Challenges of Including Wet Grasslands with Variable Groundwater Tables in Large-Area Crop Production Simulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
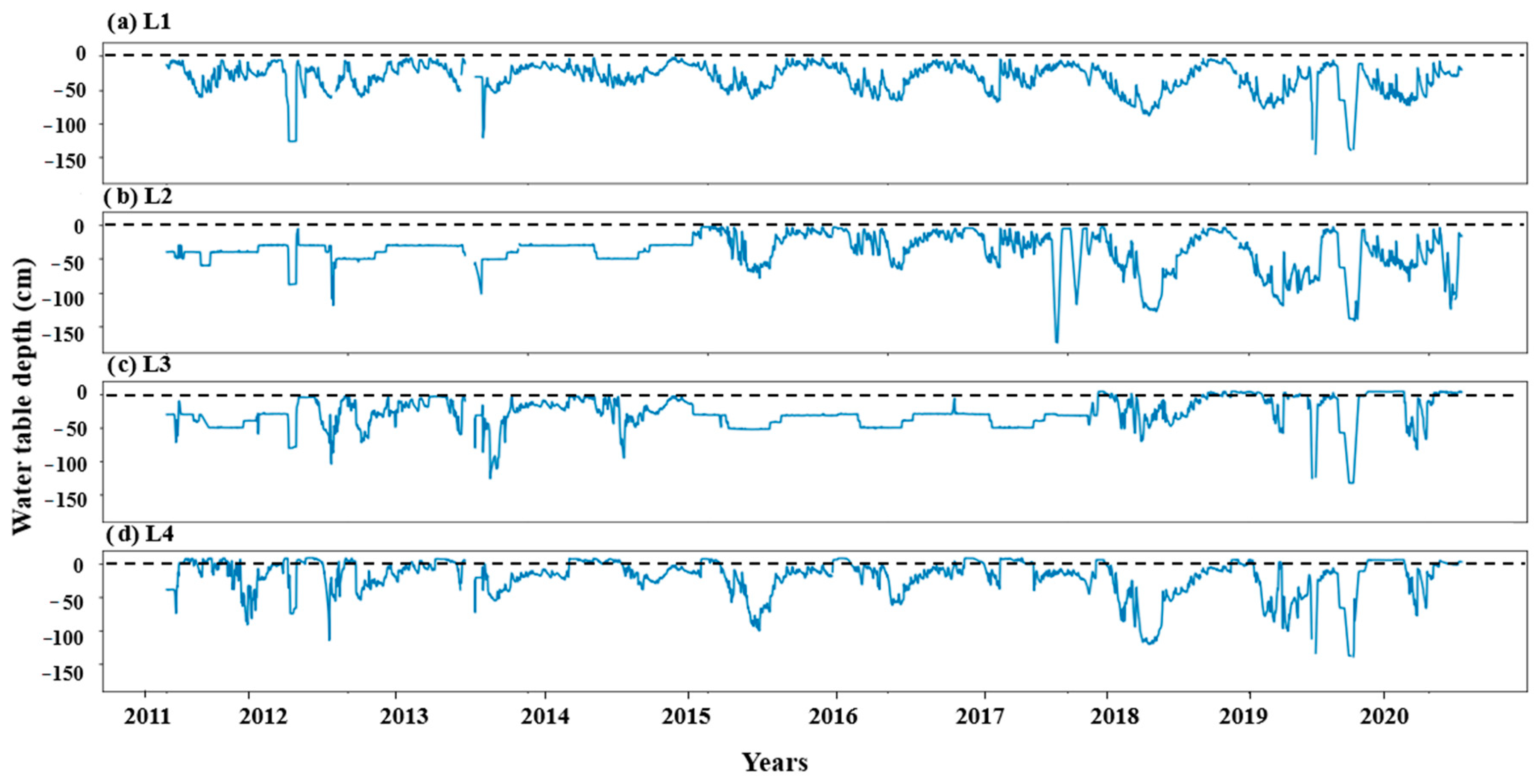
2.2. Data
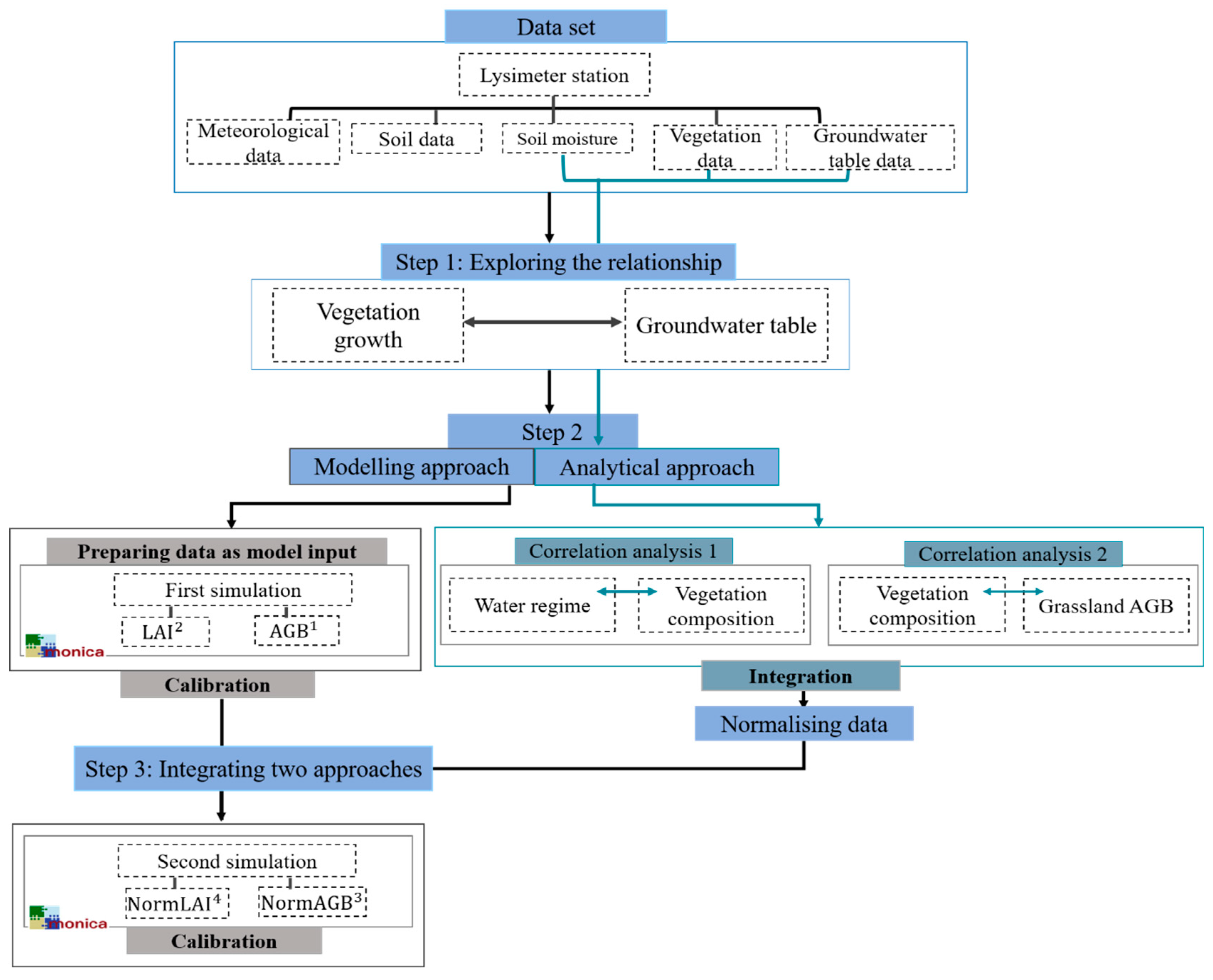
2.3. Exploring Relationships between WTD and Vegetation Growth
2.3.1. Modelling Approach
2.3.2. The Analytical Approach
2.3.3. Integrating the Simulation and the Analysis
3. Results
3.1. The Response of Above-Ground Biomass to Groundwater Table Dynamics
3.1.1. The Modelling Approach
3.1.2. The Analytical Approach
3.2. Response of Species Composition to Groundwater Table Dynamics
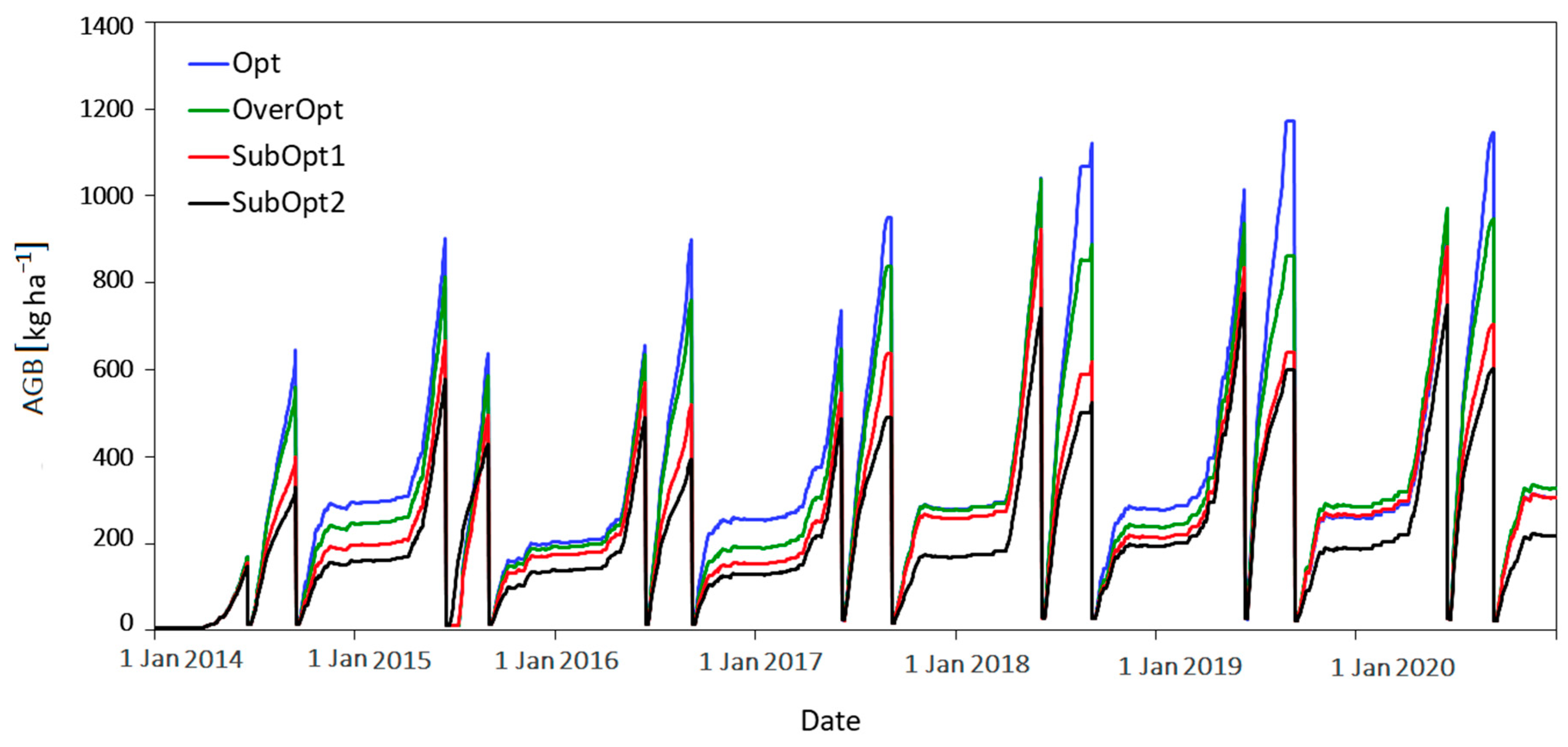
3.3. Integrating the Modelling Approach and the Correlation Analysis
4. Discussion
4.1. The Impact of Water Regime on Biomass Growth
4.2. The Impact of Water Regime on Grassland Species Composition
4.3. The Indirect Relationship between Water Regime and Biomass Growth
4.4. Simulating the Processes Leading to the Grassland Yield Pattern Observed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- White, R.P.; Murray, S.; Rohweder, M.; Prince, S.D.; Thompson, K.M. Grassland Ecosystems 2000; World Resources Institute: Washington, DC, USA, 2000; p. 81. [Google Scholar]
- Zhu, H.; Fu, B.; Wang, S.; Zhu, L.; Zhang, L.; Jiao, L.; Wang, C. Reducing soil erosion by improving community functional diversity in semi-arid grasslands. J. Appl. Ecol. 2015, 52, 1063–1072. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Wu, J. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Joyce, C.B.; Wade, P.M. European Wet Grasslands: Biodiversity, Management and Restoration; John Wiley and Sons Ltd.: Chichester, UK, 1998. [Google Scholar]
- Cebrián-Piqueras, M.Á. Trade-Offs and Synergies between Forage Production, Species Conservation and Carbon Stocks in Temperate Coastal Wet Grasslands. An Ecosystem Services and Process-Based Approach. Ph.D. Dissertation, University of Oldenburg, Oldenburg, Germany, 2017. [Google Scholar]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Gross, K.; Fritschie, K.; Flombaum, P.; Fox, J.W.; Rixen, C.; Van Ruijven, J.; Reich, P.B.; Scherer-Lorenzen, M.; Wilsey, B.J. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. J. Ecol. 2013, 94, 1697–1707. [Google Scholar] [CrossRef]
