Transformation and Sequestration of Total Organic Carbon in Black Soil under Different Fertilization Regimes with Straw Carbon Inputs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Measurement Methods
2.4. Calculations Method
- (1)
- The calculation formula [15] for the proportion of maize straw-derived carbon in SOC (Fmaize, %) is as follows:
- (2)
- The calculation formula [16] for the content of maize straw-derived carbon (Cmaize, g·kg−1) in SOC is as follows:
- (3)
- The calculation formula [17] for the residue rate of maize straw-derived carbon in soil (Rmaize, %) is as follows:
- (4)
- The calculation formula [18] for the stabilization rate of SOC (S, kg·m−2·day−1) is as follows:
- (5)
- The SOC stock is the amount of organic carbon in a fixed soil layer per unit land area, usually expressed in Mg·ha−1. The calculation for SOC stock is carried out using the following formula:
2.5. Statistical Analytics
3. Results
3.1. Changes of SOC Content and δ13C Value in Black Soil with Different Fertilization Treatments
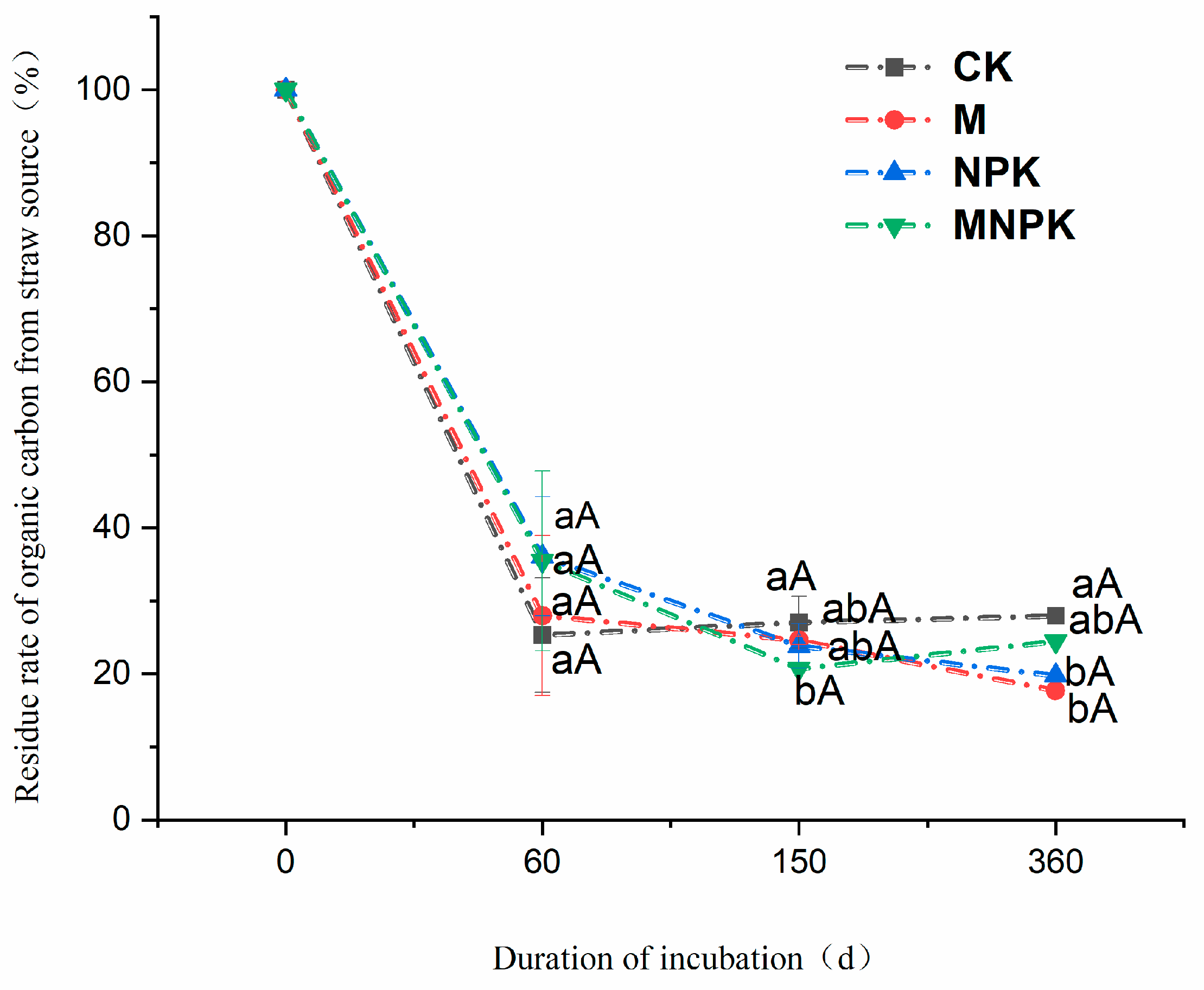
3.2. Dynamics of Straw-Derived Carbon in Black Soils
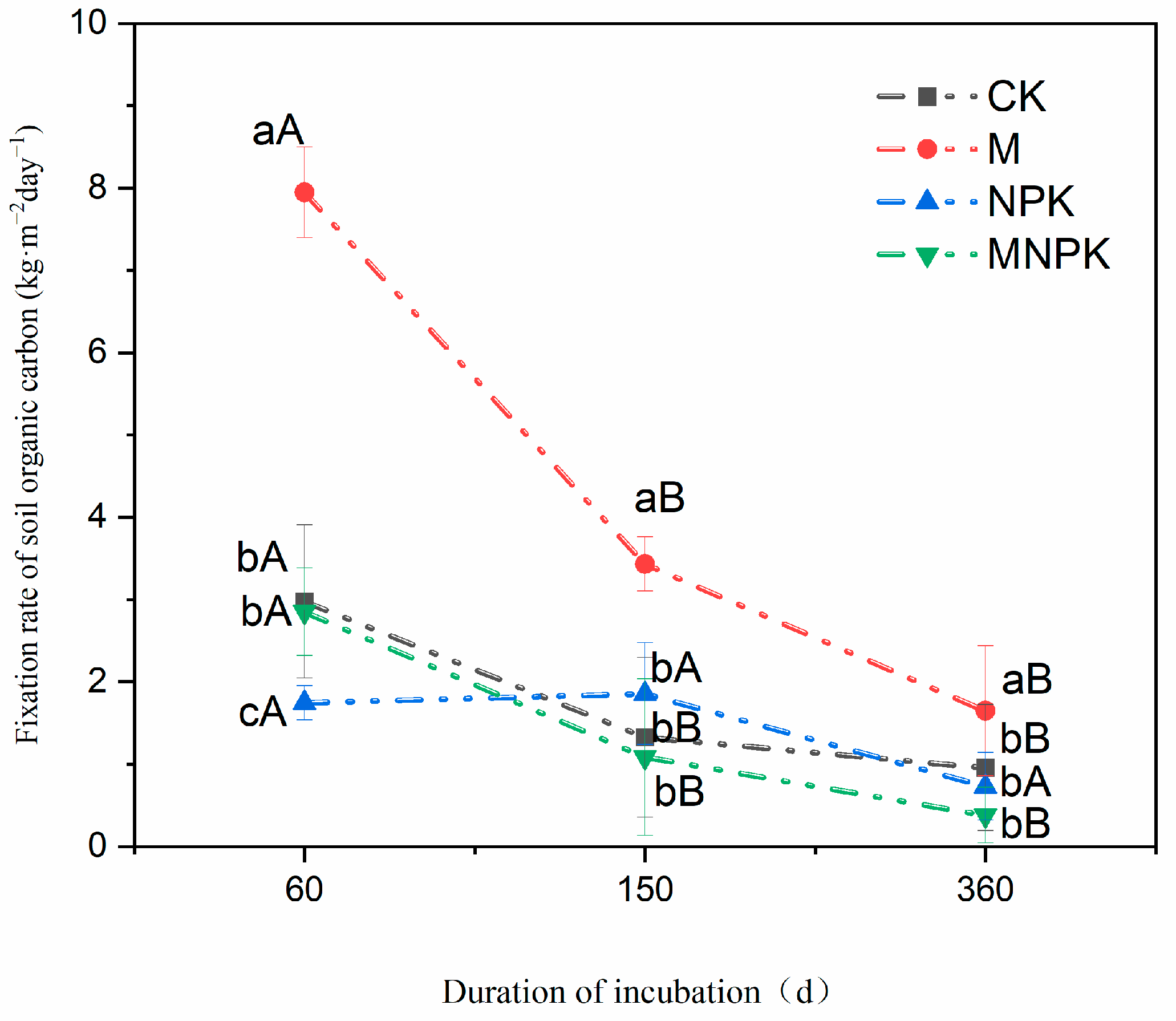
3.3. Organic Carbon Fixation Rate and Capacity of Black Soil with Different Fertilization Treatments
4. Discussion
4.1. Effects of Straw Addition on SOC and δ13C Values
4.2. Straw Carbon Residue and SOC Fixation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, M.; Torn, M.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Han, M.; Li, Y.; Wang, E. Organic carbon change and carbon sequestration potential of sloping farmland in the black soil area. Acta Ecol. Sin. 2020, 40, 5751–5760. [Google Scholar]
- Bastida, F.; Torres, I.; Hernández, T.; Bombach, P.; Richnow, H.; García, C. Can the labile carbon contribute to carbon immobilization in semiarid soils? Priming effects and microbial community dynamics. Soil Biol. Biochem. 2013, 57, 892–902. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Zhang, T.; Zhao, X.; Chen, S.; Wang, Q. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: A meta-analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Wuest, S.; Gollany, H. Soil organic carbon and nitrogen after application of nine organic amendments. Soil Sci. Soc. Am. J. 2013, 77, 237–245. [Google Scholar] [CrossRef]
