Nitrogen Addition Mitigates Drought by Promoting Soybean (Glycine Max (Linn.) Merr) Flowering and Podding and Affecting Related Enzyme Activities
Abstract
:1. Introduction
2. Materials and Method
2.1. Biological Material and Field Experiment
2.2. Sampling and Measurement
2.2.1. A Survey of Soybean Flower Pod Shedding
2.2.2. Enzyme Extraction and MDA Content of Leaves
2.3. Statistical Analysis
3. Results and Analysis
3.1. Flower Pod Abortion
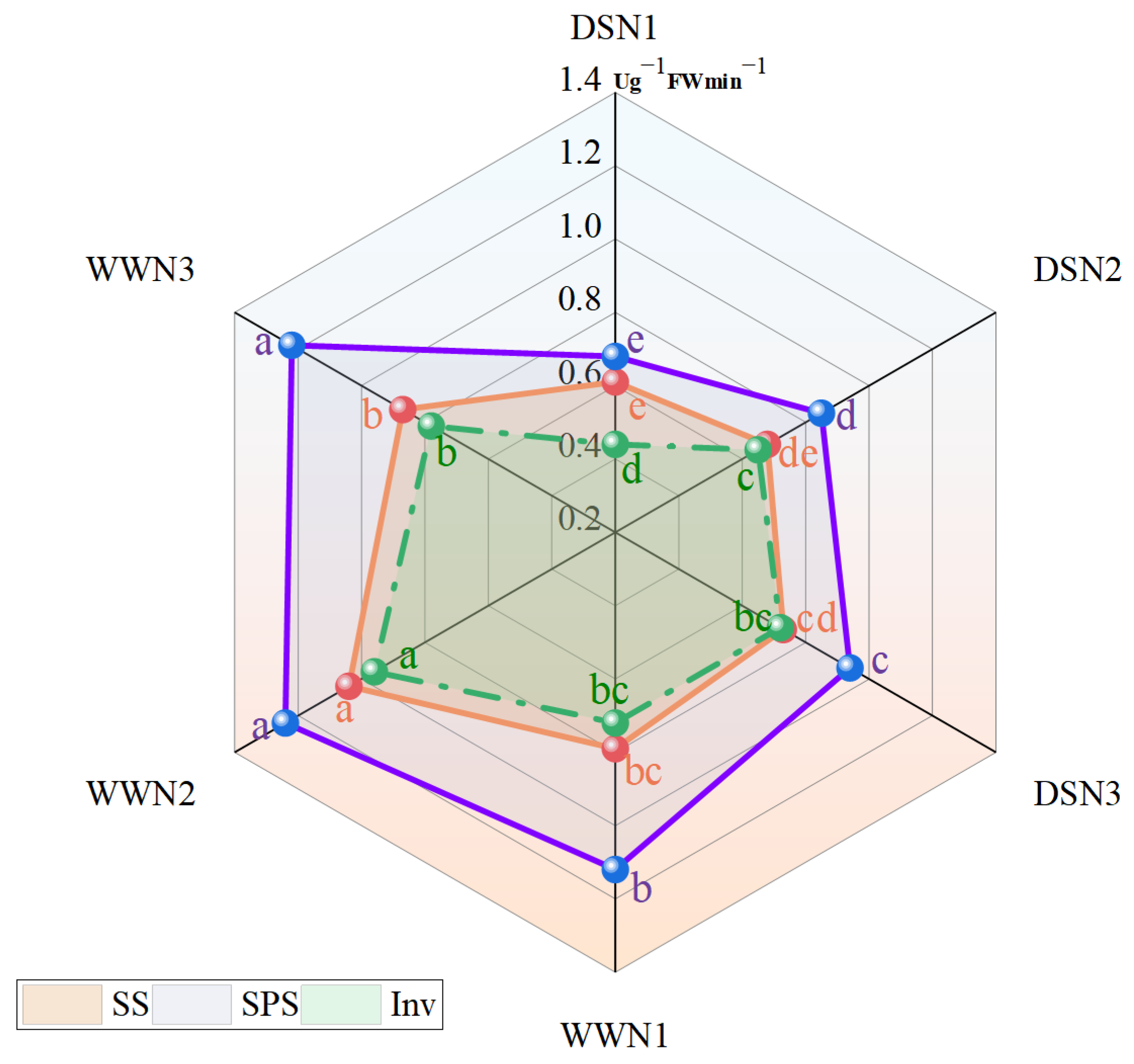
3.2. SS Activity, Inv Activity and SPS Activity in Leaves
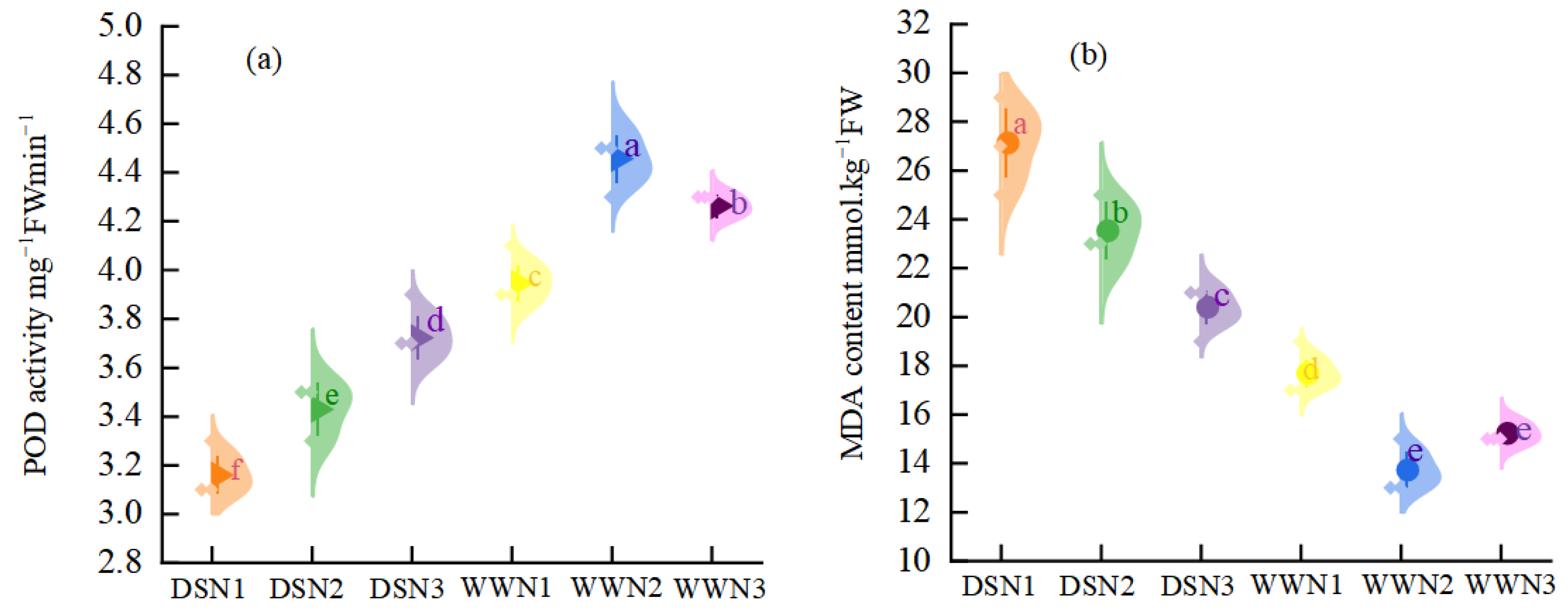
3.3. MDA Content and POD Activity
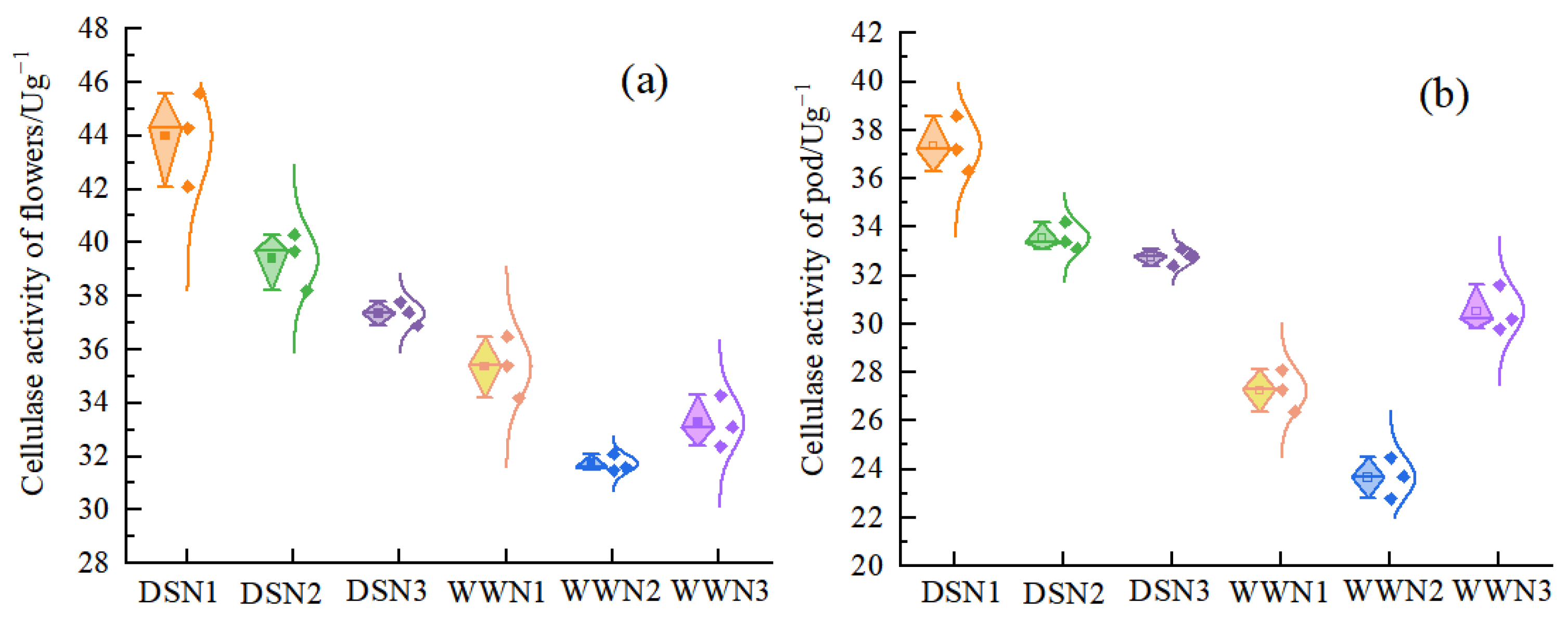
3.4. Cellulase Activity of Flowers and Pods
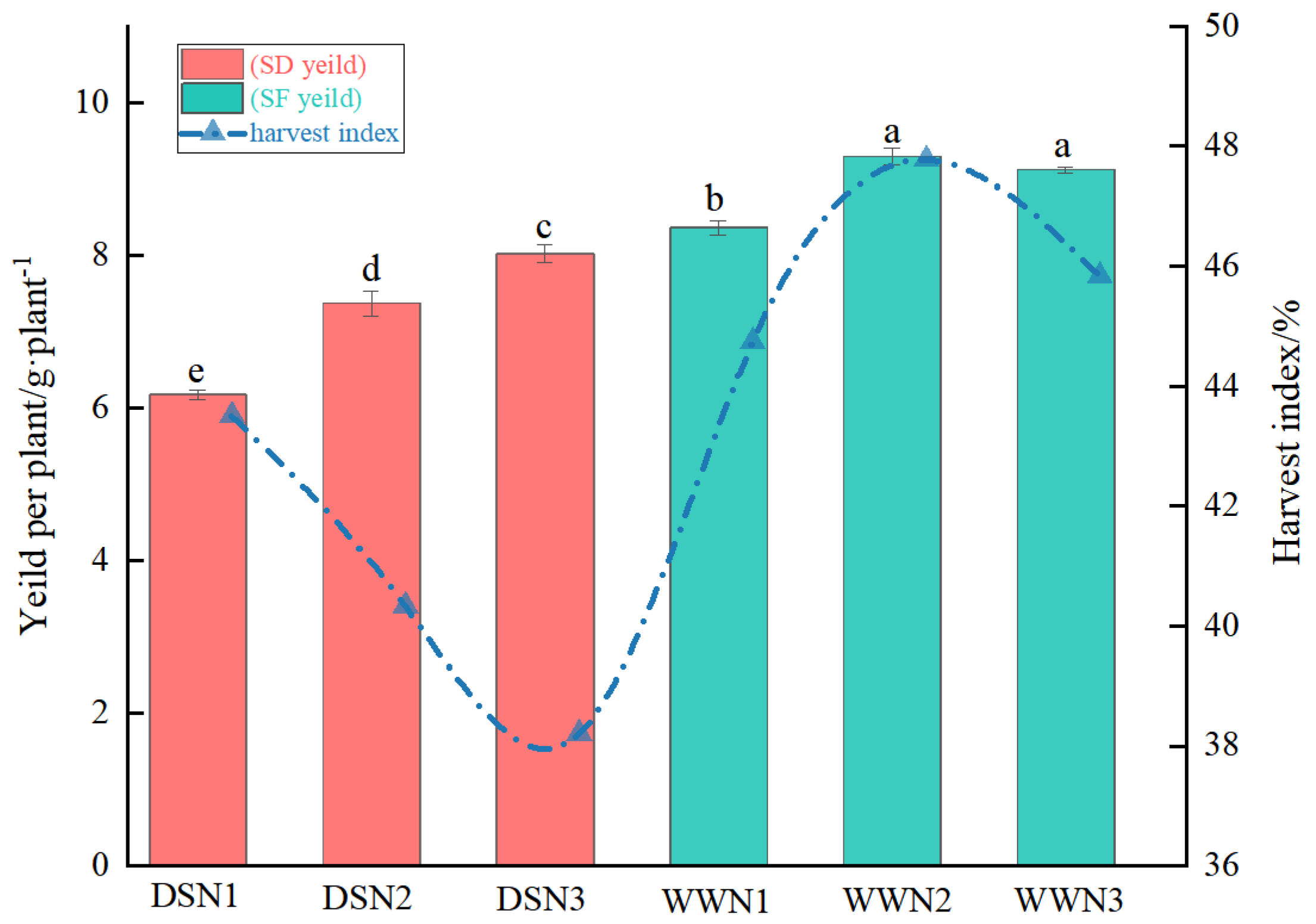
3.5. Yield Per Plant and Harvest Index
3.6. Correlation Analysis
4. Discussion
4.1. Effect of Nitrogen on Flower and Pod Abscission in Replanted Soybean under Drought Stress during Flowering and Podding Stages
4.2. Effect of Nitrogen on SS, SPS, INV, POD Activity and MDA under Drought Stress at Flowering and Podding Stage
4.3. Effect of Nitrogen on Cellulase Activity, Yield and Harvest Index under Drought Stress at Flowering and Podding Stage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Wang, S.; Wang, J.; Lu, H.; Guo, A.; Zhu, Z.; Myneni, R.B.; Shugart, H.H. Assessing spatiotemporal variation of drought in China and its impact on agriculture during 1982-2011 by using PDSI indices and agriculture drought survey data. J. Geophys. Res.-Atmos. 2016, 121, 2283–2298. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagon, D.; Arbona, V.; Gomez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotox. Environ. Safe. 2021, 216, 112195. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Zhou, B.; Cleary, D.; Xie, W. The Impact of Climate Change on China and Brazil’s Soybean Trade. Land 2022, 11, 2286. [Google Scholar] [CrossRef]