- Kennedy, M.P.; Milne, J.M.; Murphy, K.J. Experimental growth responses to groundwater level variation and competition in five British wetland plant species. Wetl. Ecol. Manag. 2003, 11, 383–396. [Google Scholar] [CrossRef]
- Reyer, C.P.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; De Lorenzi, F.; Dury, M.; Gloning, P.; Abou Jaoudé, R.; et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef]
- Dietrich, O.; Behrendt, A.; Wegehenkel, M. The water balance of wet grassland sites with shallow water table conditions in the north-eastern German lowlands in extreme dry and wet years. Water 2021, 13, 2259. [Google Scholar] [CrossRef]
- Dwire, K.A.; Kauffman, J.B.; Baham, J.E. Plant species distribution in relation to water-table depth and soil redox potential in montane riparian meadows. Wetlands 2006, 26, 131–146. [Google Scholar] [CrossRef]
- Toogood, S.E.; Joyce, C.B. Effects of raised water levels on wet grassland plant communities. Appl. Veg. Sci. 2009, 12, 283–294. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Tan, Z.; Li, Y.; Wang, X. Effects of water-table depth and soil moisture on plant biomass, diversity, and distribution at a seasonally flooded wetland of Poyang Lake, China. Chin. Geogr. Sci. 2015, 25, 739–756. [Google Scholar] [CrossRef]
- Destatis, Statistisches Bundesamt. Land- und Forstwirtschaft, Fischerei. Bodenfläche nach Art der Tatsächlichen Nutzung; Fachserie 3 Reihe 5.1; Destatis, Statistisches Bundesamt: Wiesbaden, Germany, 2019. [Google Scholar]
- Roßkopf, N.; Fell, H.; Zeitz, J. Organic soils in Germany, their distribution and carbon stocks. Catena 2015, 133, 157–170. [Google Scholar] [CrossRef]
- Söder, M.; Berg-Mohnicke, M.; Bittner, M.; Ernst, S.; Feike, T.; Frühauf, C.; Golla, B.; Jänicke, C.; Jorzig, C.; Leppelt, T.; et al. Klimawandelbedingte Ertragsveränderungen und Flächennutzung (KlimErtrag); Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2022. [Google Scholar]
- Hetzer, J.; Huth, A.; Taubert, F. The importance of plant trait variability in grasslands: A modelling study. Ecol. Model. 2021, 453, 109606. [Google Scholar] [CrossRef]
- Robertson, A.D.; Paustian, K.; Ogle, S.; Wallenstein, M.D.; Lugato, E.; Cotrufo, M.F. Unifying soil organic matter formation and persistence frameworks: The MEMS model. Biogeosciences 2019, 16, 1225–1248. [Google Scholar] [CrossRef]
