Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Cowpea Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Area Description
2.2. Experimental Design and Treatments
2.3. Cowpea Cultivation
2.4. Gas Exchange Parameters
2.5. Mineral Nutrient Analysis
2.6. Determination of Total Sugars and Sucrose in Grain
2.7. Determination of Total Amino Acids and Storage Proteins
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cakmak, I.; Kutman, U.Á. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Nardeli, A.J.; Carvalho, M.N.A.; Moura, R.M.; Wilson, L.; Young, S.D.; Reis, A.R. Agronomic biofortification of cowpea with zinc: Variation in primary metabolism responses and grain nutritional quality among 29 diverse genotypes. Plant Physiol. Biochem. 2021, 162, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Berni, C.R.; Di, C.M.; Pietravalle, A.; Aleandri, V.; Conte, F.; Curtis, M. Zinc in early life: A key element in the fetus and preterm neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wei, S.; Twardowska, I. Biofortification of soybean (Glycine max L.) with Se and Zn, and enhancing its physiological functions by spiking these elements to soil during flowering phase. Sci. Total Environ. 2020, 740, 139648. [Google Scholar] [CrossRef] [PubMed]
- Kachinski, W.D.; Ávila, F.W.; dos Reis, A.R.; Muller, M.M.L.; Mendes, M.C.; Petranski, P.H. Agronomic biofortification increases concentrations of zinc and storage proteins in common bean (Phaseolus vulgaris L.) grains. Food Res. Int. 2022, 155, 111105. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Moloto, R.M.; Moremi, L.H.; Soundy, P.; Maseko, S.T. Biofortification of common bean as a complementary approach to addressing zinc deficiency in South Africans. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 575–584. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996.
- Kumar, S.; Palve, A.; Joshi, C.; Srivastava, R.K. Crop biofortification for iron (Fe), zinc (Zn) and vitamin A with transgenic approaches. Heliyon 2019, 5, e01914. [Google Scholar] [CrossRef]
- Prom-U-Thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guo, S.; Naeem, A.; Cakmak, I. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—A review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Inacio, K.A.M.; Farfan, N.C.; Azevedo, C.E.X.; Polatto, M.A.G.; Carrion, N.S.; Mendes, P.V.S.; Santos, E.F. Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification. Agriculture 2024, 14, 268. [Google Scholar] [CrossRef]
- Lividini, K.; Fiedler, J.L.; Moura, F.F.; Moursi, M.; Zeller, M. Biofortification: A review of ex-ante models. Glob. Food Secur. 2018, 17, 186–195. [Google Scholar] [CrossRef]
- Santos, E.F.; Figueiredo, C.O.; Rocha, M.A.P.; Lanza, M.G.D.B.; Silva, V.M.; Reis, A.R. Phosphorus and Selenium Interaction Effects on Agronomic Biofortification of Cowpea Plants. J. Soil Sci. Plant Nutr. 2023, 23, 4385–4395. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture. Soil Survey Staff Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico de Campinas: Campinas, Brazil, 1997. [Google Scholar]
- Raij, B. Soil Fertility and Nutrient Management; Internatio: Piracicaba, Brazil, 2011. [Google Scholar]
- Silva, V.M.; Boleta, E.H.; Martins, J.T.; dos Santos, F.L.; Silva, A.C.d.R.; Alcock, T.D.; Wilson, L.; E de Sá, M.; Young, S.D.; Broadley, M.R.; et al. Agronomic biofortification of cowpea with selenium: Effects of selenate and selenite applications on selenium and phytate concentrations in seeds. J. Sci. Food Agric. 2019, 99, 5969–5983. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Macedo, F.G.; Zanchim, B.J.; Lima, G.P.P.; Lavres, J. Prognosis of physiological disorders in physic nut to N, P, and K deficiency during initial growth. Plant Physiol. Biochem. 2017, 115, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Pongrac, P.; Reis, A.R.; Rabêlo, F.H.S.; Azevedo, R.A.; White, P.J.; Lavres, J. Unravelling homeostasis effects of phosphorus and zinc nutrition by leaf photochemistry and metabolic adjustment in cotton plants. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2018. Available online: https://cran-archive.r-project.org/bin/windows/base/old/3.5.1/ (accessed on 1 February 2024).