- Fei, C.; Dong, Y.Q.; An, S.Z. Factors driving the biomass and species richness of desert plants in northern Xinjiang China. PLoS ONE 2022, 17, e271575. [Google Scholar] [CrossRef] [PubMed]
- Zeleke, K.; Nendel, C. Yield response and water productivity of soybean (Glycine max L.) to deficit irrigation and sowing time in south-eastern Australia. Agric. Water Manag. 2024, 296, 108815. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen Metabolism in Adaptation of Photosynthesis to Water Stress in Rice Grown under Different Nitrogen Levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen Levels Regulate Sugar Metabolism and Transport in the Shoot Tips of Crabapple Plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, R.N.; Waraich, E.A.; Ali, H.; Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Awan, M.I.; Ahmad, S.; Irfan, M.; Hussain, S.; et al. Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2016, 23, 2651–2662. [Google Scholar] [CrossRef]
- He, F.; Zhao, Q.; Huang, J.; Niu, M.; Feng, H.; Shi, Y.; Zhao, K.; Cui, X.; Wu, X.; Mi, J.; et al. External application of nitrogen alleviates toxicity of cadmium on poplars via starch and sucrose metabolism. Tree Physiol. 2021, 41, 2126–2141. [Google Scholar] [CrossRef]
- Liao, G.; Yang, Y.; Xiao, W.; Mo, Z. Nitrogen Modulates Grain Yield, Nitrogen Metabolism, and Antioxidant Response in Different Rice Genotypes. J. Plant Growth Regul. 2023, 42, 2103–2114. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ying, Y.Q.; Zhang, Y.B.; Bi, Y.F.; Wang, A.K.; Du, X.H. Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci. Rep. 