- Sándor, R.; Barcza, Z.; Hidy, D.; Lellei-Kovács, E.; Ma, S.; Bellocchi, G. Modelling of grassland fluxes in Europe: Evaluation of two biogeochemical models. Agric. Ecosyst. Environ. 2016, 215, 1–19. [Google Scholar] [CrossRef]
- Rahimi, J.; Haas, E.; Scheer, C.; Grados, D.; Abalos, D.; Aderele, M.O.; Mathiesen, G.; Butterbach-Bahl, K. Aggregation of activity data on crop management can induce large uncertainties in estimates of regional nitrogen budgets. Sustain. Agric. 2024, in press.
- Arango, M.A.; Anandhi, A.; Rice, C.W. Conceptual framework addressing timescale mismatch uncertainty: Nitrous-oxide (N2O) modeled and measured, Kansas, USA. Ecol. Model. 2023, 486, 110536. [Google Scholar] [CrossRef]
- Zhang, Y.; Lavallee, J.M.; Robertson, A.D.; Even, R.; Ogle, S.M.; Paustian, K.; Cotrufo, M.F. Simulating measurable ecosystem carbon and nitrogen dynamics with the mechanistically defined MEMS 2.0 model. Biogeosciences 2021, 18, 3147–3171. [Google Scholar] [CrossRef]
- Coffin, D.P.; Lauenroth, W.K. A gap dynamics simulation model of succession in a semiarid grassland. Ecol. Model. 1990, 49, 229–266. [Google Scholar] [CrossRef]
- Gillet, F. Modelling vegetation dynamics in heterogeneous pasture-woodland landscapes. Ecol. Model. 2008, 217, 1–18. [Google Scholar] [CrossRef]
- Siehoff, S.; Lennartz, G.; Heilburg, I.C.; Roß-Nickoll, M.; Ratte, H.T.; Preuss, T.G. Process-based modeling of grassland dynamics built on ecological indicator values for land use. Ecol. Model. 2011, 222, 3854–3868. [Google Scholar] [CrossRef]
- Thornley, J.H.M.; Verberne, E.L.J. A model of nitrogen flows in grassland. Plant Cell Environ. 1989, 12, 863–886. [Google Scholar] [CrossRef]
- Van Oijen, M.; Barcza, Z.; Confalonieri, R.; Korhonen, P.; Kröel-Dulay, G.; Lellei-Kovács, E.; Louarn, G.; Louault, F.; Martin, R.; Moulin, T.; et al. Incorporating biodiversity into biogeochemistry models to improve prediction of ecosystem services in temperate grasslands: Review and roadmap. Agronomy 2020, 10, 259. [Google Scholar] [CrossRef]
- Daleo, P.; Alberti, J.; Chaneton, E.J.; Iribarne, O.; Tognetti, P.M.; Bakker, J.D.; Borer, E.T.; Bruschetti, M.; MacDougall, A.S.; Pascual, J.; et al. Environmental heterogeneity modulates the effect of plant diversity on the spatial variability of grassland biomass. Nat. Commun. 2023, 14, 1809. [Google Scholar] [CrossRef]
