Abstract
Fungi, particularly Pleurotus eryngii, emerges as a promising solution for sustainable non-animal protein production, requiring less land and growing on waste materials. In connection with population growth, sustainable solutions must be found to increase yield and product quality without resorting to the use of synthetic chemical fertilizers. Several biobased products are currently on the market; one of the most interesting is wood distillate (WD), derived from the pyrolysis process of the woody material. WD is rich in biologically active substances such as polyphenols, alcohols, acids, and esters, and its use is authorized in organic agriculture. The study investigates the use of WD in cultivating P. eryngii. We tested different concentrations of WD: 0%, 0.1%, 0.2%, 0.5%, and 1% WD on the growth of P. eryngii. Although WD did not significantly affect the yield (fresh weight), it led to a substantial increase in total soluble protein content and antioxidant compounds, such as phenols and vitamin C, and a reduction in glycogen content, especially at 0.2% WD. The results highlight the potential of biostimulants in mushroom cultivation, providing the ground for further research to improve the nutritional properties of cultivated mushrooms through wood distillate.
1. Introduction
Since the 1950s, the global human population has experienced exponential growth, soaring from 2.5 billion to today’s 8 billion, and the United Nations estimates a raising of 8.5 billion by 2030 and 9.7 billion by 2050, with a peak of around 10.4 billion during the 2080s [1]. Feeding this rapidly expanding population demands sustainable and less impacting alternatives [2]. Protein, a key component of the human diet, influences various aspects of health, including bone structure, body weight, muscle mass, and functions such as kidney and immune performance [3]. Despite these benefits, maintaining a balanced approach is crucial. The European Food Safety Authority emphasizes the significance of balanced protein consumption, considering diverse protein sources, in European diets [4]. However, current environmental issues, exacerbated by climate change, make it increasingly challenging to find land suitable for animal farming to meet the demand for meat, so far considered the primary protein source [5]. Moreover, animal husbandry alone contributes ca. 13% of global CO2 production [6]. This underscores the need for non-animal alternatives like legumes and fungi, which have a lower environmental impact, reduce water consumption, and contribute to soil health and quality [7,8,9,10]. In addition to environmental sustainability, the economic accessibility of non-animal proteins is pivotal [11]. The production and distribution of these products can be more cost-effective than meat, making this type of protein accessible to a wider global audience [12].
Edible fungi offer an innovative and sustainable solution. Their cultivation requires less land than traditional crops and can be carried out using agricultural or industrial waste materials [10,13]. Large-scale cultivation of mushrooms is often carried out in greenhouses, using substrates that nearly eliminate the need for extensive agricultural space, ensuring rapid and continuous production of ready-to-use products [14]. Mushrooms are valued as an excellent source of vitamins and minerals, and their unique flavors and aroma make them a preferred choice in many countries [15]. While increasing mushroom consumption contributes positively to contrasting malnutrition, it is important to note that mushrooms cannot completely replace essential proteins [16,17]. Despite this, from a recent study through investigations conducted on nine different edible mushrooms belonging to the genus Agaricus, Flammulina, Lentinula and Pleurotus, it emerged that they have high levels of proteins [18,19]. These vary from 16% in P. eryngii to 37% in P. ostreatus. Regarding the contribution of consuming 100 g of mushrooms to the recommended daily intake (RDA), Bach et al. [18] report that mushrooms meet 41% and 55% of the recommended daily protein intake for men and women, respectively.
Various species of mushrooms play a crucial role in both human nutrition and modern pharmacology [20]. Among them, one of the most commercially significant and widely cultivated is Pleurotus eryngii (DC.) Quél., commonly known in Italy as “Cardoncello”. Ranked as the third most economically important cultivated mushrooms [21,22], its production is regulated in Italy [23]. P. eryngii features single or clustered carpophores with an umbrella-shaped cap (diameter: 3–20 cm) and a central or eccentric stem. Its structure is compact, firm, unalterable, tender, and elastic [24]. Like all edible mushrooms, P. eryngii is characterized by a relatively high content of proteins and various antioxidant compounds (i.e., polyphenols, flavonoids, and vitamin C), positively impacting both human dietary aspects and the extraction of phytochemicals in the pharmaceutical industry [25,26]. Scientific research on this mushroom has mainly focused on the use of different growth substrates (i.e., cotton seed shells, sawdust, sugarcane bagasse, and corn cobs) for its cultivation, in order to find the best substrate, assessing the final yield and nutritional characteristics of mushrooms [27,28,29].
