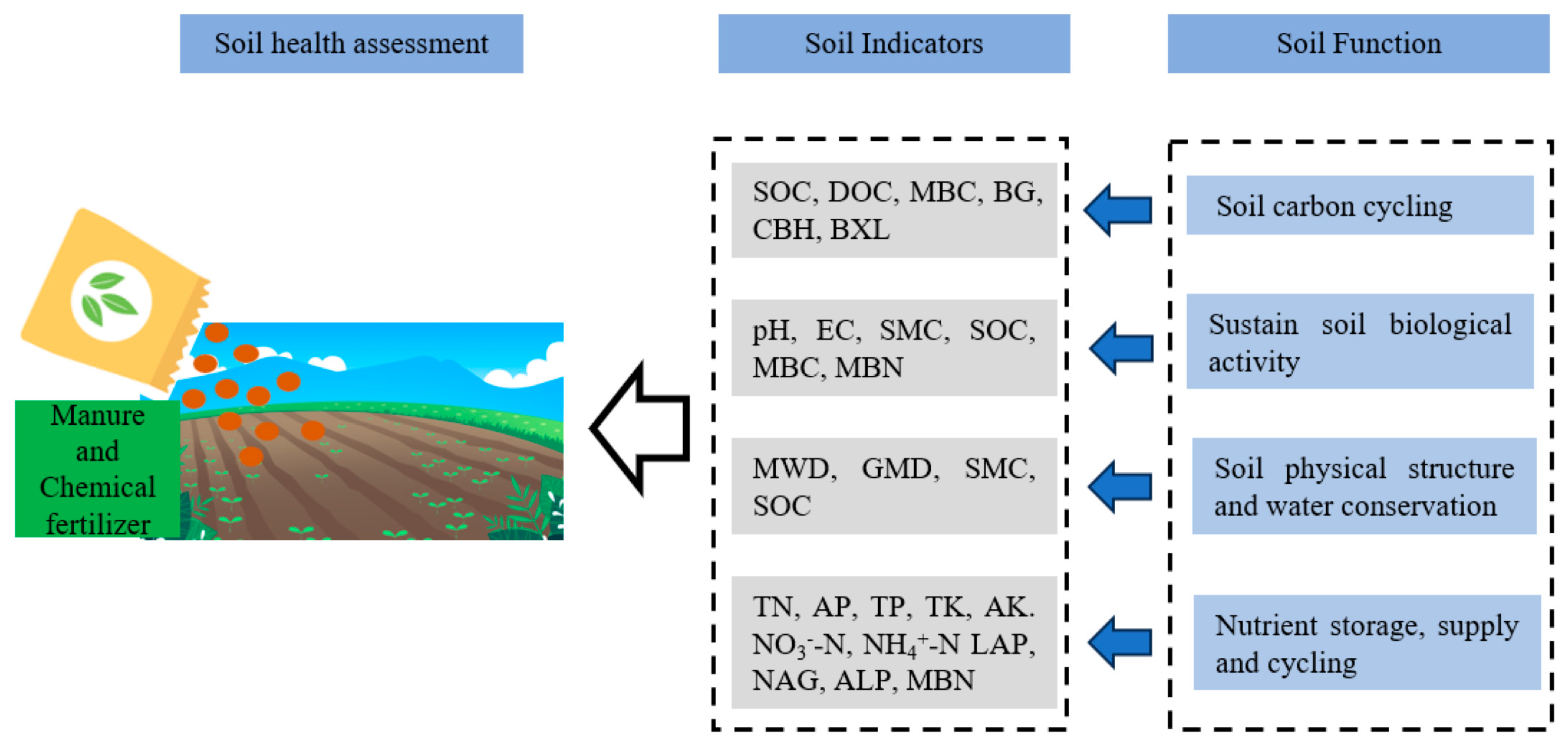
2.3. The Assessment of Farmland Soil Health
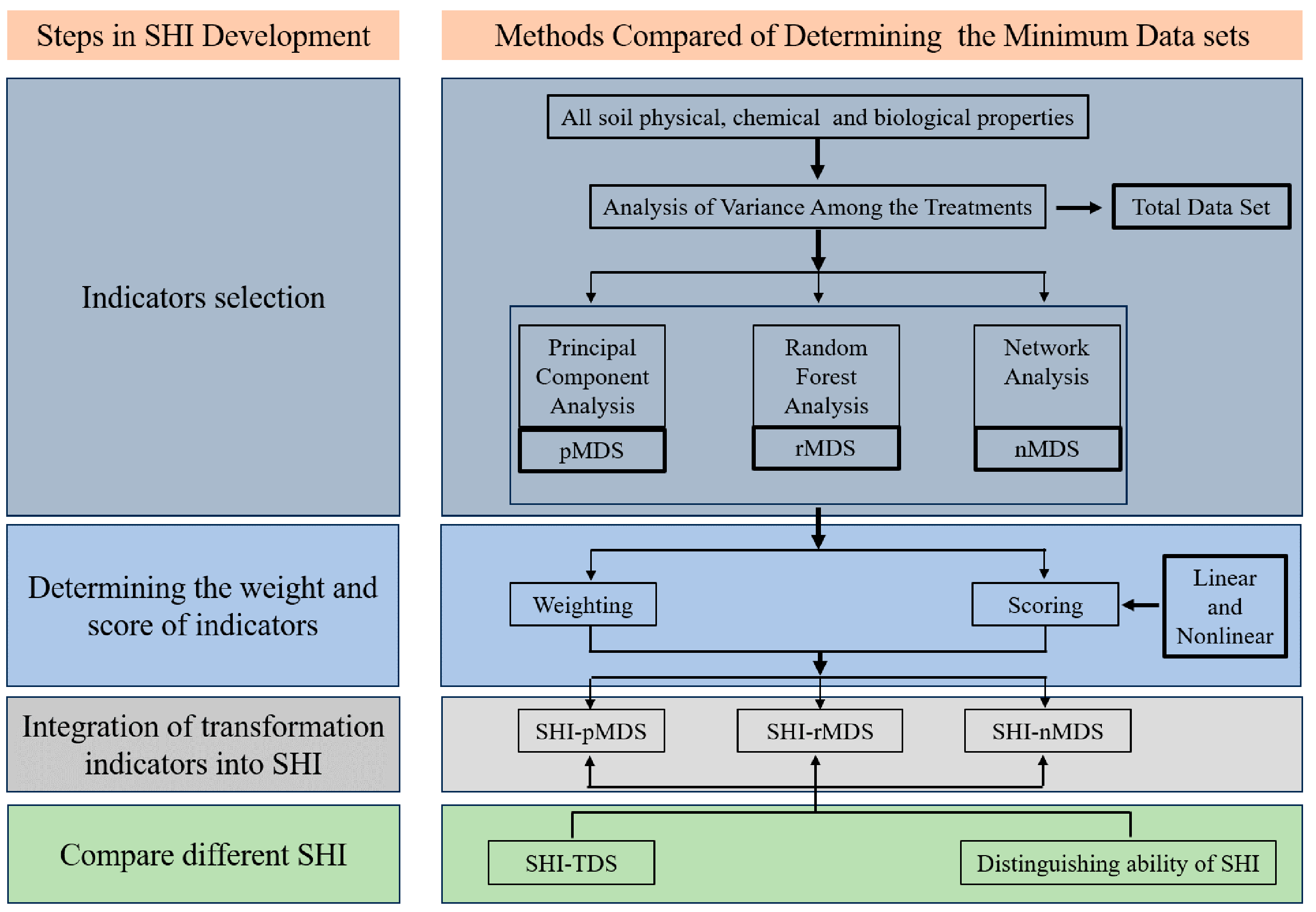
2.3.1. Developing the Minimum Dataset
2.3.2. Calculating Soil Health Indexes
2.4. Statistical Analyses
3. Results
3.1. Soil Properties of a Total Dataset and Millet Yield under Different Fertilization Treatments
3.2. Development Minimum Dataset
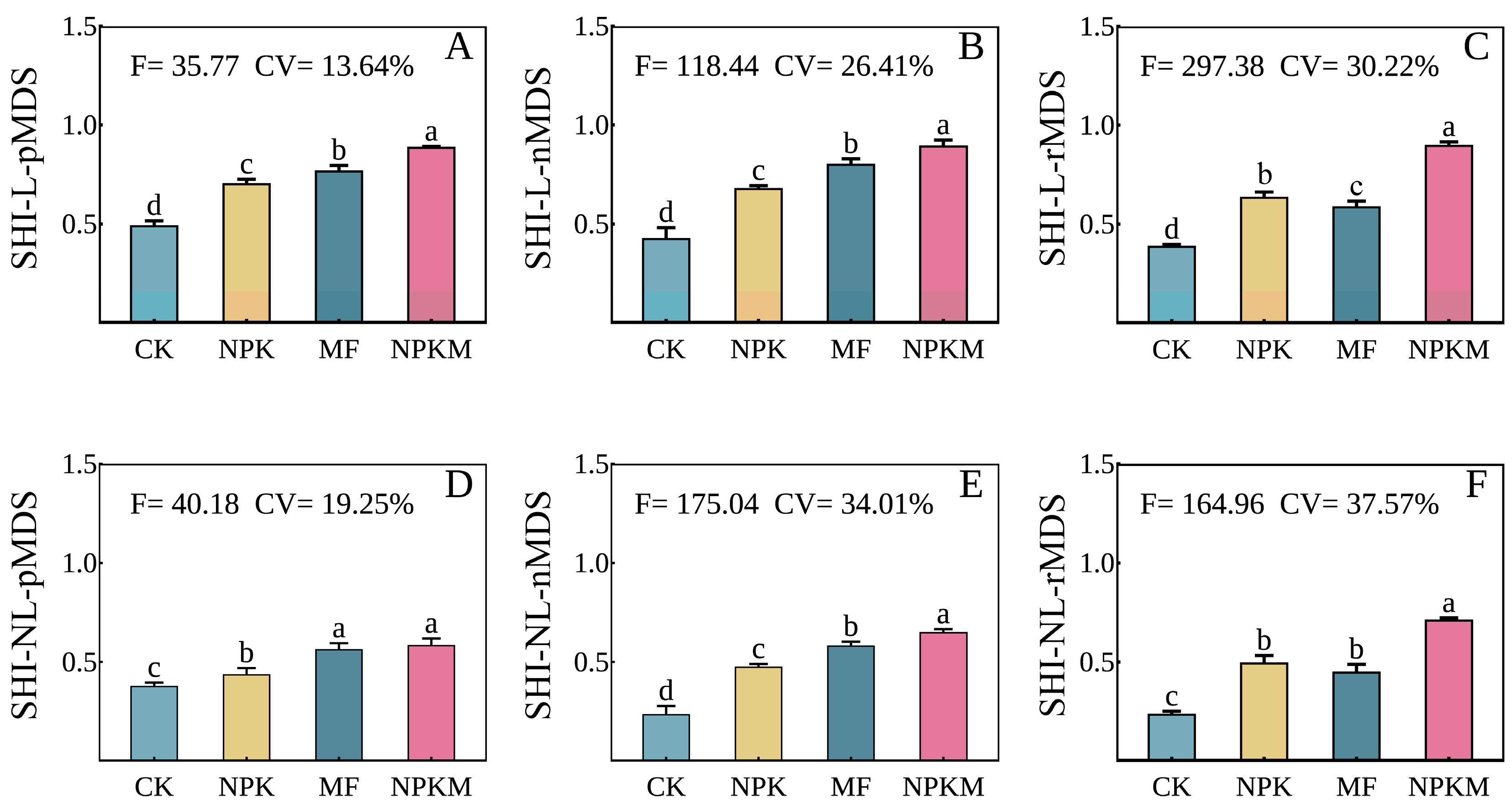
3.3. Comparison of SHIs between Fertilization Treatments
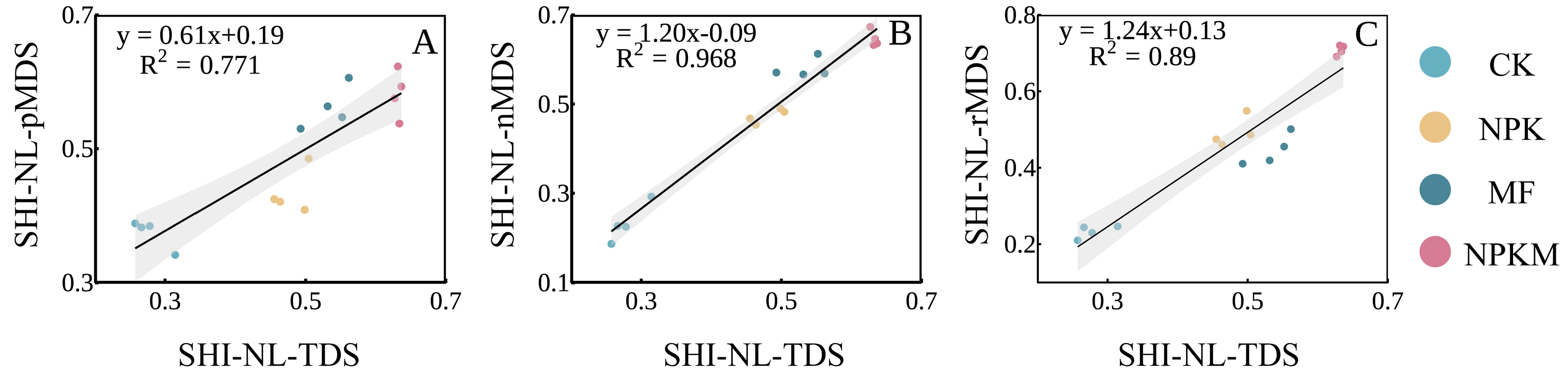
3.4. Relationships between SHIs and Crop Yields
4. Discussion
4.1. Effect of Fertilization on Soil Properties
4.2. Soil Indicator Determined for Minimum Dataset with Three Methods
4.3. Effect of Organic Fertilization on Soil Health
4.4. SHIs Have a Positive Correlation with Each Other and Crop Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rakkar, M.; Jungers, J.M.; Sheaffer, C.; Bergquist, G.; Grossman, J.; Li, F.; Gutknecht, J.L. Soil health improvements from using a novel perennial grain during the transition to organic production. Agric. Ecosyst. Environ. 2023, 341, 108164. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. A Sect. Agric. Ecosyst. Environ. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Gaffney, J.; Bing, J.; Byrne, P.F.; Cassman, K.G.; Ciampitti, I.; Delmer, D.; Habben, J.; Lafitte, H.R.; Lidstrom, U.E.; Porter, D.O.; et al. Science-based intensive agriculture: Sustainability, food security, and the role of technology. Glob. Food Secur. 2019, 23, 236–244. [Google Scholar] [CrossRef]
- Lu, M.; Powlson, D.S.; Liang, Y.; Yao, Z.; Chadwick, D.R.; Long, S.; Liu, D.; Chen, X.-P. Significant soil degradation is associated with intensive vegetable cropping in subtropical area: A case study in southwest China. SOIL Discuss. 2021, 7, 333–346. [Google Scholar] [CrossRef]
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health-a review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Huang, B.; Song, Z.; Ren, L.; Hao, B.; Liu, J.; Zhu, J.; Fang, W.; Yan, D.; et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1,3-dichloropropene fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Zhang, Y.; Liu, C.; Liu, Y.; Li, Z.; Zhang, M. Organic amendments alter microbiota assembly to stimulate soil metabolism for improving soil quality in wheat-maize rotation system. J. Environ. Manag. 2023, 339, 117927. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizers activate soil enzyme activities and promote the recovery of soil beneficial microorganisms after dazomet fumigation. J. Environ. Manag. 2022, 309, 114666. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wan, L.; Zhou, X.; Li, S.; Li, X.; Bhadauria, T. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterial community diversity in leymus chinensis steppe in inner mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jia, J.; Lu, H.; Feng, M.; Yang, W. Functional diversity of soil microbial communities in response to supplementing 50% of the mineral n fertilizer with organic fertilizer in an oat field. J. Integr. Agric. 2021, 20, 2255–2264. [Google Scholar] [CrossRef]
