Pisum sativum L. ‘Eso’: Metabolic Profiling of Yellow Seeds to Define the Optimal Harvest Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparation
2.3. NMR Experiments
2.4. Statistical Analysis
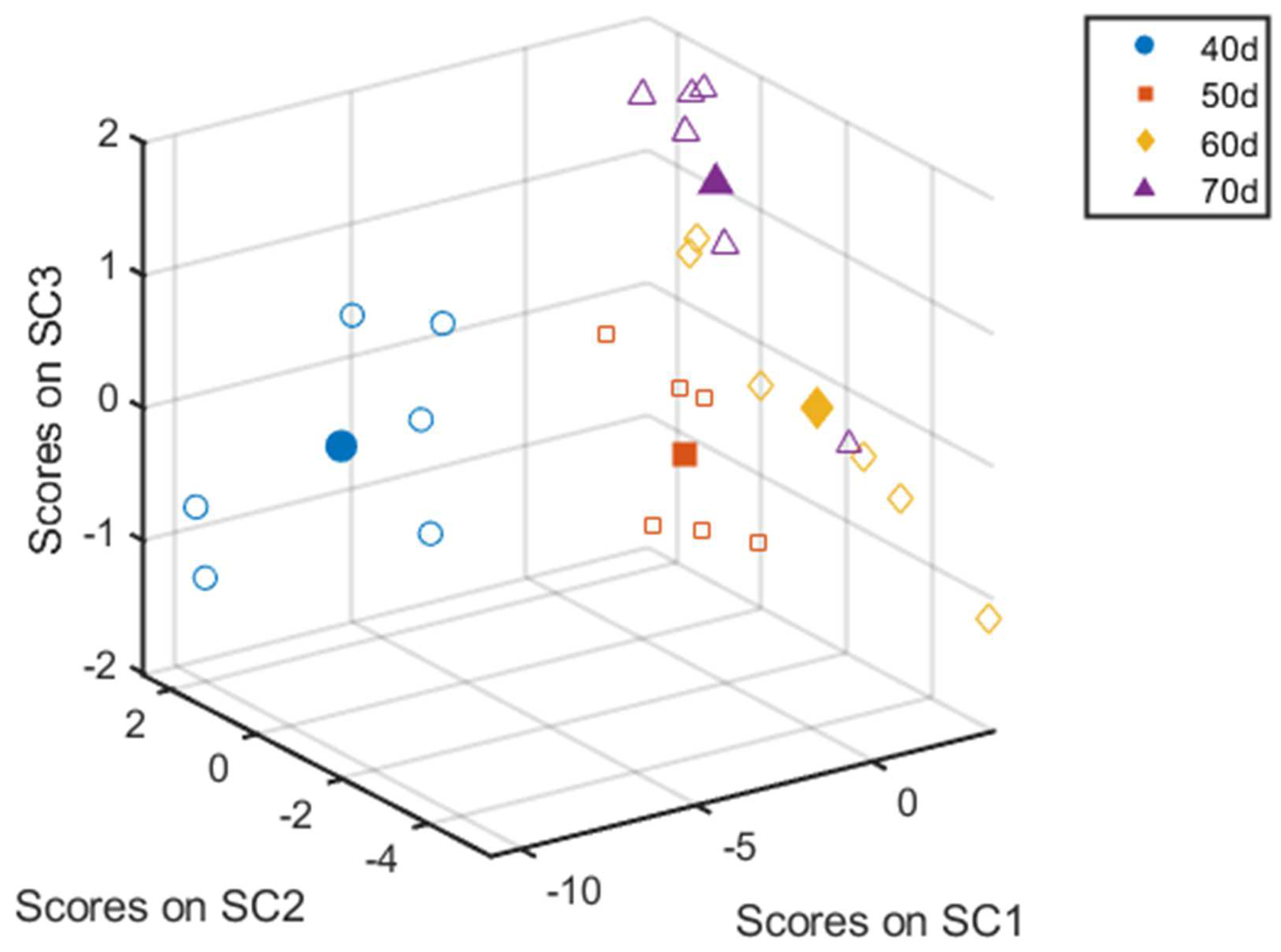
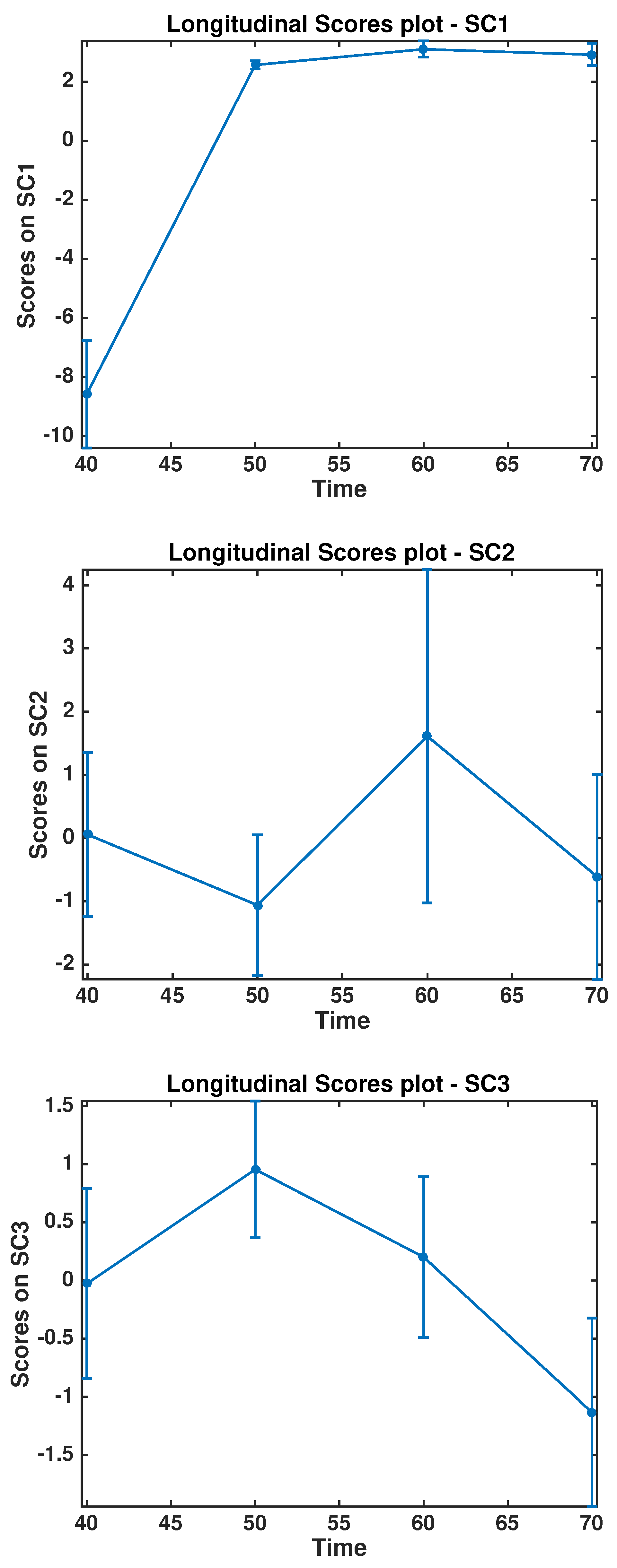
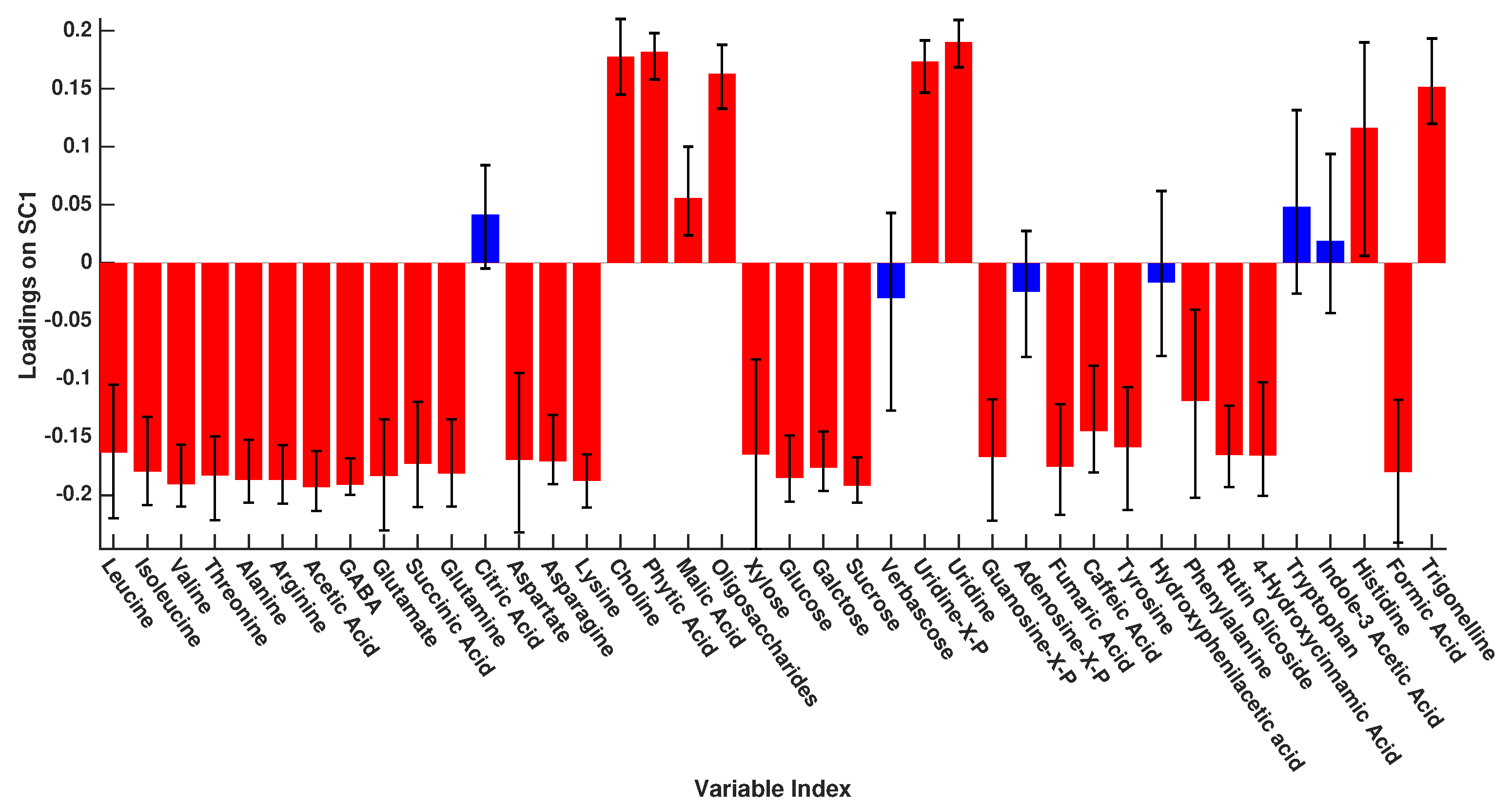