- Jouven, M.; Carrère, P.; Baumont, R. Model predicting dynamics of biomass, structure and digestibility of herbage in managed permanent pastures. 1. Model description. Grass Forage Sci. 2006, 61, 112–124. [Google Scholar] [CrossRef]
- Clark, A.T.; Ann Turnbull, L.; Tredennick, A.; Allan, E.; Harpole, W.S.; Mayfield, M.M.; Soliveres, S.; Barry, K.; Eisenhauer, N.; de Kroon, H.; et al. Predicting species abundances in a grassland biodiversity experiment: Trade-offs between model complexity and generality. J. Ecol. 2020, 108, 774–787. [Google Scholar] [CrossRef]
- Moulin, T. Perasso, Antoine., Gillet, François., Modelling vegetation dynamics in managed grasslands: Responses to drivers depend on species richness. Ecol. Model. 2018, 374, 22–36. [Google Scholar] [CrossRef]
- Movedi, E.; Bellocchi, G.; Argenti, G.; Paleari, L.; Vesely, F.; Staglianò, N.; Dibari, C.; Confalonieri, R. Development of generic crop models for simulation of multi-species plant communities in mown grasslands. Ecol. Model. 2019, 401, 111–128. [Google Scholar] [CrossRef]
- Haas, E.; Klatt, S.; Fröhlich, A.; Kraft, P.; Werner, C.; Kiese, R.; Grote, R.; Breuer, L.; Butterbach-Bahl, K. LandscapeDNDC: A process model for simulation of biosphere–atmosphere–hydrosphere exchange processes at site and regional scale. Landsc. Ecol. 2013, 28, 615–636. [Google Scholar] [CrossRef]
- Dietrich, O.; Fahle, M.; Seyfarth, M. Behavior of water balance components at sites with shallow groundwater tables: Possibilities and limitations of their simulation using different ways to control weighable groundwater lysimeters. Agric. Water Manag. 2016, 163, 75–89. [Google Scholar] [CrossRef]
- Dietrich, O. Effects of Groundwater Levels and Soil Moisture on the Development of Leaf Area Index and Biomass Yield of Wet Grasslands (Lysimeter Data) [Data Set]; Leibniz Centre for Agricultural Landscape Research (ZALF): Müncheberg, Germany, 2023. [Google Scholar]
- Bethune, M.; Wang, Q.J.A. A lysimeter study of the water balance of border-check irrigated perennial pasture. Anim. Prod. Sci. 2004, 44, 151–162. [Google Scholar] [CrossRef]
- Noory, H.; Liaghat, A.M.; Chaichi, M.R.; Parsinejad, M. Effects of water table management on soil salinity and alfalfa yield in a semi-arid climate. Irrig. Sci. 2009, 27, 401–407. [Google Scholar] [CrossRef]