- Rinot, O.; Levy, G.J.; Steinberger, Y.; Svoray, T.; Eshel, G. Soil health assessment: A critical review of current methodologies and a proposed new approach. Sci. Total Environ. 2019, 648, 1484–1491. [Google Scholar] [CrossRef]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Tillage Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Nehrani, S.H.; Askari, M.S.; Saadat, S.; Delavar, M.A.; Taheri, M.; Holden, N.M. Quantification of soil quality under semi-arid agriculture in the northwest of iran. Ecol. Indic. 2020, 108, 105770. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.; Zhao, L.; Sun, B.; Wang, B. Agricultural intensification weakens the soil health index and stability of microbial networks. Agric. Ecosyst. Environ. 2022, 339, 108118. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a soil quality index to compare soil fitness for agricultural use under different managements in the mediterranean environment. Soil Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Li, X.; Qiao, L.; Huang, Y.; Li, D.; Xu, M.; Ge, T.; Meersmans, J.; Zhang, W. Manuring improves soil health by sustaining multifunction at relatively high levels in subtropical area. Agric. Ecosyst. Environ. 2023, 353, 108539. [Google Scholar] [CrossRef]
- Shen, Q.; Suarez-Abelenda, M.; Camps-Arbestain, M.; Pereira, R.C.; Mcnally, S.R.; Kelliher, F.M. An investigation of organic matter quality and quantity in acid soils as influenced by soil type and land use. Geoderma 2018, 328, 44–55. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Li, C.; Xiao, Q.; Liu, Q.; Sun, Z.; Wang, J.; Cao, C. Soil quality indicators of integrated rice-crayfish farming in the jianghan plain, China using a minimum data set. Soil Tillage Res. 2020, 204, 104732. [Google Scholar] [CrossRef]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Kurniawati, A.; Toth, G.; Ylivainio, K.; Toth, Z. Opportunities and challenges of bio-based fertilizers utilization for improving soil health. Org. Agric. 2023, 13, 335–350. [Google Scholar] [CrossRef]
- Li, P.; Shi, K.; Wang, Y.; Kong, D.; Liu, T.; Jiao, J.; Liu, M.; Li, H.; Hu, F. Soil quality assessment of wheat-maize cropping system with different productivities in China: Establishing a minimum data set. Soil Tillage Res. 2019, 190, 31–40. [Google Scholar] [CrossRef]
- Maharjan, B.; Das, S.; Acharya, B.S. Soil health gap: A concept to establish a benchmark for soil health management. Glob. Ecol. Conserv. 2020, 23, e01116. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Tian, B.; Li, J.; Luo, J.; Wang, X.; Ai, S.; Wang, X. Assessment of soil heavy metal pollution in provinces of China based on different soil types: From normalization to soil quality criteria and ecological risk assessment. J. Hazard. Mater. 2023, 441, 129891. [Google Scholar] [CrossRef] [PubMed]
