Current Status of Haploidization in Cool-Season Grain Legume Crop Species
Abstract
1. Introduction
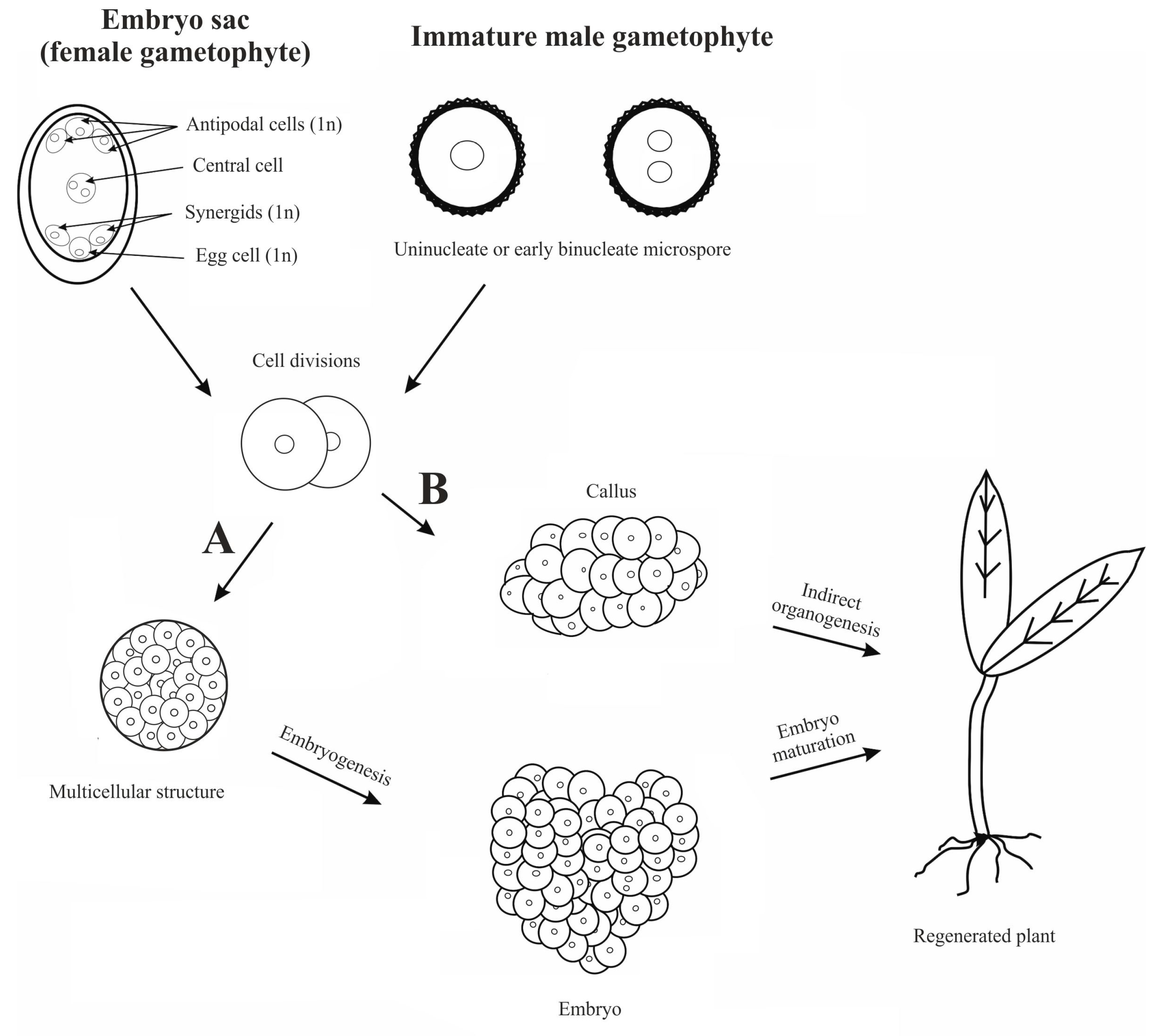
2. Origin of Haploids
2.1. In Vivo Haploidization
2.2. In Vitro Haploidization
2.2.1. Androgenesis
2.2.2. Gynogenesis
2.3. In Vivo-In Vitro Methods
2.3.1. Chromosome Elimination
2.3.2. Induced Parthenogenesis (Matromorphy)
3. Haploidization in Cool-Season Grain Legume Crops
3.1. Tribe Vicieae (Fabeae)
3.1.1. Field Pea
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| Androgenesis/ Anther culture | Anthers Uninucleate microspore | No treatments, dark | Callus | A total of 91% of the callus was identified as haploid No plant regeneration | [95] |
| Anthers Uninucleate microspore | No treatments, dark | Callus Embryo-like structures Embryos (globular and heart) Shoot regeneration | Some embryos developed into plants but no haploid plants were obtained Callus was a mixoploid. No plant regeneration from the callus | [96] | |
| Anthers Stage not specified | Anther culture kept at 4 °C for 72 h, dark | Embryoid formation and dedifferentiation into callus | Mixoploid callus No plant regeneration | [98] | |
| Anthers Stage not specified | Anthe cultures kept at 37 °C for 4 h, dark | Callus Embryo-like structures Shoot regeneration | Embryo-like structures and shoots were obtained after treating an isolated anther culture at a temperature of 37 °C for 4 h Regenerated shoots and plants. | [100] | |
| Anthers Uninucleate microspore | Flower buds kept at 4 °C for 0 to 4 days. Anther culture kept at 35 °C or 38 °C for 18 h, dark | Callus Embryos (globular) | Callus multiplication No plant regeneration | [102] | |
| Uninucleate microspore | Anther culture kept at 4 °C and 35 to 38 °C, dark | Callus Shoot regeneration | Regenerated plants were derived from the somatic cells of anthers | [103] | |
| Uninucleate microspore | Flower buds kept at 4 °C for 3 days, dark | Callus Embryos Shoot regeneration | Plant regeneration from callus-derived embryos. The ploidy level was not analyzed | [104] | |
| Androgenesis/Isolated microspore culture | Uninucleate microspore | Flower buds kept at 4 °C for 48 h, dark | Microspore-derived multinucleate structure Embryos (torpedo) | Single diploid plant regenerated from embryo | [15] |
| Tetrads, uninucleate microspores, and the later stages of microsporogenesis | Flower buds kept at 4 °C or 10 °C for 2, 4, 7, 14, 21 days or 1 month; osmotic stress provided by using mannitol or sucrose in various concentrations; electric shock | Microspore-derived micro-calli Regenerated plants | A small number of haploid plants obtained after applying stresses The best androgenic response for uninucleate microspores | [106] | |
| Uninucleate microspore | Flower buds kept at 4 °C for 0 to 28 days, later isolated microspore culture treated at 35 °C for 0 to 18 h, dark | Microspore-derived micro-calli | Micro-calli was produced after applying cold pretreatment for 10 and 16 days | [107] | |
| Induced parthenogenesis (Matromorphy) | Vicia faba pollen | No additional treatment | Embryos | After pollination with V. faba pollen, abnormal endosperm was formed | [108] |
| Lathyrus odoratus pollen, another variety of P. sativum pollen | Gamma irradiation, flower buds treated with IAA on the fifth day following emasculation | Seeds | The homozygosity of some progeny obtained from seeds | [109] |
3.1.2. Faba Bean
3.1.3. Grass Pea
3.1.4. Lentil
3.2. Tribe Genisteae
Lupins
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| In vivo/spontaneous | L. albus | - | seeds | Two sterile plants | [164] |
| Androgenesis/Anther culture | L. polyphyllus anthers | Not specified | Callus | No further development of callus | [165] |
| L. polyphyllus anthers | Not specified | Callus | - | [166] | |
| L. polyphyllus anthers | Not specified | Callus Regenerated plants | Chromosome counts showed diploid calli | [167] | |
| L. hartwegii anthers | Not specified | Callus | - | [168] | |
| L. albus anthers | No application of stress treatment, liquid culture medium | Embryo-like structures (ELSs) | secondary somatic embryogenesis on ELS | [171] | |
| L. angustifolius, L. luteus, and L. albus anthers containing microspores in uninucleate stage | Cold or heat pretreatment: flower buds kept at 4 °C for 2 or 5 days in darkness or at 32 °C for 1 or 3 days | Callus | Plant regeneration was not obtained | [169] | |
| L. albus anthers containing microspores in the late mononucleate developmental stage | Heat stress treatment: anther cultured at 30 °C in the dark, | Embryo-like structures (ELSs) | ELSs were obtained on media containing 2,4-D and BAP | [173] | |
| L. angustifolius anthers in the uninucleate stage | Cold pretreatment: inflorescences kept at 4 °C for 4 days in the darkness | Multicellular structures Embryo-like structures Callus | Calli produced roots on MS medium containing BAP and NAA | [170] | |
| Androgenesis/Isolated microspore culture | L. polyphyllus microspore. But unknown developmental stage | Not specified | No development | - | [165] |
| L. albus microspore | No application of stress treatment, liquid culture medium | No development | - | [171] | |
| L. albus, L. angustifolius, and L. luteus microspores | Cold or heat treatment: microspore culture was kept at 4 °C or 32 °C for 24 h in darkness | Induction of embryogenesis Multicellular structures Proembryos | No plant regeneration | [172] | |
| L. albus, uninucleate microspores (late stage) | Microspore culture treated at 32 °C for 3 days | Embryo-like structures (ELSs) | ELSs were obtained on media containing 2,4-D and BAP | [173] |
3.3. Tribe Cicereae
4. Conclusions
4.1. Method of Haploidization
4.2. Genotype
4.3. Stress Factors
4.4. Culture Medium
4.5. Plant Regeneration
4.6. Origin of Regenerants
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews, M.; Hodge, S. Climate Change, a Challenge for Cool Season Grain Legume Crop Production. In Climate Change and Management of Cool Season Grain Legume Crops; Yadav, S., Redden, R., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- FAOSTAT. Access Protocol. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 April 2024).