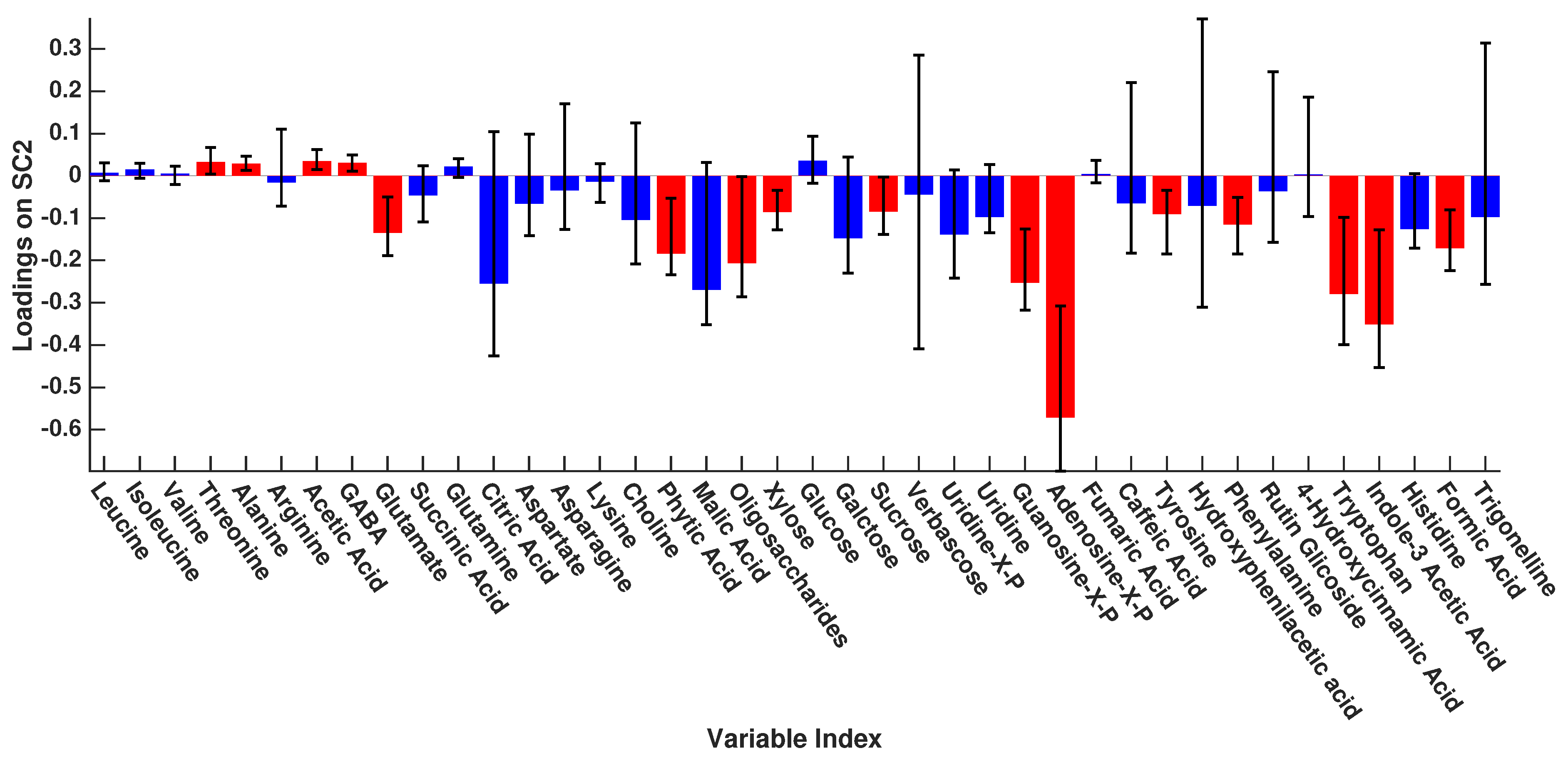
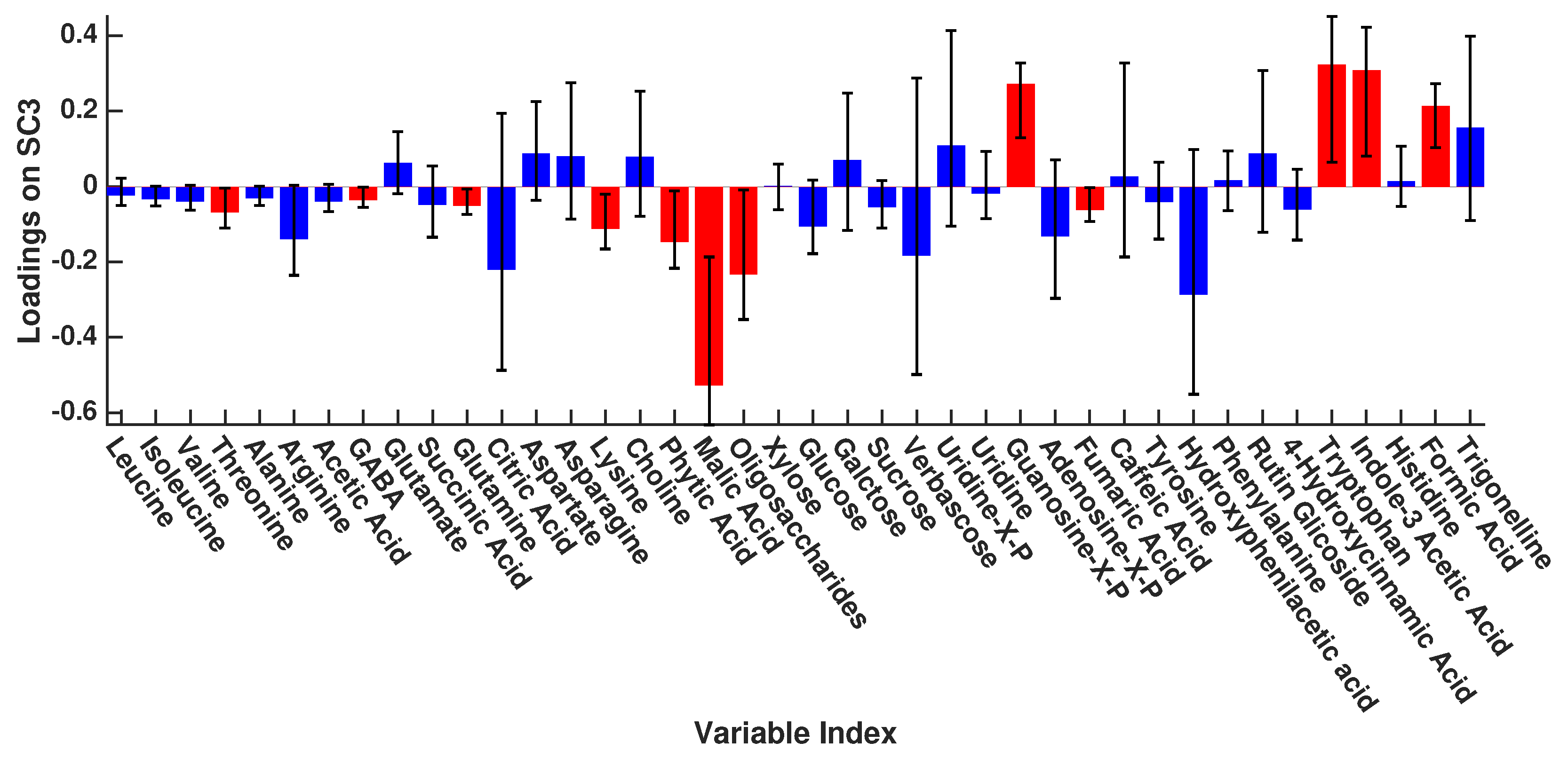
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mbow, C.; Rosenzweig, C.; Barioni, L.G.; Benton, T.G.; Herrero, M.; Krishnapillai, M.; Liwenga, E.; Pradhan, P.; Rivera-Ferre, M.G.; Sapkota, T.; et al. Climate Change and Land: Summary for Policymakers. An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems 2019; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- Zhang, H.; Yasmin, F.; Song, B.-H. Neglected Treasures in the Wild—Legume Wild Relatives in Food Security and Human Health. Curr. Opin. Plant Biol. 2019, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mendel, G. Experiments in Plant Hybridization. Verhandlungen des Naturforschenden Vereines in Brünn, Bd. IV für das Jahr Abhandlungen, 3–47. 1865. Available online: http://www.esp.org/foundations/genetics/classical/gm-65.pdf (accessed on 12 September 2023).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QV/visualize (accessed on 12 September 2023).