- Fallah, S.; Rostaei, M.; Lorigooini, Z.; Surki, A.A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead-soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crop. Prod. 2018, 115, 158–165. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Li, X.; Wang, C.; Zhang, J.; Jiang, R.; Feng, G.; Liu, X.; Zuo, Y.; Yuan, H.; et al. Field management practices drive ecosystem multifunctionality in a smallholder-dominated agricultural system. Agric. Ecosyst. Environ. 2021, 313, 107389. [Google Scholar] [CrossRef]
- Askari, M.S.; Holden, N.M. Quantitative soil quality indexing of temperate arable management systems. Soil Tillage Res. 2015, 150, 57–67. [Google Scholar] [CrossRef]
- Bünemanna, E.K.; Giulia, B.B.; Zhanguo, B.; Rachel, E.C.; Gerlinde, D.D.; de Goedeb, R.; Luuk, F.; Violette, G.; Thom, W.K.; Paul, M.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. Soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Chen, D.; Yu, Z.; Wei, C. Development of a soil quality index for camellia oleifera forestland yield under three different parent materials in southern China. Soil Tillage Res. 2018, 176, 45–50. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Deng, J.; Fu, L.; Chen, H.; Li, C.; Xu, L.; Jiao, J.; Zhang, S.; Wang, J.; et al. Cover crop by irrigation and fertilization improves soil health and maize yield: Establishing a soil health index. Appl. Soil Ecol. 2023, 182, 104727. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, C.; Li, X.; Zhang, T.; Wang, X. Soil quality evaluation for navel orange production systems in central subtropical China. Soil Tillage Res. 2016, 155, 225–232. [Google Scholar] [CrossRef]
- Karaca, S.; Dengiz, O.; Turan, D.; Özkan, B.; Dedeoğlu, M.; Gülser, F.; Sargin, B.; Demirkaya, S.; Ay, A. An assessment of pasture soils quality based on multi-indicator weighting approaches in semi-arid ecosystem. Ecol. Indic. 2021, 121, 107001. [Google Scholar] [CrossRef]
- Panico, S.C.; Esposito, F.; Memoli, V.; Vitale, L.; Polimeno, F.; Magliulo, V.; Maisto, G.; De Marco, A. Variations of agricultural soil quality during the growth stages of sorghum and sunflower. Appl. Soil Ecol. 2020, 152, 103569. [Google Scholar] [CrossRef]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Raiesi, F.; Pejman, M. Assessment of post-wildfire soil quality and its recovery in semi-arid upland rangelands in central Iran through selecting the minimum data set and quantitative soil quality index. Catena 2021, 201, 105202. [Google Scholar] [CrossRef]