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants–does it matter? Plant Soil. 2022, 476, 31–62. [Google Scholar] [CrossRef]
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The resurgence of haploids in higher plants. Trends Plant Sci. 2007, 12, 368–375. [Google Scholar] [CrossRef]
- Murovec, J.; Bohanec, B. Haploids and doubled haploids in plant breeding. In Plant Breeding; Abdurakhmonov, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 87–106. [Google Scholar]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Adamus, A.; Szklarczyk, M.; Kiełkowska, A. Haploid and doubled haploid plant production in Brassica rapa L. subsp. Pekinensis via microspore culture. In Doubled Haploid Technology; Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana Press: New York, NY, USA, 2021; Volume 2288, pp. 181–199. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M.; Jacquier, N.M.A.; Widiez, T. Overview of in vitro and in vivo doubled haploid technologies. In Doubled Haploid Technology; Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 2287, pp. 3–22. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Kiszczak, W. History and current status of haploidization in carrot (Daucus carota L.). Agronomy 2023, 13, 676. [Google Scholar] [CrossRef]
- Maluszynski, M.; Kasha, K.J.; Forster, B.P.; Szarejko, I. Doubled Haploid Production in Crop Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Żur, I.; Adamus, A.; Cegielska-Taras, T.; Cichorz, S.; Dubas, E.; Gajecka, M.; Juzon, K.; Kiełkowska, A.; Malicka, M.; Oleszczuk, S.; et al. Doubled haploids: Contribution of Poland’s academies in recognizing the mechanism of gametophyte cell reprogramming and their utilization in breeding agricultural and vegetable crops. Acta Soc. Bot. Pol. 2022, 91, 9128. [Google Scholar] [CrossRef]
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; Siddique, K.H.M. Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Li, J. Germination of embryo in soybean anther culture. Chin. Sci. Bull. 1998, 43, 1991–1995. [Google Scholar] [CrossRef]
- Kaur, P.; Bhalla, J.K. Regeneration of haploid plants from microspore culture of pigeonpea (Cajanus cajan L.). Indian J. Exp. Biol. 1998, 36, 736–738. [Google Scholar]
- Croser, J.S.; Lulsdorf, M.M. Progress towards haploid division in chickpea (Cicer arietinum L.), field pea (Pisum sativum L.) and lentil (Lens culinaris Medik.) using isolated microspore culture. In Proceedings of the European Grain Legume Conference, Paris/Dijon, France, 7–11 June 2004; AEP: Wilmington, DE, USA, 2004; p. 189. [Google Scholar]
- Reddy, V.D.; Reddy, G.M. In vivo production of haploids in chickpea (Cicer arietinum L.). J. Genet. Breed. 1996, 51, 29–32. [Google Scholar]
- Zagorska, N.; Dimitrov, B. Induced androgenesis in alfalfa (Medicago sativa L.). Plant Cell Rep. 1995, 14, 249–252. [Google Scholar] [CrossRef]
- Zagorska, N.; Dimitrov, B.; Gadeva, P.; Robeva, P. Regeneration and characterization of plants obtained from anther cultures in Medicago sativa L. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 107–110. [Google Scholar] [CrossRef]
- Gharyal, P.K.; Rashid, A.; Maheshwari, S.C. Production of haploid plantlets in anther cultures of Albizzia lebbeck L. Plant Cell Rep. 1983, 2, 308–309. [Google Scholar] [CrossRef]
- Lulsdorf, M.M.; Croser, J.S.; Ochatt, S. Androgenesis and Doubled-Haploid Production in Food Legumes. Biol. Breed. Food Legumes. 2011, 159, 159–177. [Google Scholar]
- Nowaczyk, P.; Nowaczyk, L. The influence of growth regulators on the frequency of polyembryony in pepper (Capsicum annuum L.). J. Appl. Gen. 1996, 37A, 204–207. [Google Scholar]
- Takeuchi, Y.; Kosaza, M.; Ozaki, Y.; Tomiyoshi, K.; Matsuishi, T.; Okubo, H. Origin of polyembryonic seeds and production of haploids in asparagus. Acta Hortic. 2020, 1301, 57–66. [Google Scholar] [CrossRef]
- Hand, M.L.; Koltunow, A.M. The genetic control of apomixis: Asexual seed formation. Genetics 2014, 197, 441–450. [Google Scholar] [CrossRef]
- Chase, S.S. Monoploids and monoploid-derivatives of maize (Zea mays L.). Bot. Rev. 1969, 35, 117–168. [Google Scholar] [CrossRef]
- Curtiss, J.; Rodriguez-Uribe, L.; Stewart, J.M.; Zhang, J. Identification of differentially expressed genes associated with semigamy in Pima cotton (Gossypium barbadense L.) through comparative microarray analysis. BMC Plant Biol. 2011, 16, 49. [Google Scholar] [CrossRef]
- McKone, M.J.; Halpern, S.L. The evolution of androgenesis. Am. Nat. 2003, 161, 641–656. [Google Scholar] [CrossRef]
- Palumbo, F.; Pasquali, E.; Albertini, E.; Barcaccia, G. A Review of Unreduced Gametes and Neopolyploids in Alfalfa: How to Fill the Gap between Well-Established Meiotic Mutants and Next-Generation Genomic Resources. Plants 2021, 10, 999. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T. Male gametophyte development in flowering plants: A story of quarantine and sacrifice. J. Plant Physiol. 2021, 153365, 258–259. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M. Androgenesis revisited. Bot. Rev. 2010, 76, 377–404. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Zhao, W.X.; Li, X.H.; Liu, X.C.; Gao, N.N.; Huang, J.H.; Tang, Z.H. Androgenesis, gynogenesis, and parthenogenesis haploids in cucurbit species. Plant Cell Rep. 2016, 35, 1991–2019. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Golemiec, E.; Szechyńska-Hebda, M.; Gołębiowska, G.; Wędzony, M. Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (x Triticosecale Wittm.). Plant Cell Rep. 2009, 28, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Tucker, M.R.; Maurer, H.P. Stress treatments influence efficiency of microspore embryogenesis and green plant regeneration in hexaploid triticale (× Triticosecale Wittmack, L.). In Vitro Cell. Dev. Biol.-Plant 2014, 50, 143–148. [Google Scholar] [CrossRef]
- Mikolajczyk, S.; Broda, Z.; Weigt, D. The effect of cold temperature stress on the viability of rye (Secale cereale L.) microspores. BioTechnologia. J. Biotechnol. Comput. Biol. Bionanotechnol. 2012, 93, 139–142. [Google Scholar]
- Supena, E.D.J.; Suharsono, S.; Jacobsen, E.; Custers, J.B.M. Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep. 2006, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Grozeva, S.; Todorova, V.; Stankova, G.; Anachkov, N.; Rodeva, V. Effects of low temperature, genotype and culture media on in vitro androgenic answer of pepper (Capsicum annuum L.). Acta Physiol. Plantarum. 2016, 38, 273. [Google Scholar] [CrossRef]
- Yuan, S.X.; Liu, Y.M.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Zhang, Y.Y.; Sun, P.T. Effect of combined cold pretreatment and heat shock on microspore cultures in broccoli. Plant Breed. 2011, 130, 80–85. [Google Scholar] [CrossRef]