- Taherian, A.; Mondor, M.; Lamarche, F. Enhancing Nutritional Values and Functional Properties of Yellow Pea Protein via Membrane Processing. In Peas: Cultivation, Varieties and Nutritional Uses; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 1–48. [Google Scholar]
- Kumari, T.; Deka, S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Borreani, G.; Peiretti, P.G.; Tabacco, E. Effect of Harvest Time on Yield and Pre-Harvest Quality of Semi-Leafless Grain Peas (Pisum sativum L.) as Whole-Crop Forage. Field Crops Res. 2007, 100, 1–9. [Google Scholar] [CrossRef]
- Devi, J.; Sagar, V.; Mishra, G.P.; Jha, P.K.; Gupta, N.; Dubey, R.K.; Singh, P.M.; Behera, T.K.; Prasad, P.V.V. Heat Stress Tolerance in Peas (Pisum sativum L.): Current Status and Way Forward. Front. Plant Sci. 2023, 13, 1108276. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-954906-1. [Google Scholar]
- Wray, S.; Cenkowski, S. Nutritional Changes of Yellow Peas during Infrared Processing. Trans. ASAE 2002, 45, 1023–1027. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Searching in Database of Plant Varieties/the Czech National List. Available online: https://eagri.cz/public/app/sok/odrudyNouVF.do (accessed on 12 September 2023).
- Boye, J.I.; Ma, Z. Finger on the Pulse. Food Sci. Technol. 2012, 26, 20–24. [Google Scholar]
- Smith, C.E.; Mollard, R.C.; Luhovyy, B.L.; Anderson, G.H. The Effect of Yellow Pea Protein and Fibre on Short-Term Food Intake, Subjective Appetite and Glycaemic Response in Healthy Young Men. Br. J. Nutr. 2012, 108 (Suppl. 1), S74–S80. [Google Scholar] [CrossRef]
- Bellaio, S.; Zamprogna Rosenfeld, E.; Jacobs, M.; Basu, S.; Kappeler, S. PARGEM, the Technology for a New Family of Healthy, Safe, and Convenient Food Ingredients Based on Partial Germination. In Proceedings of the AACC International Annual Meeting, Hollywood, FL, USA, 30 September–3 October 2012. [Google Scholar]
- Deshpande, S.S. (Ed.) Toxicants and Antinutrients in Plant Foods. In Handbook of Food Toxicology; Chapter 10; CRC Press: Boca Raton, FL, USA, 2002; pp. 321–386. [Google Scholar]
- Lajolo, F.M.; Genovese, M.I.; Pryme, I.F.; Dale, T.M. Beneficial (Antiproliferative) Effects of Different Substances. Recent Advances of Research in Antinutritional Factors in Legume Seeds 8 of 9 | RIB EREAU ET AL. and Oilseeds. In Proceedings of the Fourth International Workshop on Antinutritional Factors in Legume Seeds and Oilseeds, Toledo, Spain, 8–10 March 2004; Muzquiz, M., Hill, G.D., Cuadrado, C., Pedrosa, M.M., Burbano, C., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004; pp. 123–135. [Google Scholar]
- Sciubba, F.; Tomassini, A.; Giorgi, G.; Brasili, E.; Pasqua, G.; Capuani, G.; Aureli, W.; Miccheli, A. NMR-Based Metabolomic Study of Purple Carrot Optimal Harvest Time for Utilization as a Source of Bioactive Compounds. Appl. Sci. 2020, 10, 8493. [Google Scholar] [CrossRef]
- Giampaoli, O.; Sciubba, F.; Conta, G.; Capuani, G.; Tomassini, A.; Giorgi, G.; Brasili, E.; Aureli, W.; Miccheli, A. Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year. Foods 2021, 10, 1887. [Google Scholar] [CrossRef]
- Hadrich, F.; Arbi, M.E.; Boukhris, M.; Sayadi, S.; Cherif, S. Valorization of the Peel of Pea: Pisum Sativum by Evaluation of Its Antioxidant and Antimicrobial Activities. J. Oleo Sci. 2014, 63, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Li, W.-X.; Wan, J.-J.; Hu, Y.-C.; Gan, R.-Y.; Zou, L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023, 12, 2527. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, V.; Brasili, E.; Sciubba, F.; Ceci, A.; Giampaoli, O.; Miccheli, A.; Pasqua, G.; Persiani, A.M. Biostimulant Effects of Chaetomium globosum and Minimedusa polyspora Culture Filtrates on Cichorium intybus Plant: Growth Performance and Metabolomic Traits. Front. Plant Sci. 2022, 13, 879076. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-Based Metabolomic Analysis of Plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- van Rooyen, J.; Marini, F.; Orth, S.H.; Oyeyinka, S.A.; Simsek, S.; Manley, M. Effect of wheat roasting conditions and wheat type on short-wave infrared (SWIR) spectral data of whole and milled wheat by ANOVA-simultaneous component analysis. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2023, 303, 123160. [Google Scholar] [CrossRef] [PubMed]