- Romaniuk, R.; Giuffré, L.; Costantini, A.; Bartoloni, N.; Nannipieri, P. A comparison of indexing methods to evaluate quality of horticultural soils. Part ii. Sensitivity of soil microbiological indicators. Soil Res. 2014, 52, 409–418. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y.; Wei, G. Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Chang. Biol. 2022, 28, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, P.; Li, Z.; Lin, H.; Zhu, C.; Wang, J.; Zou, J. Microbial diversity and the abundance of keystone species drive the response of soil multifunctionality to organic substitution and biochar amendment in a tea plantation. Glob. Chang. Biol. Bioenergy 2022, 14, 481–495. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Zhang, D.; Li, L.; Li, W.; Sheng, L. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Vasu, D.; Tiwari, G.; Sahoo, S.; Dash, B.; Jangir, A.; Sharma, R.P.; Naitam, R.; Tiwary, P.; Karthikeyan, K.; Chandran, P. A minimum data set of soil morphological properties for quantifying soil quality in coastal agroecosystems. Catena 2021, 198, 105042. [Google Scholar] [CrossRef]
- Raiesi, F.; Beheshti, A. Evaluating forest soil quality after deforestation and loss of ecosystem services using network analysis and factor analysis techniques. Catena 2022, 208, 105778. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Xie, Y.; Jia, X.; Su, T.; Li, J.; Shen, Y. Assessment of soil quality indexes for different land use types in typical steppe in the loess hilly area, China. Ecol. Indic. 2020, 118, 106743. [Google Scholar] [CrossRef]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Howell, D.M.; Gupta, S.D.; Pinno, B.D.; Mackenzie, M.D. Reclaimed soils; fertilizer, and bioavailable nutrients: Determining similarity with natural benchmarks over time. Can. J. Soil Sci. 2016, 97, 149–158. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- von Arb, C.; Bünemann, E.K.; Schmalz, H.; Portmann, M.; Adamtey, N.; Musyoka, M.W.; Frossard, E.; Fliessbach, A. Soil quality and phosphorus status after nine years of organic and conventional farming at two input levels in the central highlands of kenya. Geoderma 2020, 362, 114112. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, J.; Wang, W.; Ma, J.; Shi, J.; Ren, W. Organic amendment effects on cropland soil organic carbon and its implications: A global synthesis. Catena 2023, 231, 107343. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Lu, W.; Hou, Z.; Li, J. Commercial organic fertilizer substitution increases wheat yield by improving soil quality. Sci. Total Environ. 2022, 851, 158132. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Ros, M. Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community c and n resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Wang, L.; Delgado-Baquerizo, M.; Wang, D.; Isbell, F.; Liu, J.; Feng, C.; Liu, J.; Zhong, Z.; Zhu, H.; Yuan, X.; et al. Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 6187–6192. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Kang, G.; Zhu, B.; Li, H.; Hu, F.; Jiao, J. Soil quality response to organic amendments on dryland red soil in subtropical China. Geoderma 2020, 373, 114416. [Google Scholar] [CrossRef]