Presently, there is a noteworthy transition in agricultural practices, characterized by the European Union’s introduction of a 10-year strategy called “Farm to Fork” [30]. This strategy aims to guide the agricultural transition towards a fair, healthy, and environmentally friendly food system. Within this framework, it has been established that there is a substantial need to reduce the use of synthetic chemical fertilizers and pesticides by 2030, replacing them with natural and environmentally friendly alternatives [30]. To the best of our knowledge, there is currently no scientific literature examining the use of bio-based products to enhance the nutritional quality of cultivated mushrooms.
Among the various types of biostimulants available in the Italian market, one of the most interesting is wood distillate (WD), also known as pyroligneous acid or wood vinegar. This product has recently been included in the list of corroborants approved for use in organic agriculture in Italy [31]. WD, together with biochar, is one of the two by-products of the pyrolysis process of woody biomass and derives from the condensation of vapors produced during this process [32,33]. The chemical characteristics are influenced by the feedstock and the operational parameters employed, but in general, it consists of >300 biologically active molecules, including polyphenols, tannins, alcohols, and organic acids [34]. Numerous scientific studies have reported an increase in yield and nutritional quality of several crops following WD spray application or fertigation [35,36,37]. Studies showed that WD is also safe for the environment [38,39] and human health [40]. The effect of WD on the cultivation of edible mushrooms has predominantly centered on two aspects: the yield of fruit bodies and the growth of mycelium. In a study conducted by Yoshimura et al. [41], varying concentrations of WD (ranging from 0.1% to 6%) were added, resulting in a notable increase in the yield of fruit bodies for Pleurotus ostreatus, ranging from 21% to 42%. Similarly, other research endeavors have elucidated that even low concentrations of WD (ranging from 0.05% to 0.25%) have a significant positive effect on the mycelial growth of various fungal species [42,43,44]. However, an overlooked area pertains to how WD influences the nutritional properties of these mushrooms. In this study, we looked at how WD affected the growth of P. eryngii. We investigated whether WD treatment affected biochemical parameters such as total soluble proteins, glycogen, phenols, flavonoids, and vitamin C levels.
2. Materials and Methods
2.1. Experimental Design
Twenty highly productive and selectively incubated substrates (height: 22 cm; width: 24 cm; depth: 12 cm; weight: 5 kg) of the “Cardoncello” mushrooms (Pleurotus eryngii (DC.) Quél.-fungal strain: DB160) were used for the experiment, and were kindly provided by the company “Azienda Agricola Micologica De Biasi Arcangelo” [45]. The substrates consisted of a mix of wheat straw (49% dw/dw), sugar beet pulp (49% dw/dw), and Ca2CO3 (2% dw/dw), which was hydrated to 70% total moisture. Subsequently, each substrate was confined in plastic bags and sterilized at 121 °C for 20 min. The fungus inoculum (DB160) was placed in a sterile area. Finally, before starting the experiment, the substrates were incubated for 60 days at 26 ± 2 °C.
In the laboratory, all substrates were getting rid of the top of the plastic cover, and a layer of approx. According to the company indication, 5 cm of growing medium (Vigor Plant Srl, Albenga, Italy) was added to allow the mushrooms to grow. Subsequently, the growing substrates were treated with five different WD concentrations (n = 4): 0% WD (control-C), 0.1% WD (0.1% WD), 0.2% WD (0.2% WD), 0.5% WD (0.5% WD), and 1% WD (1% WD). The WD treatment was applied twice (ca. 10 mL per substrate), on the first day of the experiment and on the seventh day, through a spraying method that avoided contact with the carpophores. The substrates were placed in a climatic chamber in the dark, at a temperature of 20 ± 2 °C, and relative humidity of 70 ± 5%. To maintain consistent internal humidity, substrates were periodically water-moistened. The experiment ended on the eleventh day, after >99% of the mushrooms reached full maturity.
2.2. Wood Distillate
The wood distillate (®BioDea) utilized in this experiment was kindly provided by Bio-Esperia S.r.l. (Arezzo, Italy) [46]. During the pyrolysis process of the woody material (i.e., Castanea sativa Mill., Robinia pseudoacacia L., Fraxinus L., Alnus glutinosa (L.) Gaertn., and Quercus robur L.), derived from secondary forest timber, WD is obtained through a countercurrent steam process, utilizing only the water present in the wood sap. This extraction is carried out in the pyrolitic reactor at various temperature gradients, with the output capped at a maximum of 75 °C. Subsequently, the wood extract undergoes a further step, being directed to a natural filter for the removal of any residue. It is then left to decant for a minimum of three months, allowing for the production of a WD with an amber-colored hue. Throughout all stages of the reaction process, the parameters are computer-controlled to ensure the quality of the final product. The pH ranges from 3.5 to 4.5, and the density is 1.05 kg L−1. Main components include acetic acid (2.3%), phenols (3.01 g L−1), total organic compounds (33.8 g L−1), organic acids (32.3 g L−1), and total nitrogen (0.43 g L−1). The complete chemical characterization of the WD is reported in Celletti et al. [47].