- Bos, L.; Caliari, M.; De Marchi, S.; Vianello, M. A numerical study of the Xu polynomial interpolation formula in two variables. Computing 2006, 76, 311–324. [Google Scholar] [CrossRef]
- Dietrich, O. Untersuchungen zum Wasserhaushalt Grundwassernaher Standorte Ergebnisse der Lysimeteranlage Spreewald; Programmbereich 2 “Landnutzung und Governance”, Arbeitsgruppe” Tieflandhydrologie und Wassermanagement; Leibniz-Zentrum für Agrarlandschaftsforschung (ZALF) e.V.: Müncheberg, Germany, 2021. [Google Scholar]
- Van der Maarel, E. The Braun-Blanquet approach in perspective. Vegetation 1975, 30, 213–219. [Google Scholar] [CrossRef]
- Mühlenberg, M.L. Freilandökologie, 2nd ed.; Quelle & Meyer: Heidelberg, Germany, 1989; p. 512. [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen. In Öklogischer, Dynamischer und Historischer, 6th ed.; Ulmer UTB: Stuttgart, Germany, 2010. [Google Scholar]
- Barthelheimer, M.; Poschlod, P. Functional characterizations of E llenberg indicator values–a review on ecophysiological determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Berg, C.; Welk, E.; Jäger, E.J. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. Appl. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Nendel, C.; Berg, M.; Kersebaum, K.C.; Mirschel, W.; Specka, X.; Wegehenkel, M.; Wenkel, K.O.; Wieland, R. The MONICA model: Testing predictability for crop growth, soil moisture and nitrogen dynamics. Ecol. Model. 2011, 222, 1614–1625. [Google Scholar] [CrossRef]
- Wegehenkel, M. Test of a modelling system for simulating water balances and plant growth using various different complex approaches. Ecol. Model. 2000, 129, 39–64. [Google Scholar] [CrossRef]
- Eckelmann, W.; Sponagel, H.; Grottenthaler, W.; Hartmann, K.; Hartwich, R.; Janetzko, P.; Joisten, H.; Kühn, D.; Sabel, K.; Traidl, R. Manual of Soil Mapping (KA5), 5th ed.; Federal Institute for Geosciences and Natural Resources in cooperation with the State Geological Services: Berlin, Germany, 2005. [Google Scholar]
- Kamali, B.; Stella, T.; Berg-Mohnicke, M.; Pickert, J.; Groh, J.; Nendel, C. Improving the simulation of permanent grasslands across Germany by using multi-objective uncertainty-based calibration of plant-water dynamics. Eur. J. Agron. 2022, 134, 126464. [Google Scholar] [CrossRef]