- Moreira, A.; Moraes, L.A.; Reis, A.R. The molecular genetics of zinc uptake and utilization efficiency in crop plants. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.S.P., Toru Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 87–108. [Google Scholar]
- Liu, Y.M.; Liu, D.Y.; Zhang, W.; Chen, X.X.; Zou, C.Q. Photosynthetic characteristics and productivity in a wheat–maize system under varying zinc rates. Crop Sci. 2020, 60, 3291–3300. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of zinc nutrition for increasing zinc availability, uptake, yield, and quality of maize (Zea mays L.) grains: An overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (cu, Zn, Mn, Fe, Ni, Mo, B, cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Almanza, D.J.; Ojeda-Barrios, D.L.; Hernández-Rodríguez, O.A.; Chávez, E.S.; Ruíz-Anchondo, T.; Sida-Arreola, J.P. Carbonic anhydrase and zinc in plant physiology. Chil. J. Agric. Res. 2012, 72, 140. [Google Scholar] [CrossRef]
- Das, S.K.; Avasthe, R.K.; Singh, M.; Dutta, S.K.; Roy, A. Zinc in plant-soil system and management strategy. Agrica 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Tan, Y.F.; O’Toole, N.; Taylor, N.L.; Millar, A.H. Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiol. 2010, 152, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. Zinc importance for crop production—A review. Int. J. Agron. Plant Prod. 2013, 4, 64–68. [Google Scholar]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Guoqin, H. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Esfandiari, E.; Abdoli, M.; Mousavi, S.B.; Sadeghzadeh, B. Impact of foliar zinc application on agronomic traits and grain quality parameters of wheat grown in zinc deficient soil. Indian J. Plant Physiol. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Yeboah, S.; Asibuo, J.; Oteng-Darko, P.; Asamoah Adjei, E.; Lamptey, M.; Owusu Danquah, E.; Butare, L. Impact of foliar application of zinc and magnesium aminochelate on bean physiology and productivity in Ghana. Int. J. Agron. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Oliveira, D.S.V.D.; Franco, L.J.D.; Menezes-Júnior, J.Â.N.D.; Damasceno-Silva, K.J.; Rocha, M.D.M.; Neves, A.C.D.; Sousa, F.M.D. Adaptability and stability of the zinc density in cowpea genotypes through GGE-Biplot method1. Rev. Ciênc. Agron. 2017, 48, 783–791. [Google Scholar] [CrossRef]