- Yuan, S.; Su, Y.; Liu, Y.; Li, Z.; Fang, Z.; Yang, L.; Sun, P. Chromosome doubling of microspore-derived plants from cabbage (Brassica oleracea var. capitata L.) and broccoli (Brassica oleracea var. italica L.). Front. Plant Sci. 2015, 6, 1118. [Google Scholar] [CrossRef]
- Calabuig-Serna, A.; Camacho-Fernández, C.; Mir, R.; Porcel, R.; Carrera, E.; López-Díaz, I.; Seguí-Simarro, J.M. Quantitative and qualitative study of endogenous and exogenous growth regulators in eggplant (Solanum melongena) microspore cultures. Plant Growth Regul. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Kim, M.; Jang, I.C.; Kim, J.A.; Park, E.J.; Yoon, M.; Lee, Y. Embryogenesis and plant regeneration of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep. 2008, 27, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Lantos, C.; Gémes Juhász, A.; Vági, P.; Mihály, R.; Kristóf, Z.; Pauk, J. Androgenesis induction in microspore culture of sweet pepper (Capsicum annuum L.). Plant Biotechnol. Rep. 2012, 6, 123–132. [Google Scholar] [CrossRef][Green Version]
- Shmykova, N.; Domblides, E.; Vjurtts, T.; Domblides, A. Haploid embryogenesis in isolated microspore culture of carrots (Daucus carota L.). Life 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Romanova, O.V.; Vjurtts, T.S.; Mineykina, A.I.; Tukuser, Y.P.; Kulakov, Y.V.; Akhramenko, V.A.; Domblides, E.A. Embryogenesis induction of carrot (Daucus carota L.) in isolated microspore culture. Foods Raw Mat. 2023, 11, 25–34. [Google Scholar] [CrossRef]
- Musiał, K.; Bohanec, B.; Jakse, M.; Przywara, L. The development of onion (Allium cepa L.) embryo sacs in vitro and gynogenesis induction in relation to flower size. In Vitro Cell Dev. Biol.-Plant 2005, 41, 446–452. [Google Scholar] [CrossRef]
- Lux, H.; Herrmann, L.; Wetzel, C. Production of haploid sugar-beet (Beta vulgaris L) by culturing unpollinated ovules. Plant Breed. 1990, 104, 177–183. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Zhaing, S. Study the effect of pre-treatment and exogenous hormones on embryoids induced from unpollinated ovaries of cucumber. Acta Agric. Boreali-Sin. 2008, 23, 50–53. [Google Scholar] [CrossRef]
- Diao, W.P.; Bao, S.Y.; Jiang, B.; Cui, L.; Qian, C.T.; Chen, J.F. Cytogenetic studies on microsporogenesis and male gametophyte development in autotriploid cucumber (Cucumis sativus L.): Implication for fertility and production of trisomics. Plant Syst. Evol. 2009, 279, 87–92. [Google Scholar] [CrossRef]
- Sibi, M.L.; Kobaissi, A.; Shekafandeh, A. Green haploid plants from unpollinated ovary culture in tetraploid wheat (Triticum durum Defs.). Euphytica 2001, 122, 351–359. [Google Scholar] [CrossRef]
- Alan, A.R.; Brants, A.; Cobb, E.; Goldschmied, A.; Mutschler, M.A.; Earle, E.D. Fecund gynogenic lines from onion (Allium cepa L.) breeding materials. Plant Sci. 2004, 167, 1055–1066. [Google Scholar] [CrossRef]
- Chand, S.; Sahrawat, A.K. Embryogenesis and plant regeneration from unpollinated ovary culture of Psoralea corylifolia. Biol. Plant 2007, 51, 223–228. [Google Scholar] [CrossRef]
- Tekdal, D. In vitro Regeneration Studies of Vuralia turcica Using Unpollinated Ovaries. J. Agric. Sci. 2022, 28, 115–120. [Google Scholar] [CrossRef]
- Wędzony, M. Protocol for doubled haploid production in hexaploid triticale (x Triticosecale Wittm.) by crosses with maize. In Doubled Haploid Production in Crop Plants; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Warchoł, M.; Zieliński, K.; Dubas, E. Obtaining of winter rye (Secale cereale L. ssp. cereale) haploid embryos through hybridization with maize (Zea Mays L.). Cereal Res. Commun. 2018, 46, 521–532. [Google Scholar] [CrossRef]
- Rines, H.W.; Dahleen, L.S. Haploid oat plants produced by application of maize pollen to emasculated oat florets. Crop Sci. 1990, 30, 1073–1078. [Google Scholar] [CrossRef]
- Devaux, P. The Hordeum bulbosum (L.) method. In Doubled Haploid Production in Crop Plants: A Manual; Springer: Dordrecht, The Netherlands, 2003; pp. 15–19. [Google Scholar] [CrossRef]
- Matzk, F.; Mahn, A. Improved techniques for haploid production in wheat using chromosome elimination. Plant Breed. 1994, 113, 125–129. [Google Scholar] [CrossRef]
- Bakos, F.; Jager, K.; Barnabás, B. Regeneration of haploid plants after distant pollination of wheat via zygote rescue. Acta Biol. Crac. 2005, 47, 167–171. [Google Scholar]
- Mochida, K.; Tsujimoto, H.; Sasakuma, T. Confocal analysis of chromosome behavior in wheat×maize zygotes. Genome 2004, 47, 199–205. [Google Scholar] [CrossRef]
- Ishii, T.; Karami-Ashtiyani, R.; Houben, A. Haploidization via Chromosome Elimination: Means and Mechanisms. Ann. Rev. Plant Biol. 2016, 67, 421–438. [Google Scholar] [CrossRef]
- Gałązka, J.; Niemirowicz-Szczytt, K. Review of research on haploid production in cucumber and other cucurbits. Folia Hortic. 2013, 25, 67–78. [Google Scholar] [CrossRef]
- Kasha, K.J.; Maluszynski, M. Production of doubled haploids in crop plants. An introduction. In Doubled Haploid Production in Crop Plants; Maluszynski, M., Kasha, K., Forster, B.P., Szarejko, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–4. [Google Scholar]
- Conner, J.A.; Mookkan, M.; Huo, H.; Chae, K.; Ozias-Akins, P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. USA 2015, 112, 11205–11210. [Google Scholar] [CrossRef]
- Eeniuk, A.H. Matromorphy in Brassica oleracea L. II. Differences in parthenogenetic ability and parthenogenesis inducing ability. Euphytica 1974, 23, 435–445. [Google Scholar] [CrossRef]
- Hess, D.; Wagner, G. Induction of haploid parthenogenesis in Mimulus-luteus by in vitro pollination with foreign pollen. Z. Pflanzenphysiol. 1974, 72, 466–468. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Qian, D.Q.; Cao, X.Y. Induction of parthenogenesis and chromosome behaviour in plants of parthenogenetic origin of cotton (Gossypium hirsutum). Genome 1991, 34, 255–260. [Google Scholar]
- Kiełkowska, A.; Adamus, A.; Baranski, R. An improved protocol for carrot haploid and doubled haploid plant production using induced parthenogenesis and ovule excision in vitro. In Vitro Cell Dev. Biol.–Plant 2014, 50, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiełkowska, A.; Adamus, A.; Baranski, R. Haploid and Doubled Haploid Plant Production in Carrot Using Induced Parthenogenesis and Ovule Excision In Vitro. In Plant Cell Culture Protocols; Methods in Molecular Biology; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1815, pp. 301–315. [Google Scholar] [CrossRef]