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, T.A.; Goswami, K.; Sanan-Mishra, N. Individual and Interactive Effects of Nitrogen and Phosphorus on Drought Stress Response and Recovery in Maize Seedlings. Agriculture 2023, 13, 654. [Google Scholar] [CrossRef]
- Coleman, K.; Whitmore, A.P.; Hassall, K.L.; Shield, I.; Semenov, M.A.; Dobermann, A.; Bourhis, Y.; Eskandary, A.; Milne, A.E. The potential for soybean to diversify the production of plant-based protein in the UK. Sci. Total Environ. 2021, 767, 144903. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Ghanati, F.; Ahmadi Gavlighi, H.; Sharifi, M. Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery. Biol. Plant. 2018, 62, 595–599. [Google Scholar] [CrossRef]
- Wang, W.; Kim, Y.; Lee, H.; Kim, K.; Deng, X.; Kwak, S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, L.; Li, M.; Lehmann, M.M.; Rigling, A.; Schaub, M.; Hoch, G.; Kahmen, A.; Gessler, A. Soil nutrient availability alters tree carbon allocation dynamics during drought. Tree Physiol. 2021, 41, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol.Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Batth, R.; Singh, K.; Kumari, S.; Mustafiz, A. Transcript Profiling Reveals the Presence of Abiotic Stress and Developmental Stage Specific Ascorbate Oxidase Genes in Plants. Front. Plant Sci. 2017, 8, 198. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 2016, 253, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jin, Y.; Yang, Y.; Li, G.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2-R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Chenna, S.; Koopman, W.J.H.; Prehn, J.H.M.; Connolly, N.M.C. Mechanisms and mathematical modeling of ROS production by the mitochondrial electron transport chain. Am. J. Physiol.