- Ferreiro, J.P.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Jiang, Y.; Luan, L.; Hu, K.; Liu, M.; Chen, Z.; Geisen, S.; Chen, X.; Li, H.; Xu, Q.; Bonkowski, M.; et al. Trophic interactions as determinants of the arbuscular mycorrhizal fungal community with cascading plant-promoting consequences. Microbiome 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Ovreas, L.; Thingstad, T.F. Prokaryotic diversity—Magnitude; dynamics; controlling factors. Science 2002, 296, 1064–1066. [Google Scholar] [CrossRef]
- Luo, G.; Rensing, C.; Chen, H.; Liu, M.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 2018, 32, 1103–1116. [Google Scholar] [CrossRef]
- Yu, P.; Han, D.; Liu, S.; Wen, X.; Huang, Y.; Jia, H. Soil quality assessment under different land uses in an alpine grassland. Catena 2018, 171, 280–287. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of jiangsu province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Rossi, J.P.; Franc, A.; Rousseau, G.X. Indicating soil quality and the gisq. Soil Biol. Biochem. 2009, 41, 444–445. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; Preez, C.C.D.; Botha, P.R.; Snyman, H.A.; Habig, J. Soil quality characteristics of kikuyu–ryegrass pastures in south Africa. Geoderma 2014, 232–234, 589–599. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil quality index (SQI) as a tool to evaluate crop productivity in semi-arid deccan plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Wood, S.A.; Tirfessa, D.; Baudron, F. Soil organic matter underlies crop nutritional quality and productivity in smallholder agriculture. Agric. Ecosyst. Environ. 2018, 266, 100–108. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Bastida, F.; Domínguez, S.G.; Toledo, D.M.; Faz, A. Identification of sensitive indicators to assess the interrelationship between soil quality, management practices and human health. Soil 2015, 1, 173–185. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; Wang, J.; He, L.; Wang, J.; Ren, C.; Guo, Y.; Zhao, F. Stronger microbial decay of recalcitrant carbon in tropical forests than in subtropical and temperate forest ecosystems in China. Catena 2022, 215, 106351. [Google Scholar] [CrossRef]
- Lyu, L.; Zhu, J.; Long, H.; Liao, K.; Fan, Y.; Wang, J. Effects of farmland use transition on soil organic carbon in dry farming areas. Environ. Dev. Sustain. 2023, 26, 7055–7078. [Google Scholar] [CrossRef]
- Holden, N.M.; Askari, M.S. Indices for quantitative evaluation of soil quality under grassland management. Geoderma 2014, 230, 230–231. [Google Scholar] [CrossRef]