- Liu, X.; Lee, B.; Kravchenko, Y.; Duran, A.; Huffman, T.; Morras, H.; Studdert, G.; Zhang, X.; Cruse, R.; Yuan, X. Overview of Mollisols in the world: Distribution, land use and management. Can. J. Soil Sci. 2012, 92, 383–402. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Li, X.; Sun, J.; Herzberger, A.; Wei, D.; Zhang, W.; Dou, Z.; Zhang, F. Cropping system conversion led to organic carbon change in China’s Mollisols regions. Sci. Rep. 2017, 7, 18064. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Tao, B.; Hu, N. Effect of different fertilization modes on soil organic carbon sequestration in paddy fields in South China: A meta-analysis. Ecol. Indic. 2015, 53, 144–153. [Google Scholar] [CrossRef]
- Mishra, U.; David, A.; Rattan, L. Tillage effects on soil organic carbon storage and dynamics in Corn Belt of Ohio USA. Soil Tillage Res. 2010, 107, 88–96. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Rev. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.; Evershed, R.; Bertolini, T.; Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F.; et al. Carbon input belowground is the major C flux contributing to leaf litter mass loss: Evidences from a 13C labelled-leaf litter experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; First Update 2007; FAO: Rome, Italy, 2007. [Google Scholar]
- Zhang, J.; Zhou, B.; Wei, D.; Chi, F.; Hao, X.; Jin, L.; Kuang, E. Spectroscopic characteristics of HA structure in black soil with organic and inorganic fertilizer. Spectrosc. Spectr. Anal. 2019, 39, 845. [Google Scholar]
- De, T.; Amery, F.; Van, M.; Smolders, E.; Merckx, R. Tracing the source and fate of dissolved organic matter in soil after incorporation of a 13C labelled residue: A batch incubation study. Soil Biol. Biochem. 2011, 43, 513–519. [Google Scholar]
- Werner, R.A.; Brand, W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2001, 15, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Six, J.; Bryant, D.; Denison, F.; Van, K. The relationship between carbon input, aggregation, and soil organic carbon stabilization in sustainable cropping systems. Soil Sci. Soc. Am. J. 2005, 69, 1078–1085. [Google Scholar] [CrossRef]