2.3. Yield Parameters and Samples Preparation
During sampling, carpophores were removed from the substrate, counted, cleaned of any soil residue, and weighed. Since each substrate had exactly the same weight (5 kg), the yield parameters (nr. of carpophores and fresh weight) were expressed as medians per each growing substrate. The samples were then dried for 10 h in a ventilated oven at 60 °C, following the methodology outlined by Khumlianlal et al. [48]. After drying, the samples were homogenized using a blender and sieved (2 mm) to ensure a uniform matrix for subsequent analysis. Biological efficiency and production rate were calculated following Wang et al. [19].
2.4. Total Soluble Protein Content
The total soluble protein content was assessed according to the procedure outlined by Lamaro et al. [49]. Ground samples (0.2 g) were homogenized in 4 mL of deionized water (dH2O) and subsequently centrifuged at 4000 rpm for 5 min. In total, 0.2 mL of the resulting supernatant was then mixed with 0.8 mL of Bradford solution (Sigma-Aldrich, Darmstadt, Germany). Measurements were taken at 595 nm using a UV-Vis spectrophotometer (Agilent 8453; Santa Clara, CA, USA). Quantification was carried out on a calibration curve (10–100 μg mL−1) prepared with bovine albumin (Sigma-Aldrich, Darmstadt, Germany).
2.5. Glycogen Content
The concentration of glycogen was assessed according to the procedure outlined by Fedeli et al. [50]. For the quantification of glycogen content, Lugol’s methodology, commonly used for the determination of starch content, was used. Similar to starch, glycogen forms a colored complex with Lugol’s reagent. However, the resulting color is typically red-brown rather than blue-black. This color change is used for the qualitative-quantitative determination of glycogen. Fungal samples (0.5 g) were homogenized in 2 mL of dimethyl sulfoxide (DMSO). Subsequently, 0.5 mL of 8 M HCl was added, and the samples were put inside an oven for 30 min at 60 °C. After cooling, 0.5 mL of 8 M NaOH and 7 mL of dH2O were added. The samples underwent centrifugation at 4000 rpm for 5 min, and 0.5 mL of the supernatant was mixed with 2.5 mL of Lugol’s solution (HCl 0.05 M, 0.03% I2, and 0.06% KI). After 15 min, the samples were measured at 605 nm using a UV-VIS spectrophotometer (Agilent 8453; Santa Clara, CA, USA).
2.6. Total Phenol and Total Flavonoid Content
Approximately 1 g of the dried material was immersed in 10 mL of 80% methanol (v/v) for extraction. After 30 min of orbital shaking (ASAL VDRL mod. 711, Cernusco s/N, Milan, Italy), samples were incubated at 4 °C in the dark for 48 h. The resulting solution was filtered using Whatman filter paper no. 1, and the filtrates were used for the quantification of the total phenol (TPC) and total flavonoid content (TFC).
The determination of TPC followed the procedure outlined by Azarnejad et al. [51]. 0.125 mL of filtrate extracts were mixed with 2 mL of dH2O, and 0.125 mL of Folin–Ciocalteu’s reagent was added. After 3 min in the dark, 1.25 mL of 7% (w:v) Na2CO3 and 1 mL of dH2O were introduced, shaken vigorously, and left to incubate in the dark for 90 min. The absorbance of the solutions was read at 760 nm using a UV-Vis spectrophotometer (Agilent 8543, Santa Clara, CA, USA). Quantification was based on a calibration curve (5–300 µg mL−1) prepared with pure gallic acid (98%, Thermo Fisher Scientific Inc., Rodano, Milano, Italy).
The determination of TFC followed the procedure outlined by Azarnejad et al. [51]. 0.25 mL of filtrate extracts were combined with 0.075 mL of 5% (w:v) NaNO2, followed by the addition of 0.075 mL of 10% (w:v) AlCl3. After shaking and 5 min of incubation in the dark, 0.5 mL of 1 M NaOH solution was added. The samples were left in the dark for 15 min, and absorbances were measured at 415 nm using a UV-Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). Quantification relied on a calibration curve (12.5–150 µg mL−1) prepared with quercetin (≥95%, Merck KGaA, Darmstadt, Germany).
2.7. Vitamin C Content
The vitamin C content was assessed according to the procedure outlined by Fedeli et al. [52]. Samples (0.2 g) were homogenized in 1.6 mL of 10% (w/v) trichloroacetic acid (TCA). The resulting homogenate underwent filtration using gauze, followed by 5 min cooling and subsequent centrifugation at 3000 rpm for 5 min. 0.4 mL of the supernatant were combined with 1.6 mL of distilled water and 0.2 mL of 0.2 M Folin–Ciocalteu reagent (Carlo Erba, Cornaredo, MI, Italy). After a 10 min incubation in the dark, samples were measured at 760 nm using a UV–Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). Concentration determination relied on a calibration curve established with 0.05–0.2 mL of a 100 µg mL−1 L-ascorbic acid (BioXtra, ≥99.0%, crystalline) stock solution.