- Abbaspour, K.; Rouholahnejad, E.; Vaghefi, S.; Srinivasan, R.; Yang, H.; Kløve, B. A continental-scale hydrology and water quality model for Europe: Calibration and uncertainty of a high-resolution large-scale SWAT model. J. Hydrol. 2015, 524, 733–752. [Google Scholar] [CrossRef]
- Abbaspour, K.C. SWAT-CUP: SWAT Calibration and Uncertainty Programs—A User Manual; Eawag: Dübendorf, Switzerland, 2015. [Google Scholar]
- Abbaspour, K.C.; CJohnson, A.; Van Genuchten, M.T. Estimating uncertain flow and transport parameters using a sequential uncertainty fitting procedure. Vadose Zone J. 2004, 3, 1340–1352. [Google Scholar] [CrossRef]
- Kamali, B.; Abbaspour, K.C.; Lehmann, A.; Wehrli, B.; Yang, H. Uncertainty-based auto-calibration for crop yield–the EPIC+ procedure for a case study in Sub-Saharan Africa. European J. Agron. 2018, 93, 57–72. [Google Scholar] [CrossRef]
- Willmott, C.J. On the validation of models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Lischeid, G.; Dannowski, R.; Kaiser, K.; Nützmann, G.; Steidl, J.; Stüve, P. Inconsistent hydrological trends do not necessarily imply spatially heterogeneous drivers. J. Hydrol. 2021, 596, 126096. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Tian, D.; Cong, R.; Zhang, X.; Pan, Q.; Shi, Z. Resource reallocation of two grass species during regrowth after defoliation. Front. Plant Sci. 2018, 9, 1767. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.T.; Brock, M.A. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Zhang, J.T. Quantitative Ecology, 2nd ed.; Science Press: Beijing, China, 2011; Volume 77, pp. 82–92. [Google Scholar]
- Gibson, D.J. Grasses and Grassland Ecology; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world–challenges and progress: Introduction to a Virtual Issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Baattrup-Pedersen, A.; JENSEN, K.M.; Thodsen, H.; Andersen, H.E.; Andersen, P.M.; Larsen, S.E.; Riis, T.; Andersen, D.K.; Audet, J.; Kronvang, B. Effects of stream flooding on the distribution and diversity of groundwater-dependent vegetation in riparian areas. Freshw. Biol. 2013, 58, 817–827. [Google Scholar] [CrossRef]