- Rust, A.; Marini, F.; Allsopp, M.; Williams, P.J.; Manley, M. Application of ANOVA-simultaneous component analysis to quantify and characterise effects of age, temperature, syrup adulteration and irradiation on near-infrared (NIR) spectral data of honey. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2021, 253, 119546. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.; Bucci, R.; Materazzi, S.; Marini, F. Application of near infrared (NIR) spectroscopy coupled to chemometrics for dried egg-pasta characterization and egg content quantification. Food Chem. 2013, 140, 726–734. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Stevens, J.P. Intermediate Statistics: A Modern Approach; Routledge: London, UK, 2007; ISBN 978-0-8058-5465-7. [Google Scholar]
- Peluso, A.; Glen, R.; Ebbels, T.M.D. Multiple-Testing Correction in Metabolome-Wide Association Studies. BMC Bioinform. 2021, 22, 67. [Google Scholar] [CrossRef]
- Andreoli, V.; Bagliani, M.; Corsi, A.; Frontuto, V. Drivers of Protein Consumption: A Cross-Country Analysis. Sustainability 2021, 13, 7399. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.L.R.; Dobermann, D.; Forkes, A.; House, J.; Josephs, J.; McBride, A.; Müller, A.; Quilliam, R.S.; Soares, S. Insects as Food and Feed: European Perspectives on Recent Research and Future Priorities. J. Insects Food Feed 2016, 2, 269–276. [Google Scholar] [CrossRef]
- Mancini, S.; Moruzzo, R.; Riccioli, F.; Paci, G. European Consumers’ Readiness to Adopt Insects as Food. A Review. Food Res. Int. 2019, 122, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Pursley, A.A.; Biligetu, B.; Warkentin, T.; Lardner, H.A.; Penner, G.B. Effect of Stage of Maturity at Harvest for Forage Pea (Pisum sativum L.) on Eating Behavior, Ruminal Fermentation, and Digestibility When Fed as Hay to Yearling Beef Heifers. Transl. Anim. Sci. 2019, 4, 149–158. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Tömösközi, S.; Lásztity, R.; Haraszi, R.; Baticz, O. Isolation and Study of the Functional Properties of Pea Proteins. Nahrung 2001, 45, 399–401. [Google Scholar] [CrossRef]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series No. 935; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Han, Q.; Phillips, R.S.; Li, J. Editorial: Aromatic Amino Acid Metabolism. Front. Mol. Biosci. 2019, 6, 22. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Hausler, R.E.; Ludewig, F.; Krueger, S. Amino Acids—A Life between Metabolism and Signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Veselova, T.V.; Veselovsky, V.A.; Obroucheva, N.V. Deterioration Mechanisms in Air-Dry Pea Seeds during Early Aging. Plant Physiol. Biochem. 2015, 87, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of Free Amino Acids Compared with an Equivalent Amount of Intact Protein Results in More Rapid Amino Acid Absorption and Greater Postprandial Plasma Amino Acid Availability without Affecting Muscle Protein Synthesis Rates in Young Adults in a Double-Blind Randomized Trial. J. Nutr. 2022, 152, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schwender, J.; Ohlrogge, J.; Shachar-Hill, Y. Understanding flux in plant metabolic networks. Curr. Opin. Plant Biol. 2004, 7, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Frame, D.J.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Górecki, R.J.; Zalewski, K.; Hedley, C.L. Sorbitol Accumulation during Natural and Accelerated Ageing of Pea (Pisum sativum L.) Seeds. Acta Physiol. Plant 2007, 29, 527–534. [Google Scholar] [CrossRef]