2.8. Statistical Analysis
To test for differences determined by treatment, a permutational multivariate analysis of variance (PERMANOVA) was run, followed by a pairwise permutational t-test; for post hoc comparisons. All statistical analyses were run with the R software v. 4.0.1 [53]; statistical significance was taken at p < 0.05.
3. Results
PERMANOVA analysis revealed significant differences in total soluble protein, glycogen content, total phenol content, total flavonoid content, and vitamin C content (Table 1). No disparities were noted in the number of carpophores and fresh weight (Table 1).

Table 1.
Results of permutational analyses of variance of: number of carpophores, fresh weight, total soluble protein, biological efficiency, production rate, glycogen, total phenols content, total flavonoind content, vitamin C.
The application of WD did not yield any significant impact on the number, fresh weight, biological efficiency, and production rate of carpophores across all tested concentrations (Figure 1).
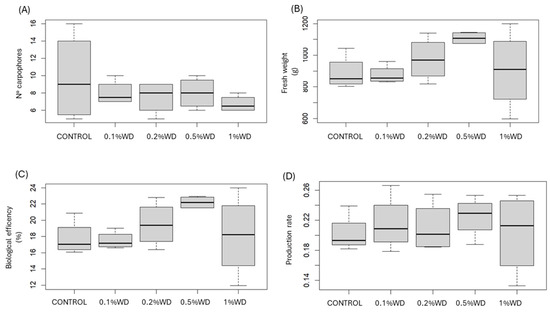
Figure 1.
Boxplot of (A) number, (B) fresh weight, (C) biological efficiency, and (D) production rate of carpophores. CONTROL: only water; 0.1% WD = treatment with 0.1% wood distillate; 0.2% WD = treatment with 0.2% wood distillate; 0.5% WD = treatment with 0.5% wood distillate; 1% WD = treatment with 1% wood distillate.
Concerning total soluble protein content, variations among treatments were negligible except for the 0.2% WD concentration, where a statistically significant increase of +95% was observed (Figure 2). For glycogen content, differences were insignificant across treatments except for the 0.2% WD concentration, which showed a statistically significant reduction of −50% (Figure 3).
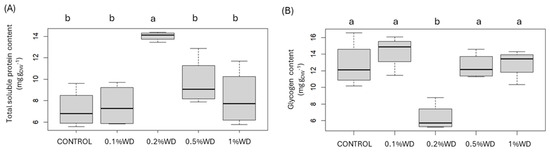
Figure 2.
Boxplot of the content of total soluble proteins (A) and glycogen (B). CONTROL: only water; 0.1% WD = treatment with 0.1% wood distillate; 0.2% WD = treatment with 0.2% wood distillate; 0.5% WD = treatment with 0.5% wood distillate; 1% WD = treatment with 1% wood distillate. Different letters indicate statistically significant (p < 0.05) differences among treatments.
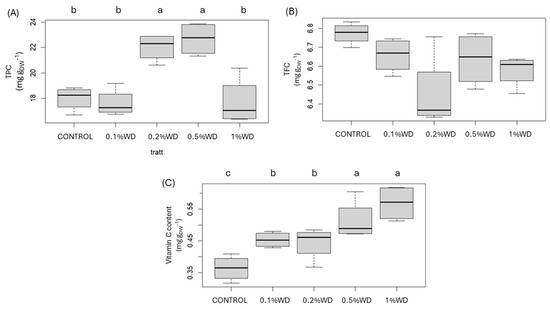
Figure 3.
Boxplot of the content of total phenols, TPC (A), total flavonoids, TFC (B), and vitamin C (C). CONTROL: only water; 0.1% WD = treatment with 0.1% wood distillate; 0.2% WD = treatment with 0.2% wood distillate; 0.5% WD = treatment with 0.5% wood distillate; 1% WD = treatment with 1% wood distillate. Different letters indicate statistically significant (p < 0.05) differences among treatments.
Regarding the antioxidant pool (Figure 3), mushrooms displayed an elevation in total phenols content only at 0.2% WD and 0.5% WD concentrations (+22% and +26%, respectively), while other concentrations maintained values similar to the controls. No discernible differences were noted in total flavonoid content across treatments, with all treatments exhibiting values akin to control samples. In contrast, vitamin C content exhibited a progressive increase across all WD treatments (0.1% WD: +10%; 0.2% WD: +17%; 0.5% WD: +32%; 1% WD: +10%) compared to control samples.