- Henszey, R.J.; Pfeiffer, K.; Keough, J.R. Linking surface-and ground-water levels to riparian grassland species along the Platte River in Central Nebraska, USA. Wetlands 2004, 24, 665–687. [Google Scholar] [CrossRef]
- Leyer, I. Predicting plant species’ responses to river regulation: The role of water level fluctuations. J. Appl. Ecol. 2005, 42, 239–250. [Google Scholar] [CrossRef]
- Brilli, F.; Hörtnagl, L.; Hammerle, A.; Haslwanter, A.; Hansel, A.; Loreto, F.; Wohlfahrt, G. Leaf and ecosystem response to soil water availability in mountain grasslands. Agric. For. Meteorol. 2011, 151, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Symstad, A.J.; Wienk, C.L.; Thorstenson, A.D. Precision, repeatability, and efficiency of two canopy-cover estimate methods in northern Great Plains vegetation. Rangel. Ecol. Manag. 2008, 61, 419–429. [Google Scholar] [CrossRef]
- Krueger, E.; Ochsner, T.E.; Levi, M.R.; Basara, J.B.; Snitker, G.J.; Wyatt, B.M. Grassland productivity estimates informed by soil moisture measurements: Statistical and mechanistic approaches. Agronomy 2021, 113, 3498–3517. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Wang, H.; Carrillo, C.M.; Ault, T.R. Unpacking the climatic drivers of US agricultural yields. Environ. Res. Lett. 2019, 14, 064003. [Google Scholar] [CrossRef]
- Haughey, E.; Suter, M.; Hofer, D.; Hoekstra, N.J.; McElwain, J.C.; Lüscher, A.; Finn, J.A. Higher species richness enhances yield stability in intensively managed grasslands with experimental disturbance. Sci. Rep. 2018, 8, 15047. [Google Scholar] [CrossRef]
- Skiba, U. Denitrification. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Oxford, UK; Academic Press: Oxford, UK, 2008; p. 866. [Google Scholar]
- Sándor, R.; Picon-Cochard, C.; Martin, R.; Louault, F.; Klumpp, K.; Borras, D.; Bellocchi, G. Plant acclimation to temperature: Developments in the pasture simulation model. Field Crop. Res. 2018, 222, 238–255. [Google Scholar] [CrossRef]
- Taubert, F.; Frank, K.; Huth, A. A review of grassland models in the biofuel context. Ecol. Model. 2012, 245, 84–93. [Google Scholar] [CrossRef]
- Taubert, F.; Hetzer, J.; Schmid, J.S.; Huth, A. The role of species traits for grassland productivity. Ecosphere 2020, 11, e03205. [Google Scholar] [CrossRef]
- Taubert, F. Modelling and Analysing the Structure and Dynamics of Species-Rich Grasslands and Forests; Helmholtz Centre for Environmental Research-UFZ, Department of Ecological: Leipzig, Germany, 2014. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