- Salvi, P.; Varshney, V.; Majee, M. Raffinose Family Oligosaccharides (RFOs): Role in Seed Vigor and Longevity. Biosci. Rep. 2022, 42, BSR20220198. [Google Scholar] [CrossRef]
- Horbowicz, M.; Obendorf, R.L. Seed Desiccation Tolerance and Storability: Dependence on Flatulence-Producing Oligosaccharides and Cyclitols—Review and Survey. Seed Sci. Res. 1994, 4, 385–405. [Google Scholar] [CrossRef]
- Kanwal, F.; Ren, D.; Kanwal, W.; Ding, M.; Su, J.; Shang, X. The Potential Role of Nondigestible Raffinose Family Oligosac-Charides as Prebiotics. Glycobiology 2023, 33, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose Family Oligosaccharides: Friend or Foe for Human and Plant Health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A. Phytate Metabolism with Special Reference to Its Myo-Inositol Component. In Mobilization of Reserves in Germination; Nozzolillo, C., Lea, P.J., Loewus, F.A., Eds.; Recent Advances in Phytochemistry; Springer: Boston, MA, USA, 1983; pp. 173–192. ISBN 978-1-4684-1167-6. [Google Scholar]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on Environment and Human Nutrition. A Challenge for Molecular Breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S330–S375. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamon, E.; Pedrosa, M.M. Bioac-Tive Compounds in Legumes: Pronutritive and Antinutritive Actions, Implications for Nutrition and Health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Wink, M. Evolution of Secondary Metabolites in Legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- De-la-Cruz Chacón, I.; Riley-Saldaña, C.A.; González-Esquinca, A.R. Secondary Metabolites during Early Development in Plants. Phytochem. Rev. 2013, 12, 47–64. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 20, e00370. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic acids in cereal grain: Occurrence, biosynthesis, metabolism and role in living organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef]
- El-El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in Islamic medicine. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 55, pp. 269–292. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Witte, C.-P.; Herde, M. Nucleotide Metabolism in Plants. Plant Physiol. 2020, 182, 63–78. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive Proteins and Peptides in Pulse Crops: Pea, Chickpea and Lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A Plant Alkaloid with Therapeutic Potential for Diabetes and Central Nervous System Disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A. Shikimate and Phenylalanine Biosynthesis in the Green Lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Akbar, M.H.; Siddiqui, S.A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S.B. Plant-Based Meat Alternatives: Compositional Analysis, Current Development and Challenges. Appl. Food Res. 2022, 2, 100154. [Google Scholar] [CrossRef]
| Molecule | Amount (mg/100 g) | |||
|---|---|---|---|---|
| 40th Day of Harvest | 50th Day of Harvest | 60th Day of Harvest | 70th Day of Harvest | |
| Amino Acids | ||||
| Arginine | 246.392 ± 32.61 a | 73.76 ± 13.54 b | 70.87 ± 27.21 b | 90.78 ± 23.28 b |
| Glutamate | 168.086 ± 67.492 a | 0.702 ± 0.294 b | 0.547 ± 0.472 b | 4.73 ± 7.17 b |
| Alanine | 162.031 ± 52.503 a | 1.647 ± 0.385 b | 1.685 ± 1.01 b | 2.421 ± 0.918 b |
| Aspartate | 105.176 ± 22.558 a | 46.88 ± 9.091 b | 38.87 ± 17.67 b | 38.85 ± 16.27 b |
| GABA | 84.714 ± 20.612 a | 0.212 ± 0.133 b | 0.476 ± 0.073 b | 1.01 ± 1.09 b |
| Threonine | 77.822 ± 28.036 a | 1.354 ± 0.152 b | 2.762 ± 1.858 b | 4.591 ± 4.359 b |
| Glutamine | 71.953 ± 10.192 a | 36.897 ± 4.994 b | 27.178 ± 6.197 b | 31.96 ± 4.95 b |
| Valine | 34.402 ± 8.278 a | 2.264 ± 0.239 b | 1.389 ± 0.257 b | 2.521 ± 1.513 b |
| Asparagine | 28.834 ± 6.777 a | 15.09 ± 2.545 b | 12.423 ± 3.855 b | 12.9 ± 2.37 b |
| Isoleucine | 19.149 ± 7.976 a | 0.792 ± 0.094 b | 0.507 ± 0.095 b | 0.902 ± 0.569 b |
| Phenylalanine | 8.662 ± 5.512 a | 4.732 ± 0.575 a | 3.446 ± 0.467 b | 4.296 ± 1.031 a |