-Cell Physiol. 2022, 323, C69–C83. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Ghassemi-Golezani, K.; Salmasi, S.Z. Changes in antioxidant enzymes activities and physiological traits of ajowan in response to water stress and hormonal application. Sci. Hortic. 2019, 246, 957–964. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of Cytokinin and Nitrogen on Drought Tolerance of Creeping Bentgrass. PLoS ONE 2016, 11, e154005. [Google Scholar] [CrossRef]
- Del Campillo, E.U.O.M.; Bennett, A.B. Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol. 1996, 111, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Chopra, J.; Kaur, N.; Gupta, A.K. Ontogenic changes in enzymes of carbon metabolism in relation to carbohydrate status in developing mungbean reproductive structures. Phytochemistry 2000, 53, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Yan, Z.; Qiao, Y.; Tian, H.; Hu, Z.; Zhang, Z.; Li, Y.; Zhao, H.; Xin, D.; et al. Exploring Soybean Flower and Pod Variation Patterns During Reproductive Period Based on Fusion Deep Learning. Front. Plant Sci. 2022, 13, 922030. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Sedri, M.H.; Roohi, E.; Niazian, M.; Niedbała, G. Interactive Effects of Nitrogen and Potassium Fertilizers on Quantitative-Qualitative Traits and Drought Tolerance Indices of Rainfed Wheat Cultivar. Agronomy 2022, 12, 30. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A. Drought and nitrogen source effects on nitrogen nutrition, seed growth, and yield in soybean. J. Plant Nutr. 1996, 19, 969–993. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Liu, Z.; Hu, Y.; Xia, Z.; Hu, B.; Rennenberg, H. Consequences of excess urea application on photosynthetic characteristics and nitrogen metabolism of Robinia pseudoacacia seedlings. Chemosphere 2024, 346, 140619. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Alamri, S.A.M.; Abd El-Mageed, T.A.; Abousekken, M.S.M.; Hashem, M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Liu, Y.; Xie, J.; Anwar, S.; Fudjoe, S.K. Nitrogen Supply Affects Yield and Grain Filling of Maize by Regulating Starch Metabolizing Enzyme Activities and Endogenous Hormone Contents. Front. Plant Sci. 2022, 12, 798119. [Google Scholar] [CrossRef]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kuehn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA Interference of LIN5 in Tomato Confirms Its Role in Controlling Brix Content, Uncovers the Influence of Sugars on the Levels of Fruit Hormones, and Demonstrates the Importance of Sucrose Cleavage for Normal Fruit Development and Fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- González-Carranza, Z.H.; Whitelaw, C.A.; Swarup, R.; Roberts, J.A. Temporal and Spatial Expression of a Polygalacturonase during Leaf and Flower Abscission in Oilseed Rape and Arabidopsis. Plant Physiol. 2002, 128, 534–543. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. High Nitrogen Enhance Drought Tolerance in Cotton through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Number of Flowers | Number of Abscission Flowers | Flower Abscission Rate/% | Number of Pods | Number of Abscission Pods | Pod Abscission Rate/% | Flower and Pod Abscission Rate/% |
|---|---|---|---|---|---|---|---|
| DSN1 | 55.33 ± 1.44 e | 30.87 ± 2.1 c | 55.72 ± 2.39 a | 24.47 ± 0.66 f | 11.40 ± 0.59 d | 46.57 ± 1.19 a | 76.36 ± 0.82 a |