- Wetterstedt, J.; Persson, T.; Agren, G. Temperature sensitivity and substrate quality in soil organic matter decomposition: Results of an incubation study with three substrates. Glob. Chang. Biol. 2010, 16, 1806–1819. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Lan, L.; Sun, Y.; Li, F. Relationship between nutrient change and acidity of soil under long-term fertilization. J. Jilin Agric. Univ. 2017, 39, 55–59. [Google Scholar]
- Xu, X.; An, T.; Zhang, J.; Sun, Z.; Schaeffer, S.; Wang, J. Transformation and stabilization of straw residue carbon in soil affected by soil types, maize straw addition and fertilized levels of soil. Geoderma 2019, 337, 622–629. [Google Scholar] [CrossRef]
- Liu, L.; Pang, D.; Wang, X.; Chen, L.; Li, X.; Wu, M.; Liu, B.; Zhu, Z.; Li, J.; Wang, J. Application of stable carbon isotope technique in soil organic carbon research: A literature review. Arid. Zone Res. 2021, 38, 123–132. [Google Scholar]
- Yu, Y. Effects of the Addition of Root, Stem and Leaf of 13C-Labeled Maize Residues on Brown Earth’s Respiration and Priming Effects. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2022. [Google Scholar]
- Poirier, V.; Angers, D.; Rochette, P.; Whalen, J. Initial soil organic carbon concentration influences the short-term retention of crop-residue carbon in the fine fraction of a heavy clay soil. Biol. Fertil. Soils 2013, 49, 527–535. [Google Scholar] [CrossRef]
- Samul, A.; Sylvie, R.; Victor, R.; Robert, O. Mine realisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol. Fertil. Soils 2005, 42, 119–128. [Google Scholar]
- Joyce, S.; Myrna, J.; Andre, J. Comparison of soil organic matter composition after incubation with maize leaves, roots and stems. Geoderma 2013, 192, 86–96. [Google Scholar]
- Meng, F.; Lal, R.; Kuang, X.; Ding, G.; Wu, W. Soil organic carbon dynamics within density and particle-size fractions of Aquic Cambisols under different land use in northern China. Geoderma 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Meng, F.; Dungait, J.; Xu, X.; Bol, R.; Zhang, X.; Wu, W. Coupled incorporation of maize (Zea mays L.) straw with nitrogen fertilizer increased soil organic carbon in Fluvic Cambisol. Geoderma 2017, 304, 19–27. [Google Scholar] [CrossRef]