- Rocha, M.M.; Damasceno-Silva, K.J.; Menezes-Júnior, J.A.N. Cultivares. In Feijão-Caupi: Do Plantio à Colheita; do Vale, J.C., Bertini, C., Borém, A., Eds.; Editora UFV: Viçosa, Brazil, 2017; pp. 113–142. [Google Scholar]
- Singh, D.; Prasanna, R. Potential of microbes in the biofortification of Zn and Fe in dietary food grains. A review. Agron. Sustain. Dev. 2020, 40, 15. [Google Scholar] [CrossRef]
- Quick, W.P.; Schaffer, A.A. Sucrose metabolism in sources and sinks. In Photoassimilate Distribution Plants and Crops Source-Sink Relationships, 1st ed.; Zamski, E., Schaffer, A.A., Eds.; Routledge: New York, NY, USA, 2017; pp. 115–158. [Google Scholar]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc biofortification improves phytochemicals and amino-acidic profile in Brassica oleracea cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Jabri, A.I.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Arslan Ashraf, M.; Ali, Q.; Abbas, Z.; Wijaya, L. Zinc-lysine supplementation mitigates oxidative stress in rapeseed (Brassica napus L.) by preventing phytotoxicity of chromium, when irrigated with tannery wastewater. Plants 2020, 9, 1145. [Google Scholar] [CrossRef]