- Lu, H.; Chen, X.; Ma, K.; Zhou, S.; Yi, J.; Qi, Y.; Hao, J.; Chen, F.; Wen, X. Soil health assessment under different soil and irrigation types in the agro-pastoral ecotone of northern China. Catena 2024, 235, 107655. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, M.; Liu, G.; Ma, L.; Zhang, S.; Xiao, T.; Peng, G. Effect of vegetation type on microstructure of soil aggregates on the loess plateau, China. Agric. Ecosyst. Environ. 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Zhang, L.; Li, Q.; Zhou, D. Selecting the minimum data set and quantitative soil quality indexing of alkaline soils under different land uses in northeastern China. Sci. Total Environ. 2018, 616–617, 564–571. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, L.; Chen, X.; Zhu, H.; Fan, H. Soil quality assessment based on a minimum data set: A case study of a county in the typical river delta wetlands. Sustainability 2020, 12, 9033. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M.; Liu, Y. Crop yield and production responses to climate disasters in China. Sci. Total Environ. 2021, 750, 141147. [Google Scholar] [CrossRef]
- Zhi, J.; Cao, X.; Zhang, Z.; Qin, T.; Qu, L.; Qi, L.; Ge, L.; Guo, A.; Wang, X.; Da, C.; et al. Identifying the determinants of crop yields in China since 1952 and its policy implications. Agric. For. Meteorol. 2022, 327, 109216. [Google Scholar] [CrossRef]
- Baldi, E.; Cavani, L.; Margon, A.; Quartieri, M.; Sorrenti, G.; Marzadori, C.; Toselli, M. Effect of compost application on the dynamics of carbon in a nectarine orchard ecosystem. Sci. Total Environ. 2018, 637–638, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Cavani, L.; Ciavatta, C.; Campanelli, G.; Burgio, G.; Marzadori, C. Conventional versus organic management: Application of simple and complex indexes to assess soil quality. Agric. Ecosyst. Environ. 2021, 322, 107673. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Jia, J.; Liao, W.; Amsili, J.P.; Schneider, R.L.; van Es, H.M.; Li, Y.; Zhang, J. Applicability of soil health assessment for wheat-maize cropping systems in smallholders’ farmlands. Agric. Ecosyst. Environ. 2023, 353, 108558. [Google Scholar] [CrossRef]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 2003, 137, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, X.; Li, Z.; Liu, M.; Xu, C.; Zhang, R.; Luo, W. Effects of vegetation restoration on soil quality in degraded karst landscapes of southwest China. Sci. Total Environ. 2019, 650, 2657–2665. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Y.; Wu, L.; Tian, Y.; Wang, Q.; Wang, B.; Xu, M.; Zhang, W. Long-term manuring increases microbial carbon use efficiency and mitigates priming effect via alleviated soil acidification and resource limitation. Biol. Fertil. Soils 2021, 57, 925–934. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of biological soil quality indicator system for subtropical China. Soil Tillage Res. 2013, 126, 112–118. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Cao, W.; Gao, Y.; Lu, Y.; Liao, Y. Long-term green manuring to substitute partial chemical fertilizer simultaneously improving crop productivity and soil quality in a double-rice cropping system. Eur. J. Agron. 2023, 142, 126641. [Google Scholar] [CrossRef]