- Han, P.F.; Zhang, W.; Wang, G.C.; Sun, W.J.; Huang, Y. Changes in soil organic carbon in croplands subjected to fertilizer management: A global meta-analysis. Sci. Rep. 2016, 6, 27199. [Google Scholar] [CrossRef]


| Treatments | SOC (g·kg−1) | δ13C (‰) | pH | Bulk Density (g·cm−3) |
|---|---|---|---|---|
| CK | 16.1 ± 1.1 | −22.91 ± 0.03 | 6.79 ± 0.16 | 1.11 ± 0.01 |
| NPK | 17.1 ± 0.1 | −23.21 ± 0.10 | 5.89 ± 0.03 | 1.12 ± 0.01 |
| M | 16.5 ± 0.9 | −23.23 ± 0.41 | 6.82 ± 0.07 | 1.13 ± 0.02 |
| MNPK | 17.6 ± 0.5 | −23.33 ± 0.11 | 5.93 ± 0.14 | 1.02 ± 0.05 |
| Treatments | N (kg·ha−1·y−1) | P2O5 (kg·ha−1·y−1) | K2O (kg·ha−1·y−1) | Horse Manure (Mg·ha−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Wheat | Soybean | Maize | Wheat | Soybean | Maize | |||
| CK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NPK | 150 | 75 | 150 | 75 | 150 | 75 | 75 | 0 |
| M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.6 |
| MNPK | 150 | 75 | 150 | 75 | 150 | 75 | 75 | 18.6 |
| Treatment | Incubation Time (d) | |||
|---|---|---|---|---|
| 0 | 60 | 150 | 360 | |
| CK | 35.74 ± 3.00 aA | 37.53 ± 1.43 cA | 37.73 ± 0.95 bA | 39.19 ± 0.55 cA |
| M | 37.29 ± 2.62 aB | 42.06 ± 0.27 aA | 42.44 ± 0.93 aA | 43.23 ± 0.37 aA |
| NPK | 38.30 ± 1.52 aA | 39.35 ± 0.27 bA | 41.09 ± 1.35 aA | 40.93 ± 0.19 bA |
| MNPK | 35.90 ± 2.07 aA | 37.61 ± 0.57 cA | 41.22 ± 0.73 aA | 40.92 ± 0.87 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yuan, J.; Zhu, Y.; Kuang, E.; Han, J.; Shi, Y.; Chi, F.; Wei, D.; Liu, J. Transformation and Sequestration of Total Organic Carbon in Black Soil under Different Fertilization Regimes with Straw Carbon Inputs. Agriculture 2024, 14, 887. https://doi.org/10.3390/agriculture14060887
Zhang J, Yuan J, Zhu Y, Kuang E, Han J, Shi Y, Chi F, Wei D, Liu J. Transformation and Sequestration of Total Organic Carbon in Black Soil under Different Fertilization Regimes with Straw Carbon Inputs. Agriculture. 2024; 14(6):887. https://doi.org/10.3390/agriculture14060887
Chicago/Turabian StyleZhang, Jiuming, Jiahui Yuan, Yingxue Zhu, Enjun Kuang, Jiaye Han, Yanxiang Shi, Fengqin Chi, Dan Wei, and Jie Liu. 2024. "Transformation and Sequestration of Total Organic Carbon in Black Soil under Different Fertilization Regimes with Straw Carbon Inputs" Agriculture 14, no. 6: 887. https://doi.org/10.3390/agriculture14060887