- Speiser, A.; Silbermann, M.; Dong, Y.; Haberland, S.; Uslu, V.V.; Wang, S.; Hell, R. Sulfur partitioning between glutathione and protein synthesis determines plant growth. Plant Physiol. 2018, 177, 927–937. [Google Scholar] [CrossRef]

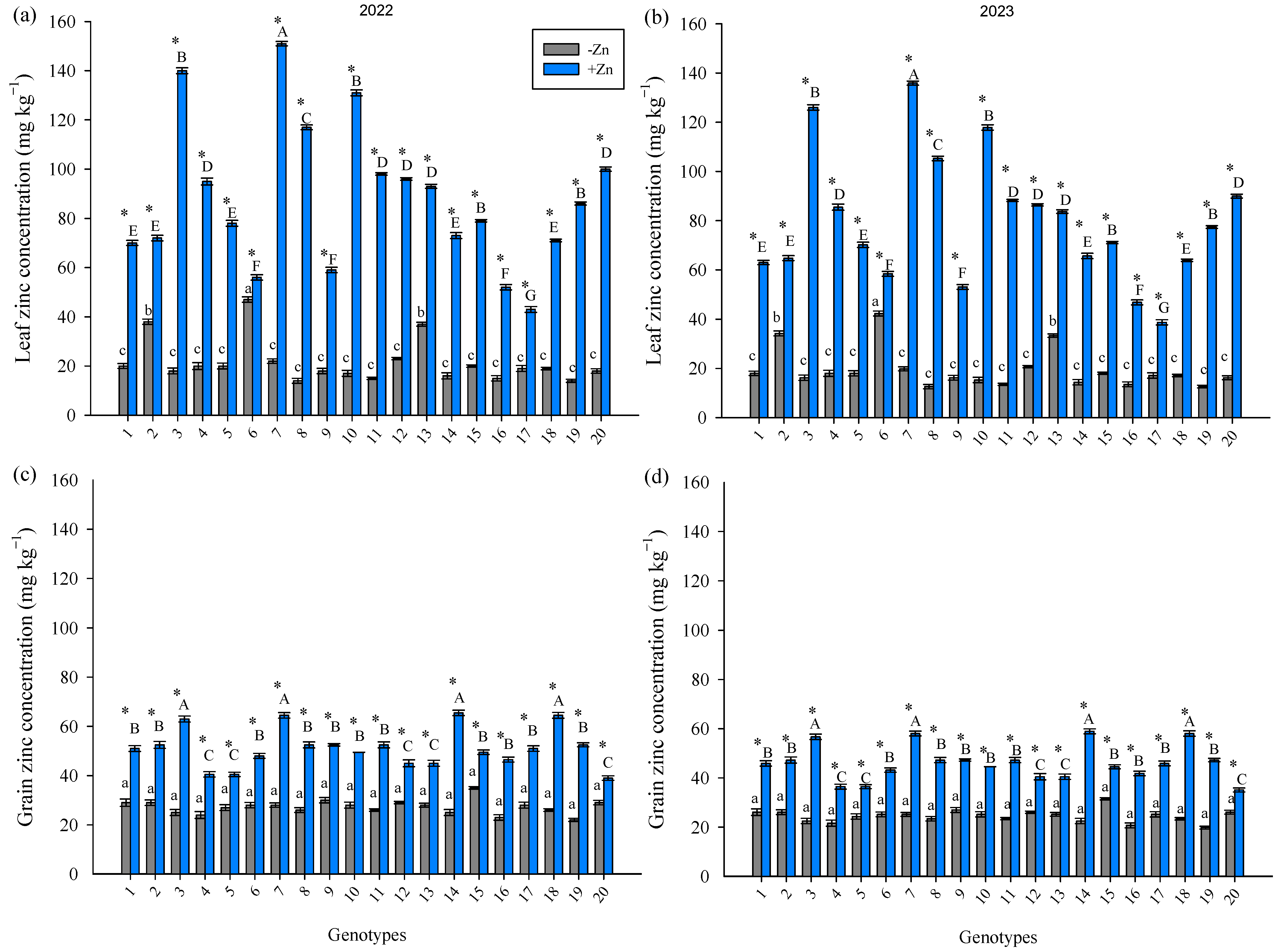
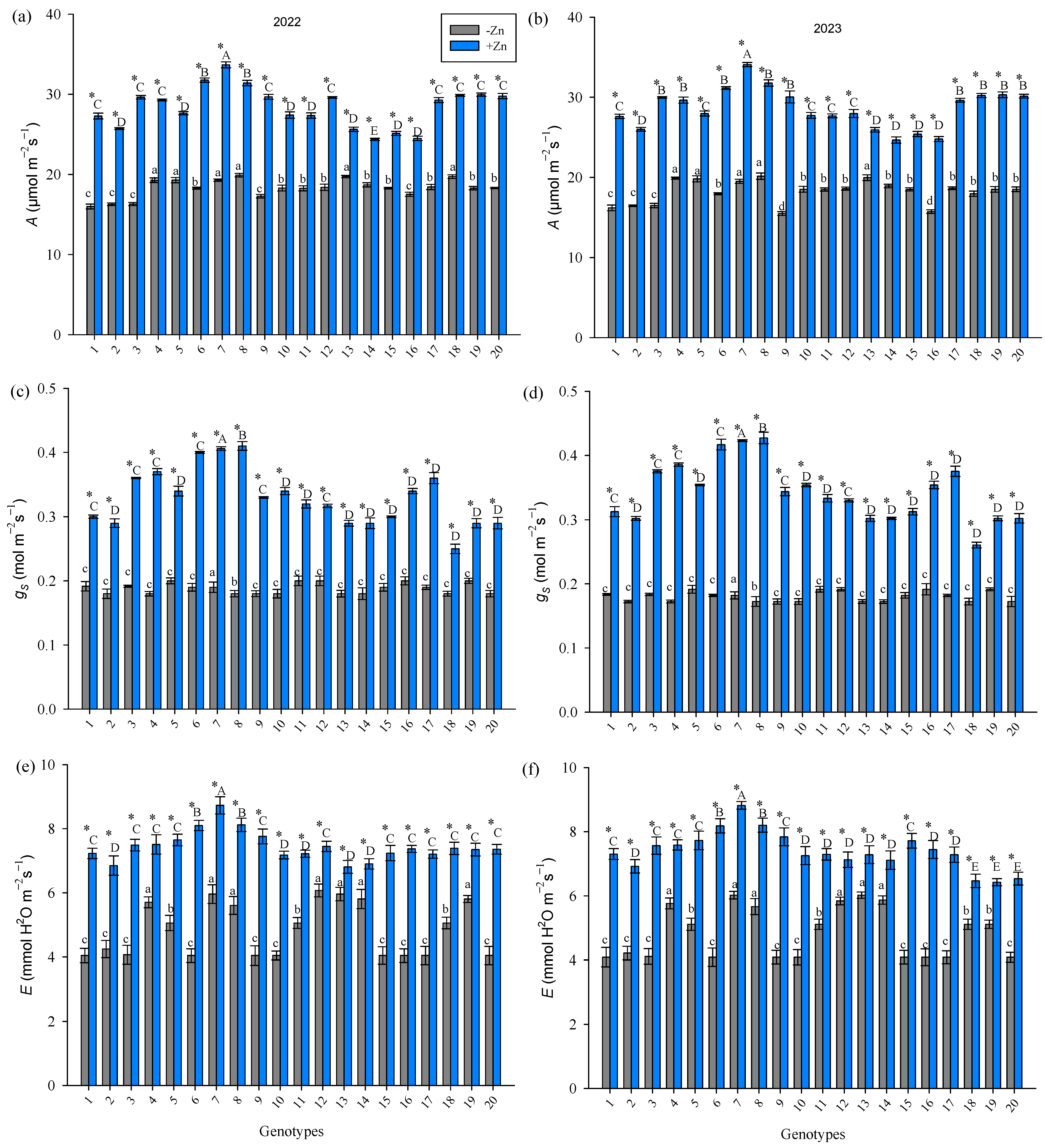
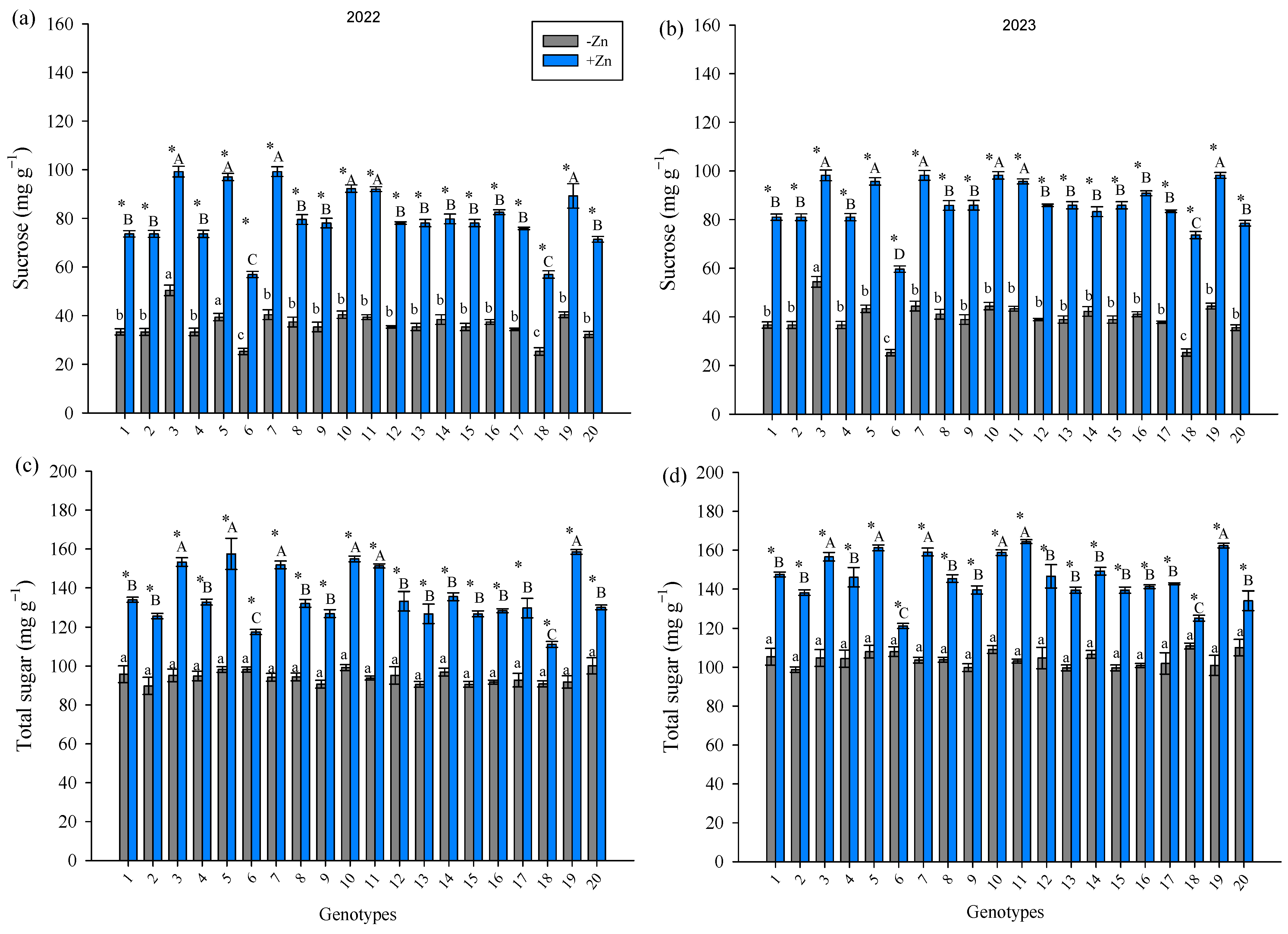

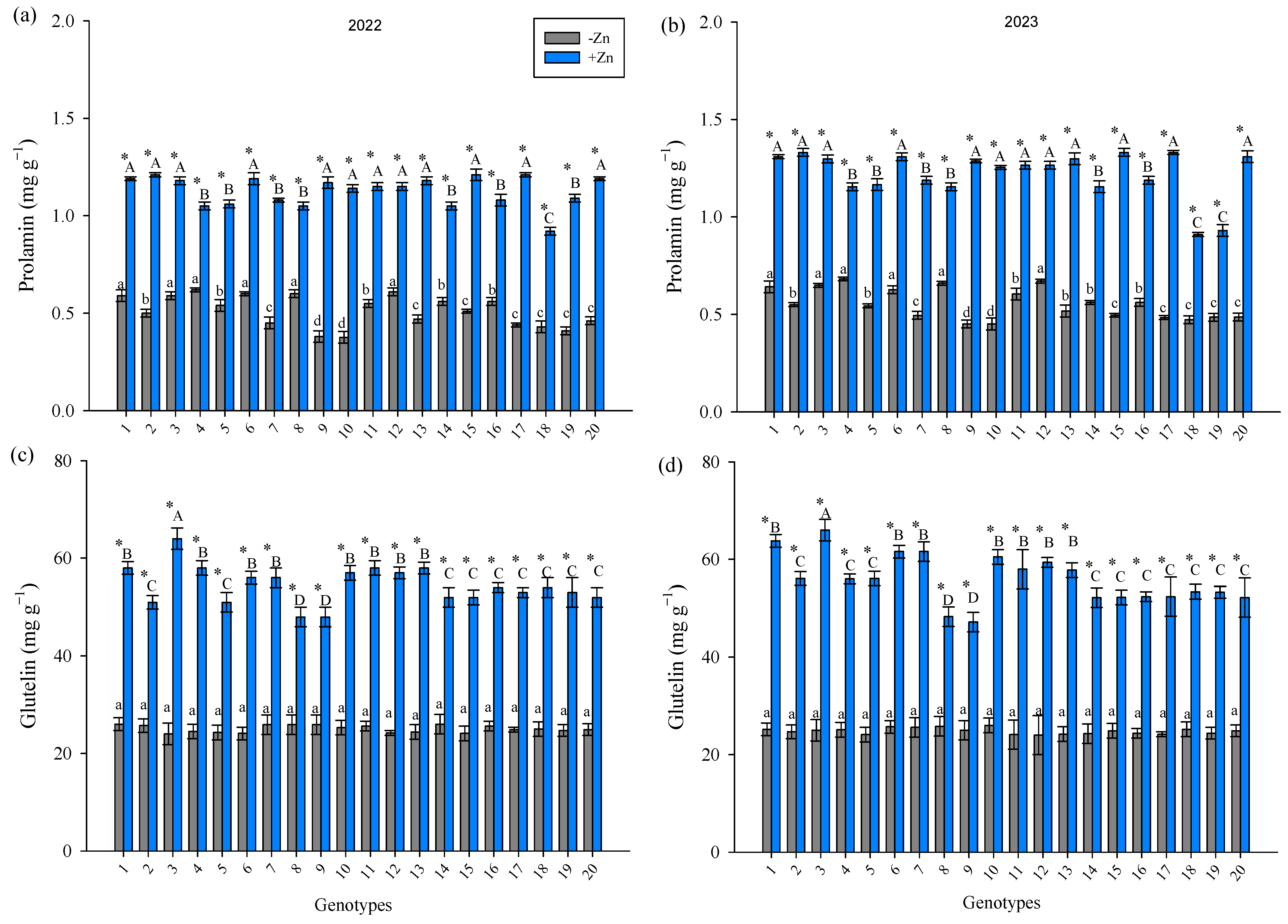
| ID | Genotype | −Zn | +Zn |
|---|---|---|---|
| 1 | MNC11-1013E-33 | 1.14 | 3.95 |
| 2 | MNC11-1013E-16 | 1.37 | 3.22 |
| 3 | MNC11-1013E-15 | 1.04 | 3.14 |
| 4 | MNC11-1013E-35 | 1.58 | 3.85 |
| 5 | MNC11-1018E-17 | 1.04 | 3.64 |
| 6 | MNC11-1019E-8 | 1.08 | 3.45 |
| 7 | MNC11-1019E-12 | 1.56 | 3.92 |
| 8 | MNC11-1019E-46 | 1.34 | 3.8 |
| 9 | MNC11-1020E-16 | 1.16 | 3.32 |
| 10 | MNC11-1022E-58 | 1.23 | 3.07 |
| 11 | MNC11-1024E-1 | 1.42 | 3.49 |
| 12 | MNC11-1026E-15 | 1.29 | 3.96 |
| 13 | MNC11-1022E-19 | 1.48 | 3.36 |
| 14 | MNC11-1031E-5 | 1.5 | 3.61 |
| 15 | MNC11-1031E-11 | 1.33 | 3.58 |