- Yeh, B.P.; Peloquin, S.J. The nucleolus-associated chromosome of Solanum species and hybrids. Am. J. Bot. 1965, 52, 626. [Google Scholar] [CrossRef]
- Badr, M.; Horn, W. Genetische untersuchungen an diploiden und tetraploiden Pelargonium zonale-Hybriden. Z Pflanzenzuchtg. 1971, 66, 203–220. [Google Scholar]
- Horn, W. Interspecific crossability and inheritance in Pelargonium. Plant Breed. 1994, 113, 3–17. [Google Scholar] [CrossRef]
- Horn, W. Induktion und züchterische Nutzung der Parthenogenese. Z. Pflanzenzüchtg. 1972, 67, 39–44. [Google Scholar]
- Emsweller, S.L.; Uhring, J. Endosperm-embryo incompatibility in Lilium species hybrids. Adv. Hort. Sci. Theor. Appl. 1962, 2, 360–367. [Google Scholar]
- Emsweller, S.L.; Uhring, J. Parthenogenesis in tetraploid Lilium longiflorum. Am. J. Bot. 1962, 49, 978–984. [Google Scholar] [CrossRef]
- Germanà, M.A.; Chiancone, B. Gynogenetic haploids of Citrus after in vitro pollination with triploid pollen grains. Plant Cell Tiss. Organ Cult. 2001, 66, 59–66. [Google Scholar] [CrossRef]
- Sato, S.; Katoh, N.; Yoshida, H.; Iwai, S.; Hagimori, M. Production of doubled haploid plants of carnation (Dianthus caryophyllus L.) by pseudofertilized ovule culture. Sci. Hortic. 2000, 83, 301–310. [Google Scholar] [CrossRef]
- Vijverberg, K.; Ozias-Akins, P.; Schranz, M.E. Identifying and Engineering Genes for Parthenogenesis in Plants. Front. Plant Sci. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Kashi, A.; Onsinejad, R. Induction of parthenogenetic embryos by irradiated pollen in cucumber. Int. Symp. Cucurbits 1997, 492, 323–328. [Google Scholar] [CrossRef]
- Faris, N.M.; Nikolova, V.; Niemirowicz-Szczytt, K. The effect of gamma irradiation dose on cucumber (Cucumis sativus L.) haploid embryo production. Acta Physiol. Plant 1999, 21, 391–396. [Google Scholar] [CrossRef]
- Lotfi, M.; Alan, A.R.; Henning, M.J.; Jahn, M.M.; Earle, E.D. Production of haploid and doubled haploid plants of melon (Cucumis melo L.) for use in breeding for multiple virus resistance. Plant Cell Rep. 2003, 21, 1121–1128. [Google Scholar] [CrossRef]
- Doré, C.; Boulidard, L.; Sauton, A.; Rode, J.C.; Cuny, F.; Niemirowiecz-Szczytt, K.; Dumas de Vaulx, R. Interest of irradiated pollen for obtaining haploid vegetables. Genetic Improvement of Horticultural Crops by Biotechnology. Acta Hort. 1994, 392, 123–128. [Google Scholar]
- Kurtar, E.S.; Sarı, N.; Abak, K. Obtention of haploid embryos and plants through irradiated pollen technique in squash (Cucurbita pepo L.). Euphytica 2002, 127, 335–344. [Google Scholar] [CrossRef]
- Musial, K.; Przywara, L. Influence of irradiated pollen on embryo and endosperm development in kiwifruit. Ann. Bot. 1998, 82, 747–756. [Google Scholar] [CrossRef]
- Todorova, M.; Ivanov, P.; Shindrova, P.; Christov, M.; Ivanova, I. Doubled haploid production of sunflower (Helianthus annuus L.) through irradiated pollen-induced parthenogenesis. Euphytica 1997, 97, 249–254. [Google Scholar] [CrossRef]
- Demmink, J.F.; Custers, J.B.M.; Bergervoet, J.H.W. Gynogenesis to bypass crossing barriers between diploid and tetraploid dianthus species. Acta Hortic. 1987, 216, 343–344. [Google Scholar] [CrossRef]
- Meynet, J.; Barrade, R.; Duclos, A.; Siadous, R. Dihaploid plants of roses (Rosa x hybrida, cv ‘Sonia’) obtained by parthenogenesis induced using irradiated pollen and in vitro culture of immature seeds. Agronomie 1994, 14, 169–175. [Google Scholar] [CrossRef]
- De Witte, K.; Keulemans, J. Homozygous plant production in apple by parthenogenesis in situ: Improvement of the pollination stimulus for parthenogenic egg cell development. Eucarpia Symp. Fruit Breed. Genet. 1999, 538, 315–320. [Google Scholar] [CrossRef]
- Höfer, M.; Grafe, C. Induction of doubled haploids in sweet cherry (Prunus avium L.). Euphytica 2003, 130, 191–197. [Google Scholar] [CrossRef]
- Myers, J.R.; Baggett, J.R.; Lamborn, C. Origin, history, and genetic improvement of the snap pea (Pisum sativum L.). Plant Breed. Rev. 2001, 21, 93–138. [Google Scholar]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World, 3rd ed.; Clarendon Press: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- Abbo, S.; Rachamim, E.; Zehavi, Y.; Zezak, I.; Lev-Yadun, S.; Gopher, A. Experimental growing of wild pea in Israel and its bearing on Near Eastern plant domestication. Ann. Bot. 2011, 107, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Uskutoğlu, D.; İdikut, L. Pea production statistics in the world and in Turkey. Inn. Res. Agric. For. Water Issues 2023, 2, 25–38. [Google Scholar]
- Ravindran, G.; Nalle, C.L.; Molan, A.; Ravindran, V. Nutritional and biochemical assessment of field peas (Pisum sativum L.) as a protein source in poultry diets. J. Poult. Sci. 2010, 47, 48–52. [Google Scholar] [CrossRef][Green Version]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef]
- Dhall, R.K. Pea cultivation. In Bulletin no PAU/2017/Elec/FB/E/29; Punjab Agricultural University: Ludhiana, India, 2017. [Google Scholar]
- Hacisalihoglu, G.; Beisel, N.S.; Settles, A.M. Characterization of pea seed nutritional value within a diverse population of Pisum sativum. PLoS ONE 2021, 16, e0259565. [Google Scholar] [CrossRef]
- Gupta, S.; Ghosal, K.K.; Gadgil, V.N. Haploid tissue culture of Triticum aestivum var. Sonalika and Pisum sativum var. B22. Indian Agric. 1972, 16, 277–278. [Google Scholar]
- Gupta, S. Morphogenetic response of haploid callus tissue of Pisum sativum (Var. B22). Indian Agric. 1975, 19, 11–21. [Google Scholar]
- White, P.R. The Cultivation of Animal and Plant Cells, 2nd ed.; Ronald Press: New York, NY, USA, 1963. [Google Scholar]
- Gosal, S.S.; Bajaj, Y.P.S. Pollen embryogenesis and chromosomal variation in anther of three food legumes—Cicer arietinum, Pisum sativum and Vigna mungo. SABRAO J. 1988, 20, 51–58. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sidhu, R.; Davies, P. Pea anther culture: Callus initiation and production of haploid plants. In Proceedings of the Australian Branch of the IAPT&B, Perth, Australia, 21–24 September 2005; pp. 180–186. [Google Scholar]
- Gamborg, O.L.; Miller, R.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Bobkov, S.V. Isolated pea anther culture. Russian Agric. Sci. 2010, 36, 413–416. [Google Scholar] [CrossRef]
- Bobkov, S. Obtaining calli and regenerated plants in anther cultures of pea. Czech J. Gen. Plant Breed. 2014, 50, 123–129. [Google Scholar] [CrossRef]
- Bermejo, C.; Guindon, M.F.; Palacios, L.T.; Cazzola, F.; Gatti, I.; Cointry, E. Comparative androgenetic competence of various species and genotypes within the genus Pisum L. Plant Cell Tiss. Organ Cult. 2020, 143, 487–497. [Google Scholar] [CrossRef]