4. Discussion
Our findings showed, for the first time, the effect of WD on the growth of P. eryngii, underscoring how the mushrooms exhibit distinct responses to varying concentrations. The motivation for testing different concentrations of WD derives from the lack of studies, making an initial screening necessary to identify the most suitable concentration. It is crucial to note that, overall, all tested WD concentrations did not adversely affect mushroom yield, both in terms of fresh biomass and the number of fruiting bodies. Differently, Yoshimura et al. [41], found that different concentrations of WD (ranging from 0.1% to 6%) significantly increase the yield of the fruit bodies of Pleurotus ostreatus, ranging from 21% to 42%. These contrasting results maybe could be influenced by the chemical profile of WD since its chemical composition is influenced by the feedstock and the operational parameters set during the pyrolysis process. In many cases, crops and weeds exhibit different responses based on the concentrations used. For instance, concentrations ranging from 0.2 to 0.5% often stimulate plant growth and enhance quality [35,36,54], whereas concentrations > 1% can have herbicidal effects [55,56]. In our study, mushrooms showed a positive response in terms of yield, with a 25% increase in fresh weight, although not statistically significant, observed at 0.5% WD.
This less sensitive effect at high concentrations of WD on mushrooms could be due to the presence of chitin in the cell wall, differently from plants, which have cellulose. Chitin plays a crucial role in fungi by providing rigidity to their cell walls and offering vital structural support for the stability of fungal cells [57]. This support is essential for maintaining the shape and integrity of the cells, enabling fungal cell walls to withstand internal osmotic pressures and preserve the overall structure of the fungus [58]. Additionally, chitin serves as protection, forming a defensive barrier in the cell walls that shields fungi from external threats such as bacteria, antagonistic fungi, or pathogens [59,60]. This defense contributes to preserving the structural and functional integrity of the fungus. In the life cycle of fungi, chitin actively participates in the growth and development of fungal structures, playing a key role during the growth of mycelium and the formation of carpophores [61,62]. The concentration of 1% WD has emerged as a critical threshold, where its application resulted in an unexpected interruption of the previously observed positive trends across all measured parameters in P. eryingii. This disruption hints at a complex interplay between the concentration of WD and its effects on fungal physiology. Notably, this adverse impact at higher concentrations may be attributed, at least in part, to the protective function of chitin present in the fungal cell wall. It is intriguing to note that at lower concentrations, particularly up to doses of 0.5% WD, the response of the fungi was notably favorable, particularly evident in the augmentation of biochemical parameters. However, as the concentration of WD increased beyond this threshold, a discernible decline was witnessed in several key parameters, including soluble proteins and phenols. This unexpected decrease suggests a potential saturation effect or an adverse response triggered by the heightened levels of WD. This underscores the necessity for precise dosage optimization to harness the beneficial effects of WD while mitigating any detrimental impacts on fungal physiology and productivity.
The increase in total soluble protein content observed in mushrooms treated with 0.2% WD is noteworthy. Fungi contain a high amount of proteins and are recognized as high-quality dietary sources due to their low-fat content, high fiber content, and functional ingredients such as phenols [63]. Moreover, mushrooms were recently included by the food industry, e.g., for the production of integrators, in view of their high nutritional value and completeness of essential amino acids [64,65,66].
As already mentioned, this is the first study investigating the use of WD on the growth of edible fungi, as scientific research on this topic is fully missing. Nevertheless, similar results have been observed in chickpea seeds, where a ca. 20% increase in soluble proteins was found following 0.2% WD spray applications [67]. The observed rise in soluble protein content could be linked to the remarkable reduction in glycogen content at 0.2% WD concentration. Similar to plants, polysaccharides in fungi play a role in storing energy, which is utilized as needed or under stress conditions [68]. In plants, it has recently been demonstrated that WD induces a precise type of stress, called eustress, stimulating the production of antioxidant substances, resulting in increased key components of crops (i.e., lycopene for tomato, proteins for chickpeas, and carbohydrates for potato) [50,54,67]. Therefore, it is plausible to speculate that the increase in proteins is indirectly connected to the reduction in glycogen since it is cleaved through an enzymatic process known as starch hydrolysis [69]. This process involves the action of specific enzymes produced by mushrooms to convert glycogen into simpler sugars such as glucose and maltose, which can then be used as a source of energy for the formation of primary and secondary metabolites [70].