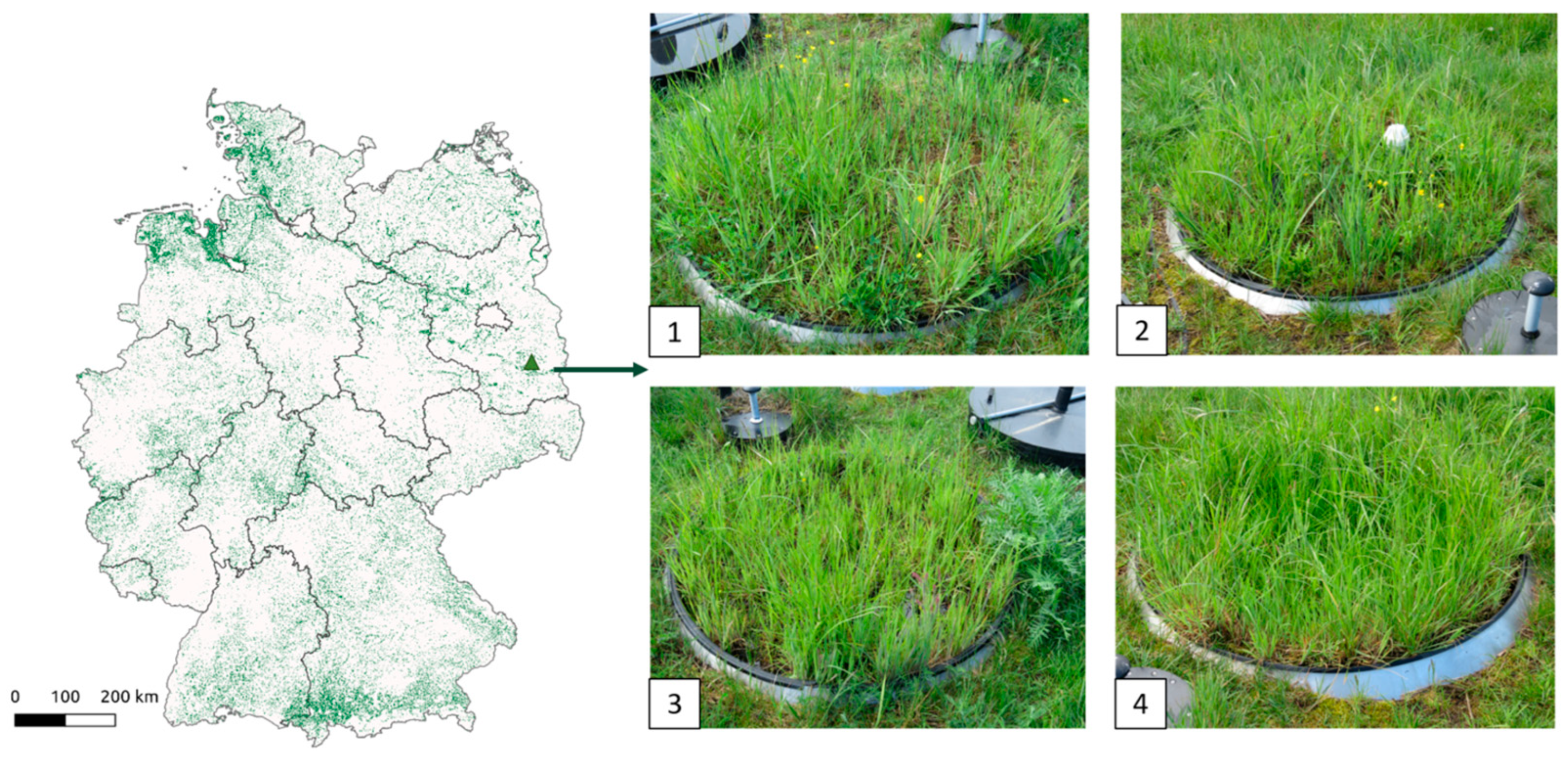

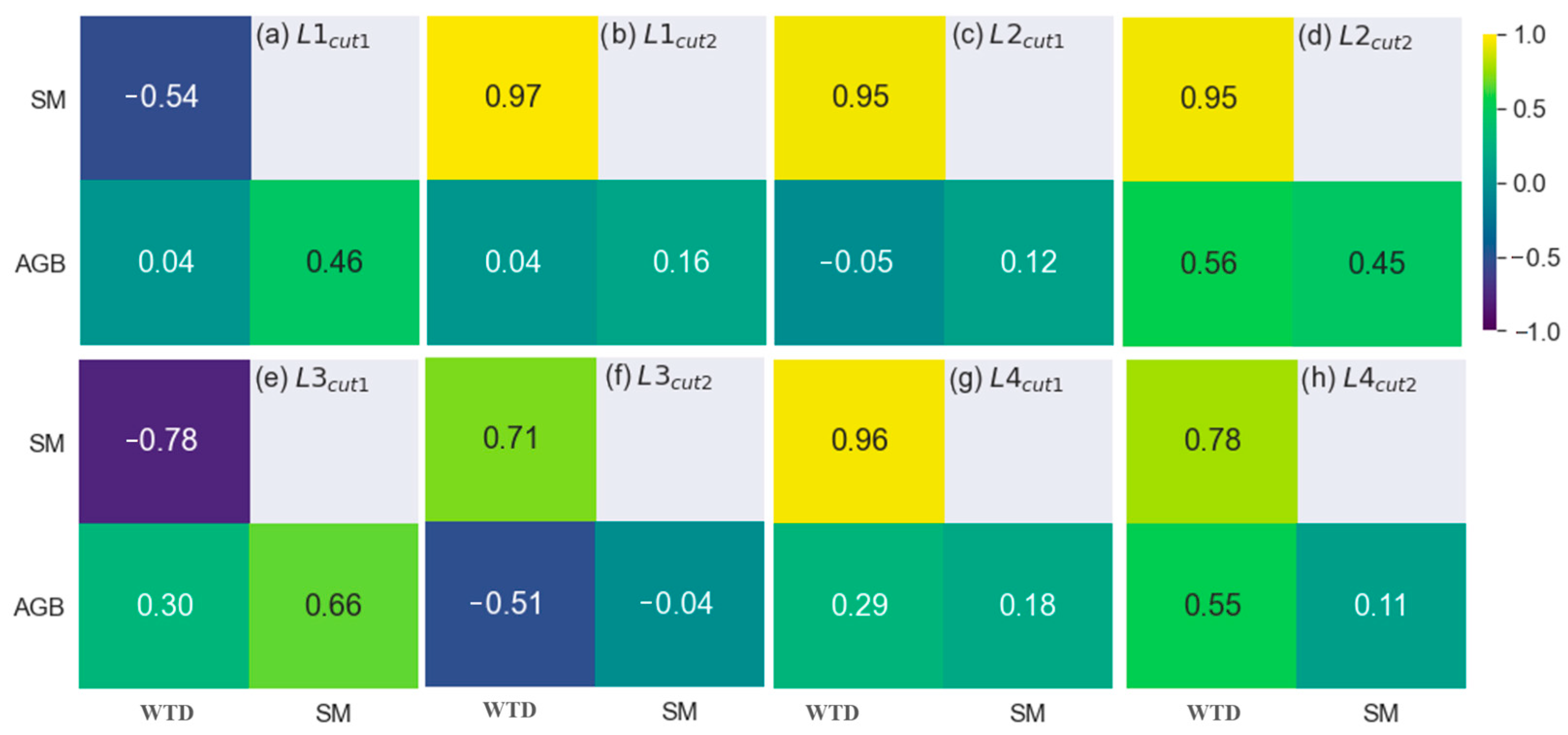
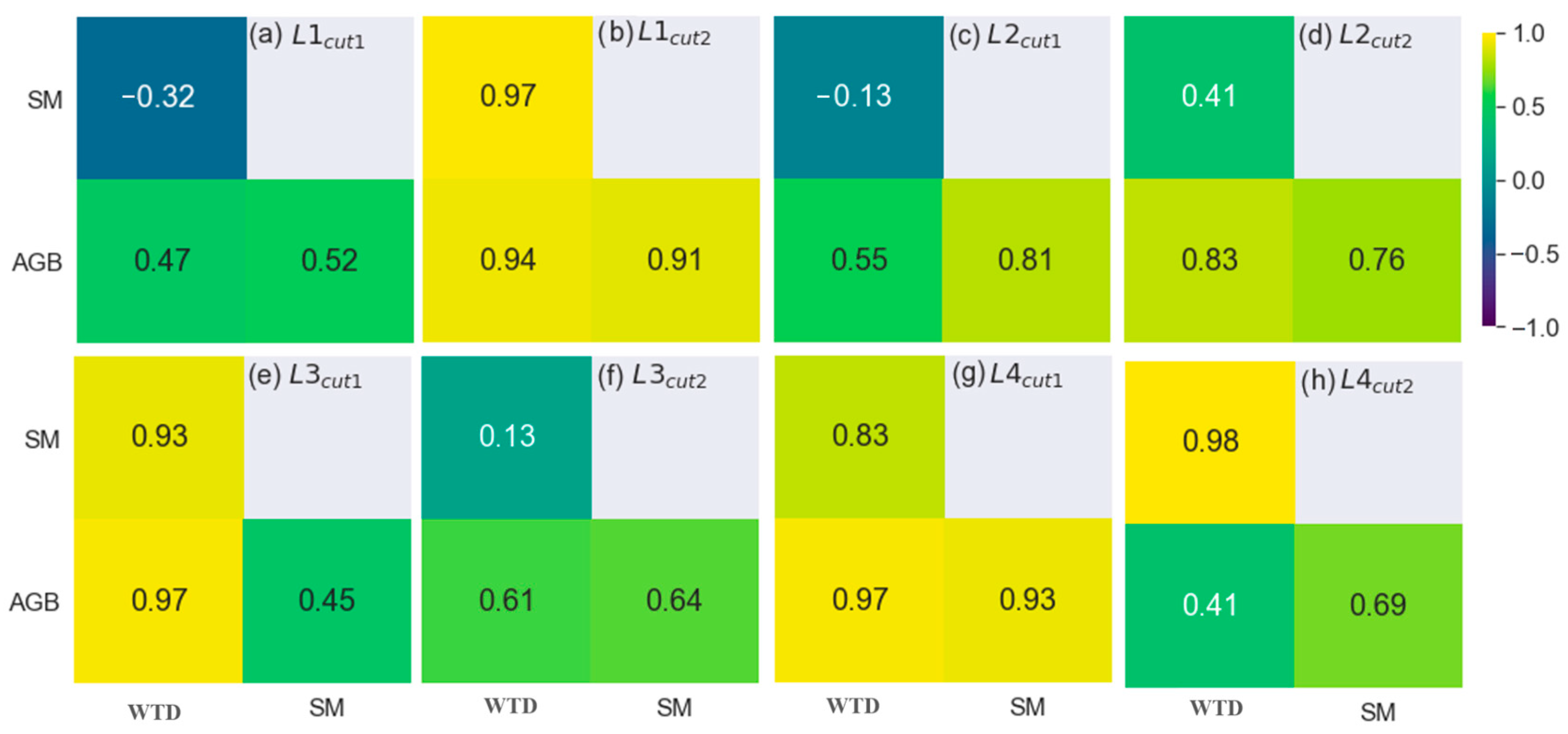
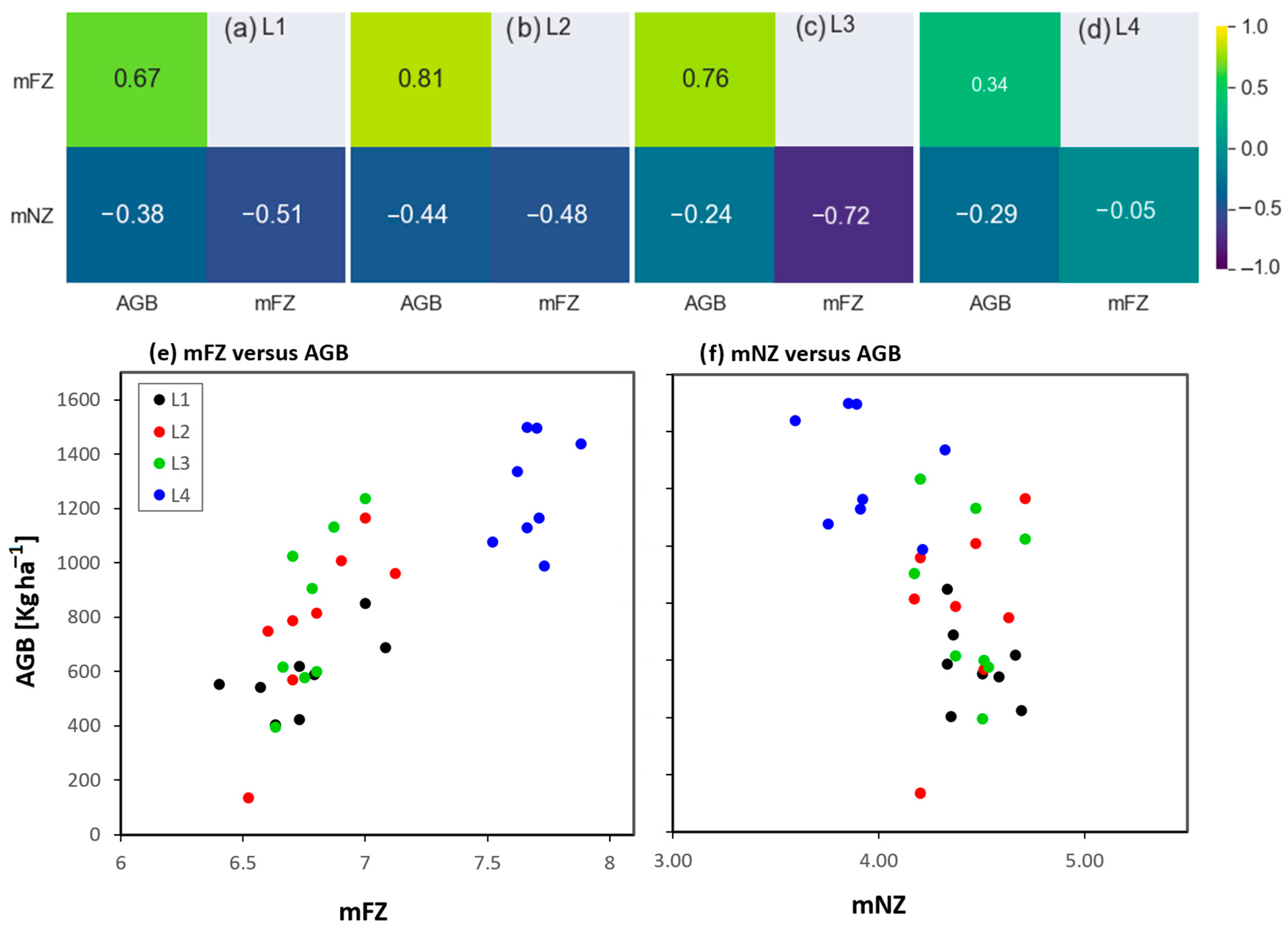
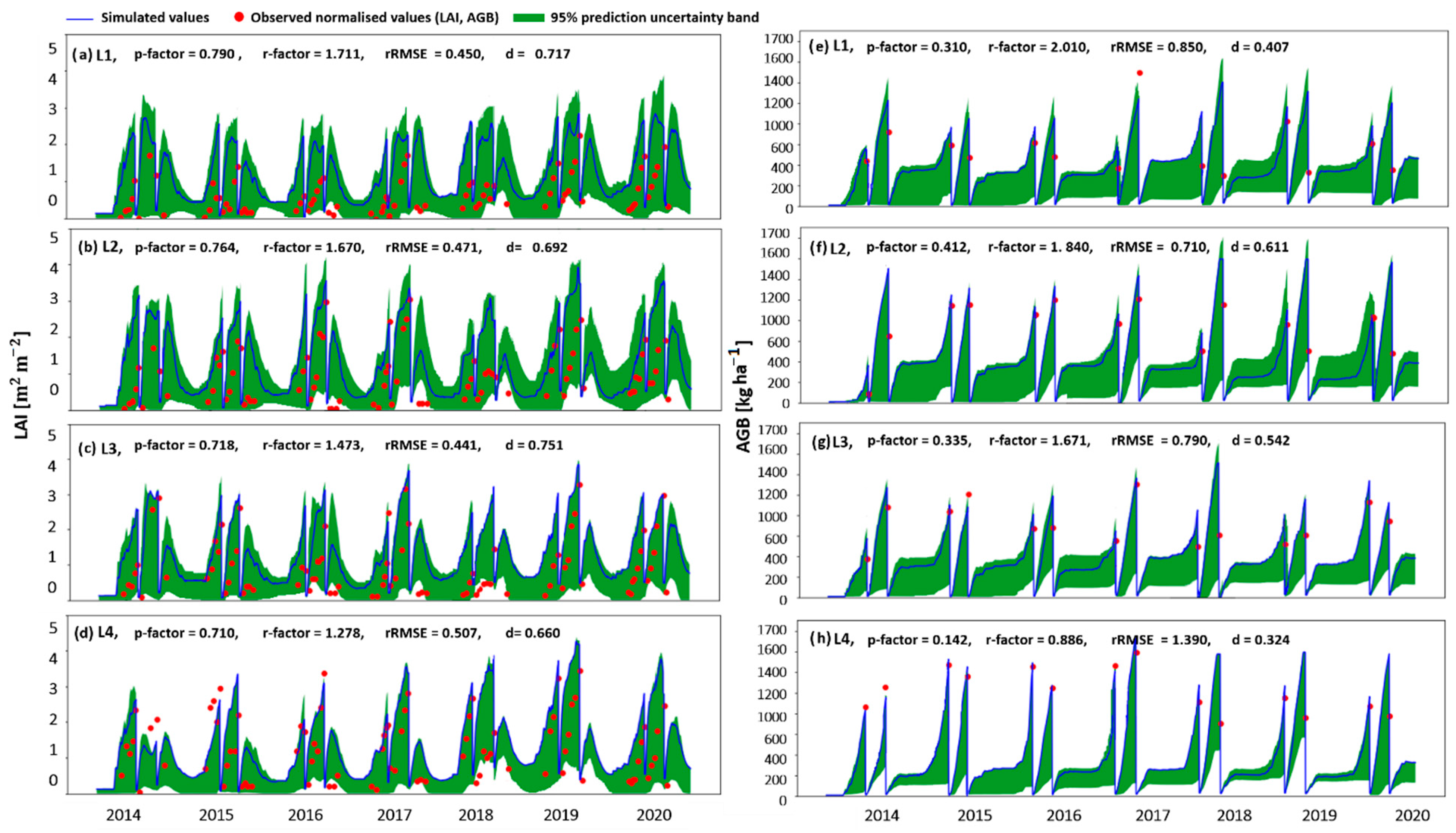
| Lysimeter | Soil Layers (cm) | Soil Type | Water Table Depth (WTD) (Shallowest to Deepest Values) (cm) |
|---|---|---|---|
| L1 | 0–14 | Sandy loam | −2.4 to −160 |
| 14–44 | Sandy loam | ||
| 44–63 | Sandy loam | ||
| 63–180 | Sand | ||
| L2 | 0–40 | Loam | −2.1 to −165 |
| 40–55 | Loamy sand | ||
| 55–180 | Sand | ||
| L3 | 0–10 | Loam | +4.7 to −130 |
| 10–27 | Loam | ||
| 27–48 | Sandy loam | ||
| 48–180 | Sand | ||
| L4 | 0–21 | Sandy loam | +8.2 to −125 |
| 21–32 | Clay loam | ||
| 32–56 | Sand | ||
| 56–180 | Sand |
| Leaf Area Index | Above-Ground Biomass | |||
|---|---|---|---|---|
| First Sim | Second Sim | First Sim | Second Sim | |
| rRMSE | 0.47 | 0.46 | 1.55 | 0.90 |
| d | 0.69 | 0.71 | 0.30 | 0.45 |
| p-factor | 0.73 | 0.75 | 0.15 | 0.30 |
| r-factor | 1.61 | 1.50 | 1.70 | 1.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaledi, V.; Kamali, B.; Lischeid, G.; Dietrich, O.; Davies, M.F.; Nendel, C. Challenges of Including Wet Grasslands with Variable Groundwater Tables in Large-Area Crop Production Simulations. Agriculture 2024, 14, 679. https://doi.org/10.3390/agriculture14050679
Khaledi V, Kamali B, Lischeid G, Dietrich O, Davies MF, Nendel C. Challenges of Including Wet Grasslands with Variable Groundwater Tables in Large-Area Crop Production Simulations. Agriculture. 2024; 14(5):679. https://doi.org/10.3390/agriculture14050679
Chicago/Turabian StyleKhaledi, Valeh, Bahareh Kamali, Gunnar Lischeid, Ottfried Dietrich, Mariel F. Davies, and Claas Nendel. 2024. "Challenges of Including Wet Grasslands with Variable Groundwater Tables in Large-Area Crop Production Simulations" Agriculture 14, no. 5: 679. https://doi.org/10.3390/agriculture14050679
APA StyleKhaledi, V., Kamali, B., Lischeid, G., Dietrich, O., Davies, M. F., & Nendel, C. (2024). Challenges of Including Wet Grasslands with Variable Groundwater Tables in Large-Area Crop Production Simulations. Agriculture, 14(5), 679. https://doi.org/10.3390/agriculture14050679