- Kumar, A.S.; Gamborg, O.L.; Nabors, M.W. Plant regeneration from cell suspension cultures of Vigna aconitifolia. Plant Cell Rep. 1988, 7, 138–141. [Google Scholar] [CrossRef]
- Ochatt, S.; Pech, C.; Grewal, R.; Conreux, C.; Lulsdorf, M.; Jacas, L. Abiotic stress enhances androgenesis from isolated microspores of some legume species (Fabaceae). J. Plant Physiol. 2009, 166, 1314–1328. [Google Scholar] [CrossRef]
- Bobkov, S. Initiation of microcalli in culture of pea (Pisum sativum L.) isolated microspores. Asia Pac. J. Mol. Biol. 2018, 26, 20–26. [Google Scholar] [CrossRef]
- Gritton, E.T.; Wierzbicka, B. An embryological study of a Pisum sativum x Vicia faba cross. Euphytica 1975, 24, 277–284. [Google Scholar] [CrossRef]
- Virk, D.S.; Gupta, A.K. Matromorphy in Pisum sativum L. Theor. Appl. Genet. 1984, 68, 207–211. [Google Scholar] [CrossRef]
- Duc, G.; Bao, S.; Baum, M.; Redden, B.; Sadiki, M.; Suso, M.J.; Zong, X. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res. 2010, 115, 270–278. [Google Scholar] [CrossRef]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar]
- Caracuta, V.; Weinstein-Evron, M.; Kaufman, D.; Yeshurun, R.; Silvent, J.; Boaretto, E. 14, 000-year-old seeds indicate the Levantine origin of the lost progenitor of faba bean. Sci. Rep. 2016, 6, 37399. [Google Scholar] [CrossRef]
- Zong, X.; Yang, T.; Liu, R. Faba bean (Vicia faba L.) breeding. Advances in Plant Breeding Strategies. Legumes 2019, 7, 245–286. [Google Scholar] [CrossRef]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Sathya Prabhu, D.; Devi Rajeswari, V. Nutritional and biological properties of Vicia faba L.: A perspective review. Intern. Food Res. J. 2018, 25, 1332–1340. [Google Scholar]
- Meng, Z.; Liu, Q.; Zhang, Y.; Chen, J.; Sun, Z.; Ren, C.; Huang, Y. Nutritive value of faba bean (Vicia faba L.) as a feedstuff resource in livestock nutrition: A review. Food Sci. Nutr. 2021, 9, 5244–5262. [Google Scholar] [CrossRef]
- Nadal, S.; Suso, M.J.; Moreno, M.T. Management of Vicia faba genetic resources: Changes associated to the selfing process in the major, equina and minor groups. Genet. Res. Crop Evol. 2003, 50, 183–192. [Google Scholar] [CrossRef]
- Maalouf, F.; Hu, J.; O’Sullivan, D.M.; Zong, X.; Hamwieh, A.; Kumar, S.; Baum, M. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 2019, 138, 465–473. [Google Scholar] [CrossRef]
- Paratasilpin, T. Vegetative development of field bean pollen grain cultured in vitro. J. Sci. Soc. Thai. 1978, 4, 139–145. [Google Scholar]
- Hesemann, C.U. Haploid cells in calli from anther cultures of Vicia faba. Z. Pflanzenziichtg. 1980, 84, 23–29. [Google Scholar]
- Shlahi, S.A.; Majeed, D.M.; Ismail, E.N. Effect of the developmental stage of microspores, growth regulator and medium type on callus indication from broad bean Vicia faba anthers culture. J. Biotechnol. Res. Center. 2012, 6, 81–90. [Google Scholar] [CrossRef]
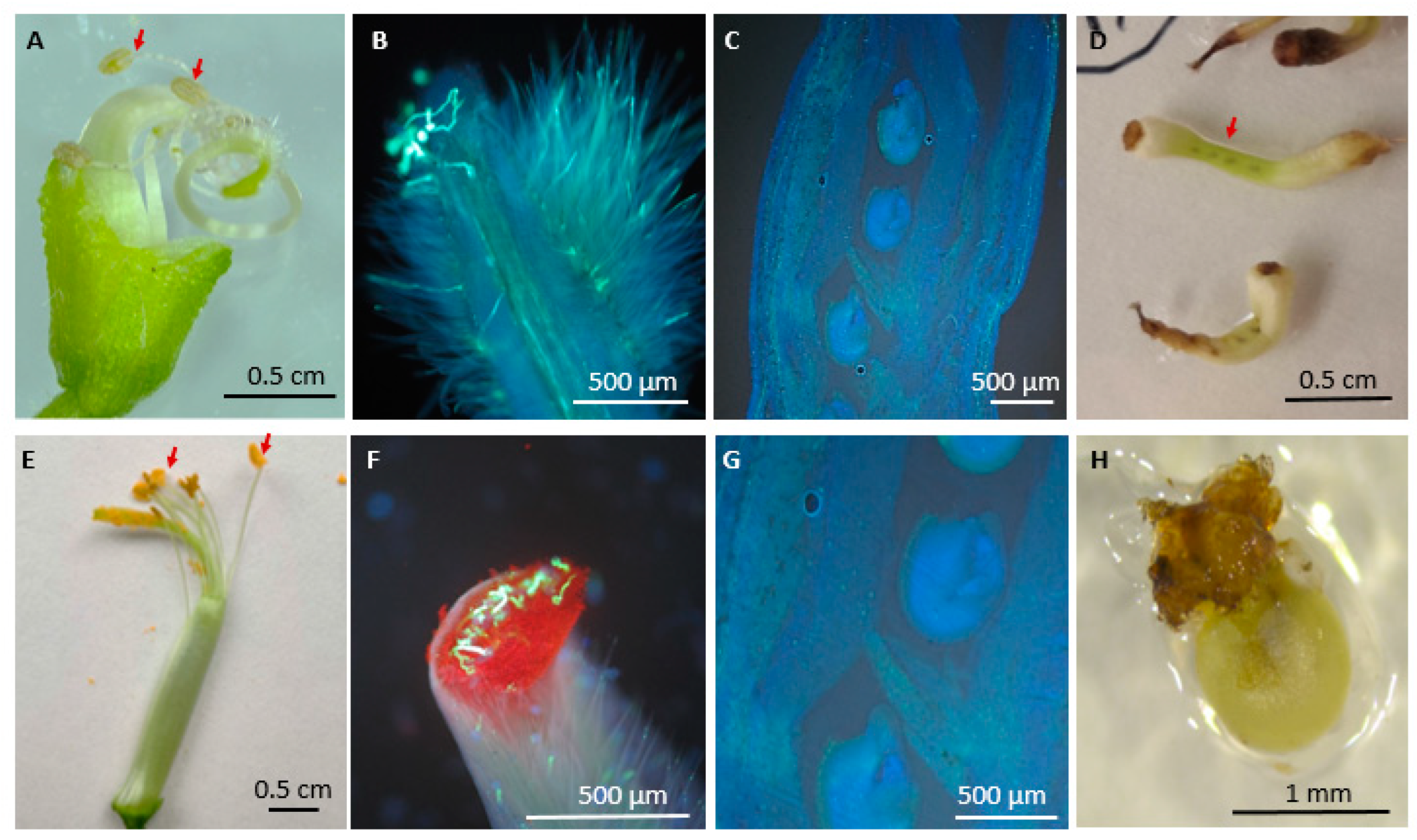
- Küçükrecep, A.; Tekdal, D. Microsporogenesis in faba bean (Vicia faba L.) grown in Mersin, Turkey. Plant Introduct. 2022, 95/96, 68–74. [Google Scholar] [CrossRef]
- Chacón, S.M.I.; Pickersgill, B.; Debouck, D.G. Domestication patterns in common bean (Phaseolus vulgaris L.) and the origin of the Mesoamerican and Andean cultivated races. Theor. Appl. Genet. 2005, 110, 432–444. [Google Scholar] [CrossRef]
- Polignano, G.B.; Uggenti, P.; Olita, G.; Bisignano, V.; Alba, V.; Perrino, P. Characterization of grass pea (Lathyrus sativus L.) entries by means of agronomically useful traits. Lathyrus Lathyrism Newsl. 2005, 4, 10–14. [Google Scholar]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef]
- Croft, A.M.; Pang, E.C.K.; Taylor, P.W.J. Molecular analysis of Lathyrus sativus L.(grasspea) and related Lathyrus species. Euphytica 1999, 107, 167–176. [Google Scholar] [CrossRef]
- Grela, E.R.; Rybiński, W.; Matras, J.; Sobolewska, S. Variability of phenotypic and morphological characteristics of some Lathyrus sativus L. and Lathyrus cicera L. accessions and nutritional traits of their seeds. Genet. Res. Crop Evol. 2012, 59, 1687–1703. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochem 2006, 67, 107–121. [Google Scholar] [CrossRef]
- Enneking, D. The nutritive value of grasspea (Lathyrus sativus) and allied species, their toxicity to animals and the role of malnutrition in neurolathyrism. Food Chem. Toxicol. 2011, 49, 694–709. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Swain, D.; Mishra, P.K.; Baisakh, B.; Dash, S. Optimization of callus induction in Lathyrus sativus L. Afr. J. Food Sci. Technol. 2014, 5, 60–66. [Google Scholar]
- Barik, D.P.; Mohapatra, U.; Chand, P.K. High frequency in vitro regeneration of Lathyrus sativus L. Biol. Plant. 2005, 49, 637–639. [Google Scholar] [CrossRef]
- Barpete, S.; Khawar, K.M.; Özcan, S. Differential competence for in vitro adventitous rooting of grass pea (Lathyrus sativus L.). Plant Cell Tiss. Organ Cult. 2014, 119, 39–50. [Google Scholar] [CrossRef]
- Roy, P.K.; Barat, G.K.; Mehta, S.L. In vitro plant regeneration from callus derived from root explants of Lathyrus sativus. Plant Cell Tiss. Organ Cult. 1992, 29, 135–138. [Google Scholar] [CrossRef]
- Ochatt, S.; Durieu, P.; Jacas, L.; Pontécaille, C. Protoplast, cell and tissue cultures for the biotechnological breeding of grass pea (Lathyrus sativus L.). Lathyrus Lathyrism Newsl. 2001, 2, 35–38. [Google Scholar]
- Lichter, R. Induction of haploid plants from isolated pollen of Brassica napus. Z. Pflanzenphysiol. 1982, 105, 427–434. [Google Scholar] [CrossRef]
- Ochatt, S.J.; Mousset-Déclas, C.; Rancillac, M. Fertile pea plants regenerate from protoplasts when calluses have not undergone endoreduplication. Plant Sci. 2000, 156, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, D.R.; Shukla, R.S. Evolutionary trend in Lens culinaris and allied species: A cytological evidence. Cytologia 2000, 65, 305–312. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Daun, J.K. Effects of variety and crude protein content on nutrients and anti-nutrients in lentils (Lens culinaris). Food Chem. 2006, 95, 493–502. [Google Scholar] [CrossRef]
- Faris, M.E.A.I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterranean J. Nutr. Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Bermejo, C.; Gatti, I.; Cointry, E. In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. 2016, 127, 585–590. [Google Scholar] [CrossRef]
- Sarker, R.H.; Das, S.K.; Hoque, M.I. In vitro flowering and seed formation in lentil (Lens culinaris Medik.). In Vitro Cell Dev. Biol.–Plant 2012, 48, 446–452. [Google Scholar] [CrossRef]