Regarding the antioxidant compounds, a notable increase in TPC was observed in mushrooms treated with 0.2% WD and 0.5% WD. Similarly, there was an increase in the content of vitamin C in mushrooms treated with concentrations > 0.2% WD. This rise in these parameters holds significant implications from a nutritional point of view, as mushrooms have long been recognized for their diverse beneficial effects, including antioxidant, antimicrobial, anticancer, and anti-inflammatory activities due to their molecules [71,72,73]. Among the secondary metabolites found in mushrooms, polyphenols have been extensively studied and proved to be effective against various health complications [74,75,76]. Additionally, vitamin C plays an important role in mushrooms, influencing cell structure, albeit to a lesser extent compared to plants and animals [77]. Moreover, the accumulation of vitamin C is recognized as a response to external environmental stress, enhancing the survival and adaptability of these organisms [78].
5. Conclusions
This study is the first to investigate the effect of wood distillate on the biochemical parameters of edible fungi. While the yield was not affected, the application of 0.2% WD led to a significant increase in the content of total soluble proteins (+95%) and antioxidant compounds (phenols: +22%; vitamin C: +17%), underscoring the potential of this biostimulant in mushroom cultivation. These findings suggest that WD can enhance certain nutritional qualities of mushrooms, making it a promising additive in the agricultural sector.
However, further research is needed to confirm and generalize these results. It is crucial to test the response of various fungal species beyond P. eryngii to gain a comprehensive understanding of the broader applicability of WD. Additionally, future studies should not only validate the observed outcomes but also investigate a wider array of biochemical parameters, such as enzyme activities, lipid profiles, and other nutritional and functional components.
Author Contributions
Conceptualization, R.F.; methodology, R.F. and S.L.; formal analysis, R.F.; investigation, R.F., I.M. and E.S.; resources, S.L.; data curation, R.F. and S.L.; visualization, R.F.; writing—original draft preparation, R.F.; writing—review and editing, R.F., I.M., C.P., E.S. and S.L.; supervision, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on reasonable request by the corresponding author.
Acknowledgments
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4-Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union–NextGenerationEU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B63C22000650007”, Project title “National Biodiversity Future Center-NBFC”. Thanks are due to Azienda Agricola Micologica De Biasi Arcangelo for kindly providing the fungal substrate, and Francesco Barbagli (BioEsperia and BioDea) for kindly providing the wood distillate.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations. 2022. Available online: https://news.un.org/en/story/2022/07/1122272#:~:text=The%20latest%20UN%20projections%20suggest%20that%20the%20world%E2%80%99s,expected%20to%20remain%20at%20that%20level%20until%202100 (accessed on 7 March 2024).
- Godfray, H.C.J.; Garnett, T. Food security and sustainable intensification. Philos. Trans. R. Soc. B Biol. 2014, 369, 20120273. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- European Commission. 2023. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 7 March 2024).
- Thavamani, A.; Sferra, T.J.; Sankararaman, S. Meet the meat alternatives: The value of alternative protein sources. Curr. Nutr. Rep. 2020, 9, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Henderson, B.; Makkar, H.P. Mitigation of Greenhouse Gas Emissions in Livestock Production: A Review of Technical Options for Non-CO2 Emissions (No. 177); Food and Agriculture Organization of the United Nations (FAO): Quebec City, AC, Canada, 2013. [Google Scholar]
- Aleksandrowicz, L.; Green, R.; Joy, E.J.M.; Smith, P.; Haines, A. The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: A systematic review. PLoS ONE 2016, 11, e0165797. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wösten, H.A. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Szczebyło, A.; Rejman, K.; Halicka, E.; Laskowski, W. Towards more sustainable diets—Attitudes, opportunities and barriers to fostering pulse consumption in Polish cities. Nutrients 2020, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. The cultivation and environmental impact of mushrooms. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Aiking, H. On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Pecoraro, L.; Altieri, R.; Esposito, A.; Perini, C.; Salerni, E.; De Dominicis, V. Impiego di substrati sperimentali a base di reflui oleari per la coltivazione di funghi eduli. Mic. Ital. 2008, 37, 34–39. [Google Scholar]
- Moore, D.; Chiu, S.W. Fungal products as food. In Bio-Exploitation of Filamentous Fungi; Fungal Diversity Press: Hong Kong, China, 2021; pp. 223–251. [Google Scholar]
- Akyuz, M.; Kirbag, S. Nutritive value of Pleurotus eryngii (DC. ex Fr.) quel. var. eryngii grown on various agrowastes. Philipp. Agric. Sci. 2009, 92, 327–331. [Google Scholar]
- Çağlarırmak, N. The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds. Food Chem. 2007, 105, 1188–1194. [Google Scholar] [CrossRef]
- Garcha, H.S.; Khanna, P.K.; Son, G.L. Nutritional importance of milk. In Mushroom Biology and Mushroom Products; Chang, S.T., Buswell, J.A., Chiu, S.W., Eds.; Chinese University Press: Hong Kong, China, 1993; pp. 227–235. [Google Scholar]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. Food Sci. Tech. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Lo, S.H. Antioxidant properties of several culinary medicinal mushrooms during postharvest storage. Int. J. Med. Mushrooms 2008, 10, 245–253. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B. Protective effect of two edible mushrooms against oxidative cell damage and their phenolic composition. Food Chem. 2011, 128, 613–619. [Google Scholar] [CrossRef]
- DPR 1995. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:presidente.repubblica:decreto:1995;376 (accessed on 8 March 2024).