| Leucine | 5.481 ± 3.046 a | 0.799 ± 0.044 b | 0.664 ± 0.153 b | 0.778 ± 0.232 b |
| Tyrosine | 5.087 ± 2.572 a | 1.375 ± 0.189 b | 0.732 ± 0.075 b | 1.346 ± 0.857 b |
| Lysine | 4.449 ± 0.989 a | 0.679 ± 0.063 b | 0.585 ± 0.143 b | 0.953 ± 0.455 b |
| Histidine | 3.544 ± 4.204 a | 7.354 ± 0.625 b | 6.608 ± 1.192 a | 7.233 ± 0.746 a |
| Tryptophan | 1.459 ± 1.366 a | 2.413 ± 0.3 a | 1.628 ± 0.489 a | 1.774 ± 0.447 a |
| Organic Acids | ||||
| Citric acid | 73.891 ± 7.924 a | 82.547 ± 17.109 a | 74.546 ± 18.95 a | 88.409 ± 16.729 a |
| Acetic acid | 46.783 ± 8.94 a | 1.137 ± 0.14 b | 1.508 ± 0.468 b | 1.688 ± 0.85 b |
| 4-Hydroxycinnamic acid | 3.436 ± 0.889 a | 1.698 ± 0.163 b | 1.666 ± 0.524 b | 1.759 ± 0.206 b |
| Succinic acid | 3.059 ± 1.287 a | 0.532 ± 0.323 b | 0.294 ± 0.232 b | 0.556 ± 0.148 b |
| Caffeic Acid | 1.545 ± 0.324 a | 1.016 ± 0.126 a | 0.921 ± 0.273 b | 0.968 ± 0.239 b |
| Malic acid | 1.415 ± 0.276 a | 1.573 ± 0.289 a | 1.46 ± 0.445 a | 1.961 ± 0.308 a |
| Hydroxyphenylacetic acid | 1.133 ± 0.173 a | 1.072 ± 0.153 a | 1.074 ± 0.212 a | 1.161 ± 0.106 a |
| Fumaric acid | 0.861 ± 0.36 a | 0.111 ± 0.01 b | 0.098 ± 0.035 b | 0.136 ± 0.043 b |
| Formic acid | 0.394 ± 0.066 a | 0.198 ± 0.04 b | 0.111 ± 0.037 b | 0.126 ± 0.02 b |
| Carbohydrates | ||||
| Sucrose | 2569 ± 241.83 a | 1017.7 ± 83.47 b | 804.3 ± 201.97 b | 1021.7 ± 134.9 b |
| Oligosaccharides | 430.86 ± 35.36 a | 1042 ± 150.99 b | 944.9 ± 241.81 b | 1193 ± 190.76 b |
| Xilose | 43.641 ± 19.669 a | 13.354 ± 3.239 b | 7.794 ± 1.967 b | 11.586 ± 2.725 b |
| Galactose | 7.125 ± 0.563 a | 4.162 ± 0.527 b | 3.231 ± 1.023 b | 3.687 ± 0.878 b |
| Glucose | 5.514 ± 1.413 a | 0.569 ± 0.151 b | 0.735 ± 0.298 b | 0.947 ± 0.668 b |
| Verbascose | 1.425 ± 0.323 a | 1.301 ± 0.126 a | 1.303 ± 0.266 a | 1.395 ± 0.349 a |
| Other Compounds | ||||
| Trigonelline | 14.27 ± 2.39 a | 30.53 ± 3.60 b | 28.059 ± 8.99 b | 27.896 ± 4.332 b |
| Choline | 6.49 ± 1.97 a | 23.852 ± 3.094 b | 21.917 ± 5.062 b | 22.654 ± 3.257 b |
| Phytate | 4.33 ± 1.78 a | 305.284 ± 38.07 b | 264.56 ± 69.41 b | 346.66 ± 55.00 b |
| Rutin-Glycoside | 4.12 ± 0.577 a | 2.385 ± 0.339 b | 2.132 ± 0.757 b | 2.124 ± 0.589 b |
| Adenosine Phosphate | 3.59 ± 0.275 a | 3.763 ± 0.542 a | 2.718 ± 0.904 c | 3.77 ± 0.537 d |
| Guanosine Phosphate | 2.65 ± 0.437 a | 1.663 ± 0.312 b | 0.937 ± 0.331 c | 1.107 ± 0.206 b |
| Indole-3-Acetic acid | 2.21 ± 1.16 a | 2.876 ± 0.324 a | 2.026 ± 0.602 a | 2.292 ± 0.408 a |
| Uridine Phosphate | 0.352 ± 0.114 a | 3.97 ± 0.477 b | 3.38 ± 0.602 b | 3.592 ± 1.266 b |
| Uridine | 0.197 ± 0.03 a | 2.85 ± 0.166 b | 2.673 ± 0.5 b | 2.921 ± 0.358 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patriarca, A.; Sciubba, F.; Tomassini, A.; Giampaoli, O.; De Rosa, M.; Marini, F.; Aureli, W.; Miccheli, A.; Brasili, E. Pisum sativum L. ‘Eso’: Metabolic Profiling of Yellow Seeds to Define the Optimal Harvest Time. Agriculture 2024, 14, 855. https://doi.org/10.3390/agriculture14060855
Patriarca A, Sciubba F, Tomassini A, Giampaoli O, De Rosa M, Marini F, Aureli W, Miccheli A, Brasili E. Pisum sativum L. ‘Eso’: Metabolic Profiling of Yellow Seeds to Define the Optimal Harvest Time. Agriculture. 2024; 14(6):855. https://doi.org/10.3390/agriculture14060855
Chicago/Turabian StylePatriarca, Adriano, Fabio Sciubba, Alberta Tomassini, Ottavia Giampaoli, Michele De Rosa, Federico Marini, Walter Aureli, Alfredo Miccheli, and Elisa Brasili. 2024. "Pisum sativum L. ‘Eso’: Metabolic Profiling of Yellow Seeds to Define the Optimal Harvest Time" Agriculture 14, no. 6: 855. https://doi.org/10.3390/agriculture14060855
APA StylePatriarca, A., Sciubba, F., Tomassini, A., Giampaoli, O., De Rosa, M., Marini, F., Aureli, W., Miccheli, A., & Brasili, E. (2024). Pisum sativum L. ‘Eso’: Metabolic Profiling of Yellow Seeds to Define the Optimal Harvest Time. Agriculture, 14(6), 855. https://doi.org/10.3390/agriculture14060855