- Sultana, T.; Majeed, N.; Khan, F.; Rehman, A.; Naqvi, S.S. Direct regeneration and efficient in vitro root development studies in lentil (Lens culinaris Medik). Pak. J. Bot. 2016, 48, 1999–2004. [Google Scholar]
- Bagheri, A.; Ghasemi Omraan, V.O.; Hatefi, S. Indirect in vitro regeneration of lentil (Lens culinaris Medik.). J. Plant Mol. Breed. 2012, 1, 43–50. [Google Scholar] [CrossRef]
- Das, S.K.; Shethi, K.J.; Hoque, M.I.; Sarker, R.H. Agrobacterium-mediated genetic transformation in lentil (Lens culinaris Medik.) followed by in vitro flowering and seed formation. Plant Tissue Cult. Biotechnol. 2012, 22, 13–26. [Google Scholar] [CrossRef]
- Saha, S.; Tullu, A.; Yuan, H.Y.; Lulsdorf, M.M.; Vandenberg, A. Improvement of embryo rescue technique using 4-chloroindole-3 acetic acid in combination with in vivo grafting to overcome barriers in lentil interspecific crosses. Plant Cell Tiss. Organ Cult. 2015, 120, 109–116. [Google Scholar] [CrossRef]
- Deswal, K. Progress and opportunities in double haploid production in lentil (Lens culinaris Medik.), soybean (Glycine max L. Merr.) and chickpea (Cicer arietinum L.). J. Pharmac. Phytochem. 2018, 7, 3105–3109. [Google Scholar]
- Gupta, D.; Dady, R.H.; Sambasivam, P.; Bar, i.; Azad, M.; Beera, N.; Ford, R.; Biju, S. Conventional and Biotechnological Approaches for Targeted Trait Improvement in Lentil. In Accelerated Plant Breeding; Gosal, S.S., Wani, S.H., Eds.; Springer: Cham, Switzerland, 2020; Volume 3. [Google Scholar] [CrossRef]
- Vijaipandurangam, K.D. Microspore culture effects on double haploid production in lentil (Lens culinaris Medik.). Int. J. Agric. Sci. Res. 2019, 8, 147–152. [Google Scholar]
- Bold, H.C.; Wynne, M.J. Introduction to the algae: Structure and reproduction. In Cultivation of Algae in the Laboratory; PrenticeHall INC: Englewood Cliffs, NJ, USA, 1978; pp. 571–578. [Google Scholar]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1964, 18, 100–127. [Google Scholar] [CrossRef]
- FAOSTAT. Access Protocol. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 April 2024).
- Van de Noort, M. Lupin: An important protein and nutrient source. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2024; pp. 219–239. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Hasenkopf, K.; Mittermaier, S.; Jahreis, G. Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. LWT-Food Sci. Technol. 2014, 59, 587–590. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Internat. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Resta, D.; Boschin, G.; D’Agostina, A.; Arnoldi, A. Quantification of quinolizidine alkaloids in lupin seeds, lupin-based ingredients and foods. In Lupins for Health and Wealth Proceedings of the 12th International Lupin Conference, 14–18 September 2008, Fremantle, Australia; Palta, J.A., Berger, J.B., Eds.; International Lupin Assocation: Canterbury, New Zealand, 2008; ISBN 0-86476-153-8. [Google Scholar]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Pasqualone, A. Lupine (Lupinus spp.) proteins: Characteristics, safety and food applications. Eur. Food Res. Technol. 2022, 248, 345–356. [Google Scholar] [CrossRef]
- Vishnyakova, M.A.; Kushnareva, A.V.; Shelenga, T.V.; Egorova, G.P. Alkaloids of narrow-leaved lupine as a factor determining alternative ways of the crop’s utilization and breeding. Vavilov J. Genet. Breed. 2020, 24, 625. [Google Scholar] [CrossRef]
- Osorio, C.E.; Till, B.J. A bitter-sweet story: Unraveling the genes involved in quinolizidine alkaloid synthesis in Lupinus albus. Front. Plant Sci. 2022, 12, 795091. [Google Scholar] [CrossRef]
- Atkins, C.A.; Smith, P.H.C.; Gupta, S.; Jones, M.C.K.; Caligary, P.D.S. Genetics, cytology and biotechnology. In Lupins as Crop Plants: Biology, Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Oxon, NY, USA, 1998; pp. 67–92. [Google Scholar]
- Rybczyński, J. An assessment of the present status of in vitro culture of lupins. In Proceedings of the 8th International Lupin Conference Asilomar, Asilomar, CA, USA, 11–16 May 1996; pp. 222–235. [Google Scholar]
- Święcicki, W.; Rybczyński, J.J.; Święcicki, W.K. Domestication and genetics of the yellow lupin (Lupinus luteus L.) and the biotechnological improvement of lupins. J. Appl. Genet. 2000, 41, 11–34. [Google Scholar]
- Kazmierski, T.; Kazmierska, E.M. Meiosis in spontaneous haploids of white lupin (Lupinus albus L.). Genet. Polon. 1989, 30, 47–60. [Google Scholar]
- Palada, M.; Sator, C. Observatii asupra incipients ale evolutiei androgenetice a polenului in vitro. Probl. Genet. Theor. App. XM/2 1981, 115–119. [Google Scholar]
- Sator, C.; Mix, G.; Menge, U. Investigations on anther culture of Lupinus polyphyllus. Plant Res. Dev. 1983, 18, 37–46. [Google Scholar]
- Sator, C. Regeneration of lupin plants from anthers. Landbauforsch. Volk. 1985, 35, 5–7. [Google Scholar]
- Campos-Andrada, M.P.; Mota, M. Callus differentiation from anther culture of Lupinus hartwegii Lindl. In Proceedings of the Advances in Lupin Research, Proceedings 7th International Lupin Conference, Evora, Portugal, 18–23 April 1994; Technical University of Lisbon: Lisbon, Portugal, 1994; Volume 262. [Google Scholar]
- Skrzypek, E.; Czyczyło-Mysza, I.; Marcińska, I.; Wędzony, M. Prospects of androgenetic induction in Lupinus spp. Plant Cell Tiss. Organ Cult. 2008, 94, 131–137. [Google Scholar] [CrossRef]
- Kozak, K.; Galek, R.; Waheed, M.T.; Sawicka-Sienkiewicz, E. Anther culture of Lupinus angustifolius: Callus formation and the development of multicellular and embryo-like structures. Plant Growth Regul. 2012, 66, 145–153. [Google Scholar] [CrossRef]
- Ormerod, A.J.; Caligari, P.D.S. Anther and microspore culture of Lupinus albus in liquid culture medium. Plant Cell Tiss. Organ Cult. 1994, 36, 227–236. [Google Scholar] [CrossRef]
- Bayliss, K.L.; Wroth, J.M.; Cowling, W.A. Pro-embryos of Lupinus spp. produced from isolated microspore culture. Aust. J. Agric. Res. 2004, 55, 589–593. [Google Scholar] [CrossRef]
- Simioniuc, D.; Burlacu-Arsene, M.C.; Morariu, A.; Lipșa, F.D. Induction of the embryogenesis process in anther and microspores cultures at the Lupinus albus species. Lucrări Științifice, Universitatea de Stiinte Agricole Și Medicină Veterinară “Ion Ionescu de la Brad” Iași, Seria. Agronomie 2010, 53, 60–63. [Google Scholar]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Nutritional characteristics and value-added products of Chickpea (Cicer arietinum)—A review. J. Postharvest Technol. 2021, 9, 1–13. [Google Scholar]
- Khan, S.K.; Ghosh, P.D. In vitro induction of androgenesis and organogenesis in Cicer arietinum L. Curr. Sci. 1983, 52, 891–893. [Google Scholar]