- Bruno, G.L.; Lafortezza, M.A.; Tommasi, F. Il Cardoncello, Pleurotus eryngii (DC.) Quél. una risorsa del territorio: Caratterizzazione di ceppi pugliesi tra fisiologia e nutraceutica. Not. Della Soc. Bot. Ital. 2020, 4, 1–4. [Google Scholar]
- Calabretti, A.; Mang, S.M.; Becce, A.; Castronuovo, D.; Cardone, L.; Candido, V.; Camele, I. Comparison of bioactive substances content between commercial and wild-type isolates of Pleurotus eryngii. Sustainability 2021, 13, 3777. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Sankaran, M. Pleurotus ostreatus: An oyster mushroom with nutritional and medicinal properties. J. Biochem. Technol. 2014, 5, 718–726. [Google Scholar]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Xie, C.; Yan, L.; Gong, W.; Zhu, Z.; Tan, S.; Chen, D.; Peng, Y. Effects of different substrates on lignocellulosic enzyme expression, enzyme activity, substrate utilization and biological efficiency of Pleurotus eryngii. Cell Physiol. Biochem. 2016, 39, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.L.; Lin, J.F.; Guo, L.Q.; Cao, R.W.; Zeng, W.Q. Evaluation of Burma reed as substrate for production of Pleurotus eryngii. Indian. J. Microbiol. 2013, 53, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Food Safety Authority. Scientific opinion on establishing food-based dietary guidelines. EFSA J. 2010, 8, 1460. [Google Scholar] [CrossRef]
- Italian Ministerial Decree 6793. 18 July 2018. Available online: https://www.gazzettaufficiale.it/eli/id/2018/09/05/18A05693/sg (accessed on 13 March 2024).
- Fedeli, R.; Vannini, A.; Djatouf, N.; Celletti, S.; Loppi, S. Can lettuce plants grow in saline soils supplemented with biochar? Heliyon 2024, 10, e26526. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. JAAP 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H.; Abbey, L. Seed priming with pyroligneous acid mitigates aluminum stress, and promotes tomato seed germination and seedling growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Fedeli, R.; Fiaschi, T.; de Simone, L.; Vannini, A.; Angiolini, C.; Maccherini, S. Effects of wood distillate on seedling emergence and first-stage growth in five threatened arable plants. Diversity 2022, 14, 669. [Google Scholar] [CrossRef]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-Dependent and Species-Specific Effects of Wood Distillate Addition on the Germination Performance of Threatened Arable Plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the safety profile of sweet chestnut wood distillate employed in agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Yoshimura, H.; Washio, H.; Yoshida, S.; Seino, T.; Otaka, M.; Matsubara, K.; Matsubara, M. Promoting effect of wood vinegar compounds on fruit-body formation of Pleurotus ostreatus. Mycoscience 1995, 36, 173–177. [Google Scholar] [CrossRef]
- Chang, H.Y.; Kang, A.S.; Cha, D.Y.; Sung, J.M.; Morinaga, T. Effects of wood vinegar on the mycelial growth promotion of some edible mushrooms and Trichoderma pathogen inhibition. RDA J. Agric. Sience Farm Manag. Agric. Eng. Seric. Mycol. Farm Prod. Util. 1995, 37, 766–771. [Google Scholar]
- Ohta, A.; Zhang, L.J. Acceleration of mycelial growth and fruiting body production of edible mushrooms by wood vinegar fractions. J. Jpn. Wood Res. Soc. 1994, 40, 429–433. [Google Scholar]
- Yoshimura, H.; Hayakawa, T. Acceleration effect of wood vinegar from Quercus crispula on the mycelial growth of some basidiomycetes. Trans. Mycol. Soc. Jpn. 1991, 32, 55–64. [Google Scholar]
- De Biasi, 2024. Available online: https://www.fungocardoncello.it/ (accessed on 7 March 2024).
- BioDea. Available online: https://biodea.bio/bio-wood-distillate/?lang=en (accessed on 1 March 2024).