| MWD | GMD | SMC | pH | EC | SOC | TN | AP | TP | |
|---|---|---|---|---|---|---|---|---|---|
| CK | 1.57 ± 0.74 b | 1.77 ± 0.42 b | 6.49 ± 0.26 d | 8.81 ± 0.09 a | 71.16 ± 1.71 c | 3.04 ± 0.59 b | 0.45 ± 0.03 b | 2.70 ± 0.05 d | 0.02 ± 0.02 b |
| NPK | 1.87 ± 1.19 b | 2.30 ± 0.57 b | 8.46 ± 0.53 c | 8.49 ± 0.13 b | 99.57 ± 6.92 b | 4.53 ± 0.77 b | 0.50 ± 0.09 b | 7.57 ± 0.76 b | 0.06 ± 0.01 a |
| MF | 1.79 ± 1.06 b | 3.41 ± 0.67 a | 9.30 ± 0.49 b | 8.54 ± 0.07 b | 105.11 ± 4.61 b | 7.43 ± 1.54 a | 0.52 ± 0.15 b | 4.15 ± 0.16 c | 0.07 ± 0.01 a |
| NPKM | 4.24 ± 0.56 a | 3.83 ± 0.72 a | 11.90 ± 0.53 a | 8.52 ± 0.08 b | 117.43 ± 117.43 a | 6.15 ± 0.65 | 0.70 ± 0.07 a | 8.96 ± 0.64 a | 0.07 ± 0.01 a |
| TK | -N | -N | AK | DOC | MBC | MBN | BG | CBH | |
| CK | 1.46 ± 0.03 c | 2.84 ± 0.88 b | 1.30 ± 0.09 c | 119.00 ± 7.87 c | 97.33 ± 5.91 c | 355.67 ± 49.13 c | 15.40 ± 1.55 c | 5.56 ± 1.45 c | 46.88 ± 13.55 b |
| NPK | 1.46 ± 0.04 b | 6.80 ± 2.76 a | 1.56 ± 0.31 bc | 215.00 ± 12.57 b | 127.33 ± 20.82 b | 43.47 ± 3.52 b | 43.47 ± 3.52 b | 7.84 ± 0.45 b | 78.54 ± 2.24 a |
| MF | 1.49 ± 0.02 ab | 6.38 ± 1.73 a | 2.01 ± 0.33 b | 244.75 ± 14.15 a | 157.67 ± 8.01 a | 45.17 ± 2.18 b | 45.17 ± 2.18 b | 7.86 ± 0.23 b | 84.16 ± 2.74 a |
| NPKM | 1.57 ± 0.11 a | 6.89 ± 1.77 a | 3.30 ± 0.65 a | 242.00 ± 10.71 a | 158.67 ± 7.32 a | 60.37 ± 10.36 a | 60.37 ± 10.36 a | 7.78 ± 0.18 a | 79.82 ± 2.36 a |
| BXL | LAP | NAG | ALP | Yield | |||||
| CK | 37.20 ± 1.30 c | 24.21 ± 3.61 c | 6.07 ± 0.61 c | 11.02 ± 1.52 c | 2715.22 ± 343.44 c | ||||
| NPK | 41.59 ± 1.97 b | 38.89 ± 1.81 b | 7.02 ± 0.58 b | 12.31 ± 1.07 b | 3943.18 ± 59.72 b | ||||
| MF | 41.61 ± 0.74 b | 38.34 ± 1.44 b | 5.97 ± 0.79 b | 12.88 ± 0.77 b | 3732.44 ± 31.98 b | ||||
| NPKM | 43.95 ± 3.39 a | 42.18 ± 1.39 a | 6.94 ± 0.66 a | 14.43 ± 1.74 a | 4802.50 ± 172.18 a | ||||
| Soil Indicators | Soil Ecosystem Function | %IncMSE |
|---|---|---|
| SOC | Soil carbon cycling | 1.58 |
| DOC | 2.56 | |
| MBC | 4.51 | |
| BG | 6.26 | |
| CBH | 3.72 | |
| BXL | 8.19 | |
| AP | Nutrient storage, supply, and cycling | 5.71 |
| AK | 4.88 | |
| LAP | 4.83 | |
| -N | 4.63 | |
| TK | 3.73 | |
| -N | 3.05 | |
| TP | 2.67 | |
| TN | 2.48 | |
| ALP | 1.75 | |
| NAG | 1.61 | |
| SMC | Sustain soil biological activity | 6.63 |
| MBN | 5.68 | |
| MBC | 4.51 | |
| EC | 4.41 | |
| pH | 3.82 | |
| SOC | 1.58 | |
| SMC | Soil physical structure and water conservation | 6.63 |
| MWD | 4.52 | |
| SOC | 1.58 | |
| GMD | 1.32 |
| Soil Indicators | Soil Ecosystem Function | Eigenvector Centrality |
|---|---|---|
| SOC | Soil carbon cycling | 0.87 |
| DOC | 0.98 | |
| MBC | 1.00 | |
| BG | 0.89 | |
| CBH | 0.92 | |
| BXL | 0.89 | |
| TN | Nutrient storage, supply, and cycling | 0.87 |
| AP | 0.64 | |
| TP | 0.92 | |
| TK | 0.87 | |
| -N | 0.52 | |
| -N | 0.89 | |
| AK | 0.83 | |
| LAP | 0.79 | |
| NAG | 0.48 | |
| ALP | 0.89 | |
| pH | Sustain soil biological activity | 0.13 |