- Altaf, N.; Ahmad, M.S. Plant regeneration and propagation of chickpea (Cicer arietinum L.) through tissue-culture techniques. In Nuclear Techniques and In Vitro Culture for Plant Improvement, Food and Agriculture Organisation and International Atomic Energy Agency; International Atomic Energy Agency: Vienna, Austria, 1986; pp. 407–417. [Google Scholar]
- Bajaj, Y.P.S.; Gosal, S.S. Pollen embryogenesis and chromosomal variation in cultured anthers of chickpea. Int. Chickpea Newsl. 1987, 17, 12–13. [Google Scholar]
- Huda, S.; Islam, R.; Bari, M.A.; Asaduzzaman, M. Anther culture of chickpea. Int. Chickpea Pigeonpea Newsl. 2001, 8, 24–26. [Google Scholar]
- Vessal, S.R.; Bagheri, A.; Safarnejad, A. The possibility of in vitro haploid production in chickpea (Cicer arietinum L.). J. Sci. Technol. Agric. Nat. Resour. 2002, 6, 67–76. [Google Scholar]
- Grewal, R.K.; Lulsdorf, M.; Croser, J.; Ochatt, S.; Vandenberg, A.; Warkentin, T.D. Doubled-haploid production in chickpea (Cicer arietinum L.): Role of stress treatments. Plant Cell Rep. 2009, 28, 1289–1299. [Google Scholar] [CrossRef]
- Panchangam, S.S.; Mallikarjuna, N.; Gaur, P.M.; Suravajhala, P. Androgenesis in chickpea: Anther culture and expressed sequence tags derived annotation. Ind. J. Exp. Biol. 2014, 52, 181–188. [Google Scholar]
- Abdollahi, M.R.; Rashidi, S. Production and conversion of haploid embryos in chickpea (Cicer arietinum L.) anther cultures using high 2, 4-D and silver nitrate containing media. Plant Cell Tiss. Organ Cult. 2018, 133, 39–49. [Google Scholar] [CrossRef]
- Croser, J. Haploid and Zygotic Embryo Culture of Chickpea (Cicer arietinum L.). Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2002. [Google Scholar]
- Croser, J.S.; Lulsdorf, M.M.; Grewal, R.K.; Usher, K.M.; Siddique, K.H. Isolated microspore culture of chickpea (Cicer arietinum L.): Induction of androgenesis and cytological analysis of early haploid divisions. In Vitro Cell. Dev. Biol.–Plant 2011, 47, 357–368. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Jadhav, D.R.; Clarke, H.; Coyne, C.; Muehlbauer, F.J. Induction of Androgenesis as a Consequence of Wide crossing in chickpea. J. SAT Agric. Res. 2005, 1, 1–3. [Google Scholar]
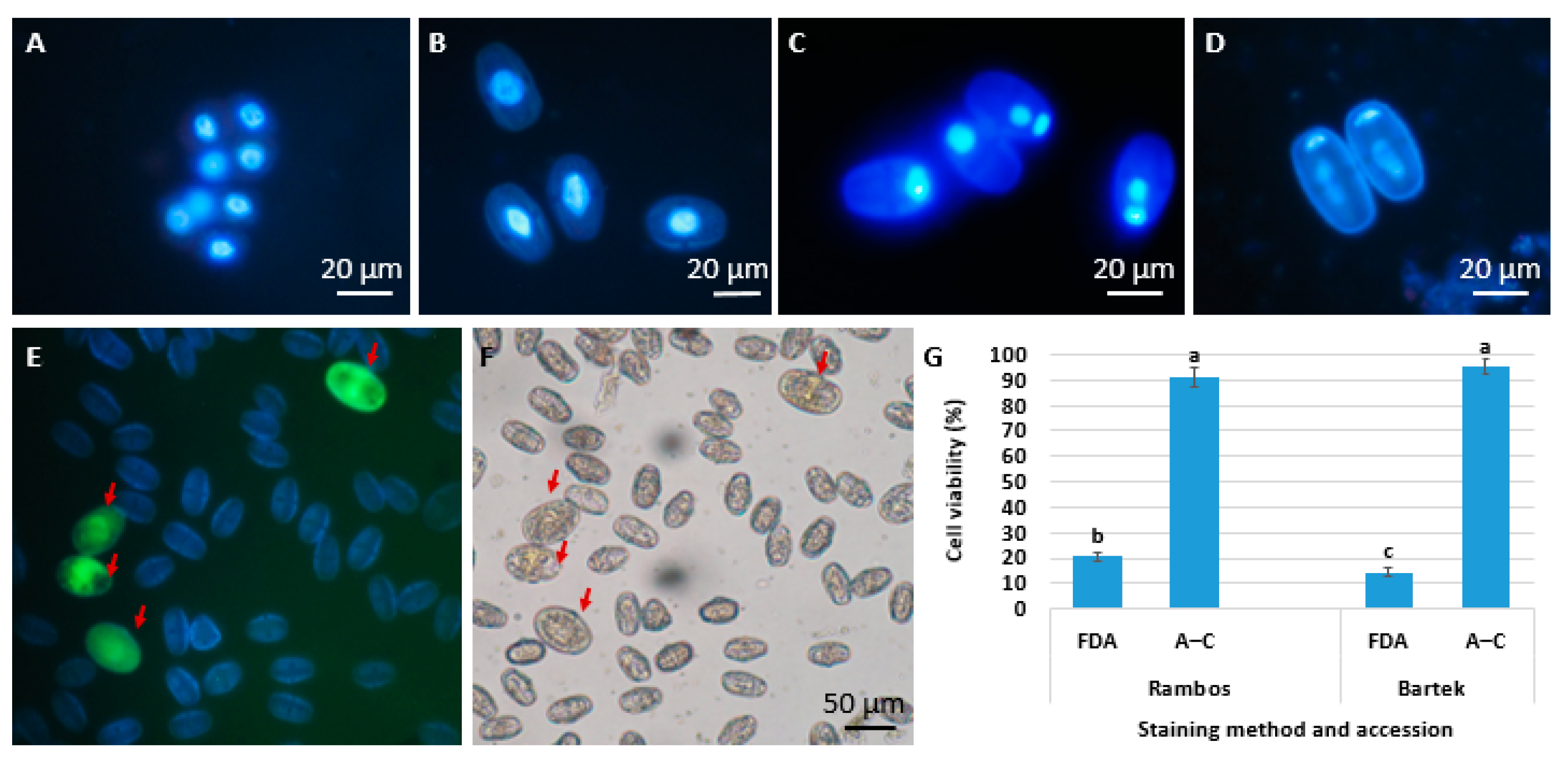
- Skrzypkowski, W.; Galek, R.; Adamus, A.; Kiełkowska, A. Pollen Development and Stainability in Vicia faba L. and Lupinus angustifolius L. Agriculture 2023, 13, 2065. [Google Scholar] [CrossRef]
- Clarke, G.C.S.; Kupicha, F.K. The relationships of the genus Cicer, L. (Leguminosae): The evidence from pollen morphology. Bot. J. Linn. Soc. 1976, 72, 35–44. [Google Scholar] [CrossRef]
- Jiang, Y.; Lahlali, R.; Karunakaran, C.; Kumar, S.; Davis, A.R.; Bueckert, R.A. Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ. 2015, 38, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Lichter, R. Anther culture of Brassica napus in a liquid culture medium. Z. Pflanzenphysiol. 1981, 103, 229–237. [Google Scholar] [CrossRef]
- Asif, M.; Eudes, F.; Randhawa, H.; Amundsen, E.; Spaner, D. Induction medium osmolality improves microspore embryogenesis in wheat and triticale. Vitr. Cell. Dev. Biol.–Plant 2014, 50, 121–126. [Google Scholar] [CrossRef]
- Mestinšek, M.Š.; Kunej, U.; Vogrinčič, V.; Jakše, J.; Murovec, J. The effect of phytosulfokine alpha on haploid embryogenesis and gene expression of Brassica napus microspore cultures. Front. Plant Sci. 2024, 15, 1336519. [Google Scholar] [CrossRef] [PubMed]
- Ochatt, S.; Conreux, C.; Moussa Mcolo, R.; Despierre, G.; Magnin-Robert, J.B.; Raffiot, B. Phytosulfokine-alpha, an enhancer of in vitro regeneration competence in recalcitrant legumes. Plant Cell 2018, 135, 189–201. [Google Scholar] [CrossRef]
- Solís, M.T.; El-Tantawy, A.A.; Cano, V.; Risueño, M.C.; Testillano, P.S. 5-azacytidine promotes microspore embryogenesis initiation by decreasing global DNA methylation, but prevents subsequent embryo development in rapeseed and barley. Front. Plant Sci. 2015, 6, 472. [Google Scholar] [CrossRef]
- Nowicka, A.; Juzoń, K.; Krzewska, M.; Dziurka, M.; Dubas, E.; Kopeć, P.; Zieliński, K.; Żur, I. Chemically-induced DNA de-methylation alters the effectiveness of microspore embryogenesis in triticale. Plant Sci. 2019, 287, 110189. [Google Scholar] [CrossRef] [PubMed]
- Ochatt, S.J.; Sangwan, R.S.; Marget, P.; Ndong, Y.A.; Rancillac, M.; Perney, P.; Röbbelen, G. New approaches towards the shortening of generation cycles for faster breeding of protein legumes. Plant Breed. 2002, 121, 436–440. [Google Scholar] [CrossRef]
- Gurusamy, V.; Bett, K.E.; Vandenberg, A. Grafting as a tool in common bean breeding. Can. J. Plant Sci. 2010, 90, 299–304. [Google Scholar] [CrossRef]
- Haddon, L.; Northcote, D.H. The effect of growth conditions and origin of tissue on the ploidy and morphogenic potential of tissue cultures of bean (Phaseolus vulgaris L.). J. Exp. Bot. 1976, 27, 1031–1051. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Oliveira, J.M.S.; Mariath, J.E.; Bodanese-Zanettini, M.A. Histology of embryogenic responses in soybean anther culture. Plant Cell Tiss. Organ Cult. 2005, 80, 129–137. [Google Scholar] [CrossRef]
- Mousa, M.A.A.; Abo-Elyousr, K.A.M.; Ibrahim, O.H.M. Evaluation of Genetic Variability within a Collection of Cumin Genotypes Using RAPD, ISSR, SRAP and SCoT Markers and Variability of In Vitro Callus Induced Therefrom. Horticulturae 2023, 9, 742. [Google Scholar] [CrossRef]
- Ravi, M.; Chan, S.W. Haploid plants produced by centromere-mediated genome elimination. Nature 2010, 464, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Starr, D.; Wang, W.; McCuiston, J.; Zhong, H.; Nuccio, M.L.; Martin, B. Maternal haploids are preferentially induced by CENH3-tailswap transgenic complementation in maize. Front. Plant Sci. 2016, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Navratilova, A.; Schroeder-Reiter, E.; Koblížková, A.; Steinbauerova, V.; Chocholova, E.; Macas, J. Stretching the rules: Monocentric chromosomes with multiple centromere domains. PLoS Genet. 2012, 8, e1002777. [Google Scholar] [CrossRef]
- Neumann, P.; Pavlíková, Z.; Koblížková, A.; Fuková, I.; Jedličková, V.; Novák, P.; Macas, J. Centromeres off the hook: Massive changes in centromere size and structure following duplication of CenH3 gene in Fabaceae species. Mol. Biol. Evol. 2015, 32, 1862–1879. [Google Scholar] [CrossRef]
- Ávila Robledillo, L.; Koblížková, A.; Novák, P.; Böttinger, K.; Vrbová, I.; Neumann, P.; Macas, J. Satellite DNA in Vicia faba is characterized by remarkable diversity in its sequence composition, association with centromeres, and replication timing. Sci. Rep. 2018, 8, 5838. [Google Scholar] [CrossRef]