| DSN2 | 67.70 ± 1.28 d | 37.80 ± 0.85 b | 55.84 ± 1.21 a | 29.90 ± 1.15 e | 12.80 ± 0.57 c | 42.70 ± 0.37 b | 74.74 ± 0.66 ab |
| DSN3 | 71.93 ± 0.48 c | 39.40 ± 0.41 ab | 54.78 ± 0.87 a | 32.53 ± 0.83 d | 13.43 ± 0.21 bc | 41.27 ± 0.62 bc | 73.45 ± 0.77 b |
| WWN1 | 78.13 ± 1.13 b | 42.20 ± 1.4 a | 54.00 ± 1.14 a | 35.93 ± 0.61 c | 14.40 ± 0.24 b | 40.07 ± 1.35 c | 72.43 ± 1.25 b |
| WWN2 | 84.47 ± 2.98 a | 42.47 ± 1.96 a | 50.26 ± 0.59 b | 42.00 ± 1.04 a | 16.00 ± 0.85 a | 36.37 ± 0.88 d | 69.21 ± 1.14 c |
| WWN3 | 80.47 ± 1.56 b | 42.17 ± 0.54 a | 52.41 ± 0.35 ab | 38.30 ± 1.02 b | 14.10 ± 0.24 bc | 36.83 ± 0.33 d | 69.93 ± 0.38 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, K.; Liu, J.; Nizam ul Din, G. Nitrogen Addition Mitigates Drought by Promoting Soybean (Glycine Max (Linn.) Merr) Flowering and Podding and Affecting Related Enzyme Activities. Agriculture 2024, 14, 852. https://doi.org/10.3390/agriculture14060852
Li M, Zhang K, Liu J, Nizam ul Din G. Nitrogen Addition Mitigates Drought by Promoting Soybean (Glycine Max (Linn.) Merr) Flowering and Podding and Affecting Related Enzyme Activities. Agriculture. 2024; 14(6):852. https://doi.org/10.3390/agriculture14060852
Chicago/Turabian StyleLi, Mengjiao, Kangxu Zhang, Jianguo Liu, and Ghulam Nizam ul Din. 2024. "Nitrogen Addition Mitigates Drought by Promoting Soybean (Glycine Max (Linn.) Merr) Flowering and Podding and Affecting Related Enzyme Activities" Agriculture 14, no. 6: 852. https://doi.org/10.3390/agriculture14060852
APA StyleLi, M., Zhang, K., Liu, J., & Nizam ul Din, G. (2024). Nitrogen Addition Mitigates Drought by Promoting Soybean (Glycine Max (Linn.) Merr) Flowering and Podding and Affecting Related Enzyme Activities. Agriculture, 14(6), 852. https://doi.org/10.3390/agriculture14060852