| SMC | 0.99 | |
| SOC | 0.87 | |
| MBC | 1.00 | |
| MBN | 0.97 | |
| EC | 0.52 | |
| MWD | Soil physical structure and water conservation | 0.27 |
| GMD | 0.88 | |
| SMC | 0.99 | |
| SOC | 0.87 |
| Indictors | Scoring Curve | Linear Curve | Nonlinear Curve | Weight | |||||
|---|---|---|---|---|---|---|---|---|---|
| Xmax | Xmin | Mean | Slope | TDS | pMDS | nMDS | rMDS | ||
| MWD | More is better | 4.99 | 0.51 | 2.37 | −2.50 | 0.041 | 0.147 | ||
| GMD | More is better | 4.87 | 1.38 | 2.83 | −2.50 | 0.038 | 0.133 | ||
| SMC | More is better | 12.61 | 6.25 | 9.03 | −2.50 | 0.052 | 0.149 | 0.216 | |
| pH | Less is better | 8.89 | 8.34 | 8.59 | 2.50 | 0.047 | 0.328 | ||
| EC | Less is better | 129.62 | 69.41 | 98.32 | 2.50 | 0.049 | 0.343 | ||
| SOC | More is better | 9.30 | 2.46 | 5.29 | −2.50 | 0.047 | |||
| TN | More is better | 0.79 | 0.30 | 0.54 | −2.50 | 0.040 | 0.186 | ||
| AP | More is better | 9.57 | 2.63 | 5.85 | −2.50 | 0.050 | |||
| TP | More is better | 0.07 | 0.01 | 0.05 | −2.50 | 0.045 | 0.138 | ||
| TK | More is better | 1.66 | 1.16 | 1.44 | −2.50 | 0.045 | |||
| -N | More is better | 9.98 | 1.84 | 5.73 | −2.50 | 0.030 | |||
| -N | More is better | 3.80 | 1.17 | 2.04 | −2.50 | 0.047 | 0.329 | ||
| AK | More is better | 261.00 | 112.00 | 205.19 | −2.50 | 0.051 | |||
| DOC | More is better | 169.00 | 89.00 | 135.25 | −2.50 | 0.048 | 0.148 | ||
| MBC | More is better | 772.00 | 317.00 | 514.25 | −2.50 | 0.050 | 0.150 | ||
| MBN | More is better | 74.70 | 13.50 | 41.10 | −2.50 | 0.048 | 0.145 | 0.185 | |
| BG | More is better | 10.96 | 3.81 | 7.70 | −2.50 | 0.045 | |||
| CBH | More is better | 91.33 | 33.88 | 73.41 | −2.50 | 0.050 | 0.138 | ||
| BXL | More is better | 61.45 | 29.45 | 42.71 | −2.50 | 0.049 | 0.266 | ||
| LAP | More is better | 43.55 | 19.46 | 35.91 | −2.50 | 0.051 | |||
| NAG | More is better | 7.82 | 3.70 | 6.12 | −2.50 | 0.036 | |||
| ALP | More is better | 15.55 | 8.52 | 12.22 | −2.50 | 0.041 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Ma, K.; Chen, X.; Zhou, S.; Li, Y.; Zhang, Z.; Wang, C.; Chen, F.; Wen, X. Multiple Soil Health Assessment Methods for Evaluating Effects of Organic Fertilization in Farmland Soil of Agro-Pastoral Ecotone. Agriculture 2024, 14, 572. https://doi.org/10.3390/agriculture14040572
Lu H, Ma K, Chen X, Zhou S, Li Y, Zhang Z, Wang C, Chen F, Wen X. Multiple Soil Health Assessment Methods for Evaluating Effects of Organic Fertilization in Farmland Soil of Agro-Pastoral Ecotone. Agriculture. 2024; 14(4):572. https://doi.org/10.3390/agriculture14040572
Chicago/Turabian StyleLu, Huayu, Ke Ma, Xiangyang Chen, Shiwei Zhou, Yiwei Li, Zecheng Zhang, Chu Wang, Fu Chen, and Xinya Wen. 2024. "Multiple Soil Health Assessment Methods for Evaluating Effects of Organic Fertilization in Farmland Soil of Agro-Pastoral Ecotone" Agriculture 14, no. 4: 572. https://doi.org/10.3390/agriculture14040572