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| Androgenesis/Anther culture | Anthers, tetrads, uninucleate microspore, and binucleate pollen | Anther cultures kept at 28 °C and exposed to light (16 h photoperiod and 8 h darkness) | Callus | Multinuclear structures obtained from uninucleate and binucleate microspores—Kinetin had the highest influence on microspores | [119] |
| Anthers Stage not specified | Unknown | Callus | Haploid cells in anther-derived callus | [120] | |
| Anthers isolated from different size flower buds | Unknown | Callus | Haploid and diploid anther-derived callus | [121] | |
| Anther microspores in different developmental stages | No stresses applied, dark | Callus Embryo-like structures (heart) | Callus was obtained from the anthers containing microspores in the uninucleate stage Embryo-like structures callused in time No ploidy was analyzed | [122] |
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| Androgenesis/Isolated microspore culture | Uninucleate microspores | Flower buds kept at 4 °C or 10 °C for 2, 4, 7, 14, 21 days, or 1 month; osmotic stress provided by using mannitol or sucrose in various concentrations; Electric shock | Microspore-derived micro-calli Regenerated plants | Plants obtained after cold stress stress (2 pcs from embryogenic callus and 1 pc from organogenic callus) | [106] |
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| Androgenesis/Anther culture | Anthers Uninucleate microspore | Heat and cold stresses | Callus Proembryos | Anther derived calli with a few pro-embryos were obtained but no plants regenerated Applied stresses were not effective | [147] |
| Androgenesis/Isolated microspore culture | Not specified | Flower buds kept at 4 °C for 96 h | Cell divisions 8-cell structures | No embryos were regenerated | [15] |
| Uninucleate microspores | Flower buds kept at 4 °C for 72 h | Callus | LS media with various concentrations of 2,4-D and BAP were used but hormonal treatment affected no significant differences | [148] |
| Method/ Technique | Material/Stage of Development | Conditions and Treatments | Development | Remarks | References |
|---|---|---|---|---|---|
| Androgenesis/Anther culture | Anthers Uninucleate and binucleate microspores | Anthers kept in the dark for 3 days at 25 ± °C, then incubated for 10 h in light | Callus Embryoids | Various ploidy levels in callus: haploid (28%), diploid (38%), and aneuploid (38%) Rhizogenesis from callus. | [176] |
| Greenish-white anthers containing tetrads and cells at the first mitosis | Flower buds kept at 4 °C for 3 to 7 days. | Callus No shoot regeneration | Callus produced on media containing BAP + 2,4-D + NAA in different combinations. Unknown ploidy level. | [177] | |
| Anthers, uninucleate microspores | Floral buds kept at 4 °C 72 h | Callus Embryoids Rhizogenesis from callus | Callus with different chromosome numbers. | [178] | |
| Anthers, uninucleate microspores | A total of 72 h cold pretreatment. | Callus Embryoids Rhizogenesis from callus | Embryoids did not develop further. Callus had no haploid cells. | [98] | |
| Anthers, microspores at mid to late uninucleate stage | Cold pretreatment: flower buds kept at 4–5 °C for 3 to 7 days. | Shoots regenerated from anther-derived callus, callus-derived globular embryos | Unstated ploidy level of regenerated shoots. | [179] | |
| Not specified | Not applied | Callus Plant regeneration | Regenerated plants were diploid. | [184,185] | |
| Anthers, uninucleate microspores | Cold pretreatment: buds kept in cold for 7 to 10 days | Callus Organogenesis and embryogenesis | Callus obtained on MS media supplemented with 2,4-D and kinetin. Mature embryos obtained on modified Blayd’s media containing kinetin and 10% sucrose. | [180] | |
| Anthers, uninucleate microspores | Cold pretreatment: buds kept at 4 °C for 3 to 4 days, electric shock, centrifugation, and high osmotic pressure | Embryos Callus Plant regeneration | The highest number of embryos per anther was obtained when stresses were applied combined (cold pretreatment + centrifugation + electroporation). | [181] | |
| Anthers, mid–late uninucleate microspores | Cold pretreatment: buds kept at 4 °C for 2 to 4 days; stress induced by centrifugation and osmotic stress | Multicellular structures | The combination of stress factors resulted in a positive effect on the frequency of divisions. | [182] | |
| Anthers, uninucleate microspores | Cold and heat pretreatment in different combinations: cultured anthers stored at 4 °C for 4 and 7 days and then transferred to 30 °C for 10 days, 32° for 2 days, or 35 °C for 8 h | Callus Embryos Haploid and diploid plants regenerated from embryos | The highest percentage of regenerated plants from embryos was observed for anthers treated with a combination of cold and heat stress. | [183] | |
| Androgenesis/Isolated microspores culture | Not specified | - | Callus | No plants regenerated | [184] |
| Microspores at the uninucleate stage | Heat stress: flower buds kept at 32 °C for 16 h | Proembryo structures | No plants regenerated | [15] | |
| Microspores at the uninucleate stage | Cold or heat pretreatment: flower buds kept at 4 °C for 24 h or at 32 °C for 12 h | Multicellular structures Embryos (globular) | No plants regenerated | [185] | |
| In vivo pollination | Male-sterile mutant used as a female parent and fertile variety as a male parent | - | Haploid plants obtained from seeds | The haploid plants constituted 34% of the plants obtained from seeds | [16] |
| Wide crossing | Cicer aretinum and Cicer pinnatifidum | Multicellular microspores produced as a result of wide crossing | - | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypkowski, W.; Kiełkowska, A. Current Status of Haploidization in Cool-Season Grain Legume Crop Species. Agriculture 2024, 14, 1031. https://doi.org/10.3390/agriculture14071031
Skrzypkowski W, Kiełkowska A. Current Status of Haploidization in Cool-Season Grain Legume Crop Species. Agriculture. 2024; 14(7):1031. https://doi.org/10.3390/agriculture14071031
Chicago/Turabian StyleSkrzypkowski, Wiktor, and Agnieszka Kiełkowska. 2024. "Current Status of Haploidization in Cool-Season Grain Legume Crop Species" Agriculture 14, no. 7: 1031. https://doi.org/10.3390/agriculture14071031
APA StyleSkrzypkowski, W., & Kiełkowska, A. (2024). Current Status of Haploidization in Cool-Season Grain Legume Crop Species. Agriculture, 14(7), 1031. https://doi.org/10.3390/agriculture14071031