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Aseka, J.M.; Loppi, S. Exploring sustainable alternatives: Wood distillate alleviates the impact of bioplastic in basil plants. Sci. Total Environ. 2023, 900, 166484. [Google Scholar] [CrossRef]
- Khumlianlal, J.; Sharma, K.C.; Singh, L.M.; Mukherjee, P.K.; Indira, S. Nutritional profiling and antioxidant property of three wild edible mushrooms from north east India. Molecules 2022, 27, 5423. [Google Scholar] [CrossRef] [PubMed]
- Gaur, T.; Rao, P.B.; Kushwaha, K.P.S. Nutritional and anti-nutritional components of some selected edible mushroom species. Indian J. Nat. Prod. Resour. 2016, 7, 155–161. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of Yield and Nutraceutical Traits of Orange-Fleshed Sweet Potato Storage Roots in Two Agro-Climatic Zones of Northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Grattacaso, M.; Loppi, S. Wood distillate (pyroligneous acid) boosts nutritional traits of potato tubers. Ann. Appl. Biol. 2023, 183, 135–140. [Google Scholar] [CrossRef]
- Azarnejad, N.; Celletti, S.; Ghorbani, M.; Fedeli, R.; Loppi, S. Dose-Dependent Effects of a Corn Starch-Based Bioplastic on Basil (Ocimum basilicum L.): Implications for Growth, Biochemical Parameters, and Nutrient Content. Toxics 2024, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Celletti, S.; Loppi, S.; Vannini, A. Comparison of the Effect of Solid and Liquid Digestate on the Growth of Lettuce (Lactuca sativa L.) Plants. Agronomy 2023, 13, 782. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Fedeli, R.; Marotta, L.; Frattaruolo, L.; Panti, A.; Carullo, G.; Fusi, F.; Loppi, S. Nutritionally enriched tomatoes (Solanum lycopersicum L.) grown with wood distillate: Chemical and biological characterization for quality assessment. J. Food Sci. 2023, 88, 5324–5338. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Liu, H.; Zhang, Z.; Zhan, Y.; Wang, K.; Yang, D.; Yu, J. Evaluation of wood vinegar as an herbicide for weed control. Agronomy 2022, 12, 3120. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Bagavathiannan, M.; Yu, J. The use of wood vinegar as a non-synthetic herbicide for control of broadleaf weeds. Ind. Crops Prod. 2021, 173, 114105. [Google Scholar] [CrossRef]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A “hidden figure” in the fungal cell wall. In The Fungal Cell Wall: An Armour and a Weapon for Human Fungal Pathogens; Springer: Cham, Switzerland, 2020; pp. 83–111. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, L.; Vafaei, A.; Rösner, J.; Merzendorfer, H. Chitin prevalence and function in bacteria, fungi and protists. In Targeting Chitin-Containing Organisms; Springer: Singapore, 2019; pp. 19–59. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C.S. Chitin and its effects on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Göhre, V. Fungal chitinases: Function, regulation, and potential roles in plant/pathogen interactions. Curr. Genet. 2016, 62, 243–254. [Google Scholar] [CrossRef]
- Thakur, D.; Bairwa, A.; Dipta, B.; Jhilta, P.; Chauhan, A. An overview of fungal chitinases and their potential applications. Protoplasma 2023, 260, 1031–1046. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional quality and biological application of mushroom protein as a novel protein alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Ache, N.T.; Manju, E.B.; Lawrence, M.N.; Tonjock, R.K. Nutrient and mineral components of wild edible mushrooms from the KilumIjim forest, Cameroon. Afr. J. Food Sci. 2021, 15, 152–161. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of nutritional composition in 23 kinds of edible fungi. J. Food Qual. 2020, 8821315. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2023, 182, 57–64. [Google Scholar] [CrossRef]
- Wang, B.T.; Hu, S.; Yu, X.Y.; Jin, L.; Zhu, Y.J.; Jin, F.J. Studies of cellulose and starch utilization and the regulatory mechanisms of related enzymes in fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; White, N.A. Introduction to fungal physiology. In Fungi: Biology and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 1–35. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, V.W.; Lubbe, A.; Qin, L.; Deng, S.; Kennedy, M.; Glass, N.L. A fungal transcription factor essential for starch degradation affects integration of carbon and nitrogen metabolism. PLoS Genet. 2017, 13, e1006737. [Google Scholar] [CrossRef] [PubMed]
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of phen-olic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends Food Sci. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Muszynska, B.; Sułkowska-Ziaja, K.M.; Ekiert, H. Phenolic acidsin selected edible Basidiomycota species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol.-Hortorum Cultus 2013, 12, 107–116. [Google Scholar]
- Ferreira, I.C.; Barros, L.; Abreu, R.M. Antioxidants in wildmushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- G̨asecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bio-active compounds content and antioxidant properties of soil-grow-ing and wood-growing edible mushrooms. J. Food Process. Preserv. 2018, 42, e13386. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capaci-ties and metal chelating ability of edible mushrooms commonly con-sumed in China. LWT 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free. Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).