| 16 | MNC11-1034E-2 | 1.47 | 3.41 |
| 17 | MNC11-1052E-3 | 1.36 | 3.01 |
| 18 | BRS Pajéu | 1.21 | 3.86 |
| 19 | BRS Marataoã | 1.38 | 3.17 |
| 20 | BRS Rouxinol | 1.29 | 3.08 |
| Average | 1.34 b | 3.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.F.; Silva, M.G.; Silva, G.N.; Ducatti, K.R.; Rocha, M.d.M.; Reis, A.R.; Rabêlo, F.H.S.; Lavres, J.; Santos, E.F. Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Cowpea Grains. Agriculture 2024, 14, 911. https://doi.org/10.3390/agriculture14060911
Oliveira CF, Silva MG, Silva GN, Ducatti KR, Rocha MdM, Reis AR, Rabêlo FHS, Lavres J, Santos EF. Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Cowpea Grains. Agriculture. 2024; 14(6):911. https://doi.org/10.3390/agriculture14060911
Chicago/Turabian StyleOliveira, Caroline Figueiredo, Matheus Gomes Silva, Gutierres Nelson Silva, Karina Renostro Ducatti, Maurisrael de Moura Rocha, André Rodrigues Reis, Flávio Henrique Silveira Rabêlo, José Lavres, and Elcio Ferreira Santos. 2024. "Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Cowpea Grains" Agriculture 14, no. 6: 911. https://doi.org/10.3390/agriculture14060911







