Physiological, Biochemical, and Ultrastructural Changes in Naturally Aged Sweet Corn Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Germination Assay
2.3. Measurements of Physiological and Biochemical Indictors
2.4. Ultrastructure Observation
2.5. Statistical Analysis
3. Results
3.1. Comparison of Seed Viability between 0M and 8M
3.2. Evaluation of Phytohormones, Total Protein, and Starch Content
3.3. Evaluation of MDA Content, LOX, and Antioxidant Enzyme Activity
3.4. Evaluation of Amylase Activities and Ultrastructural Observations
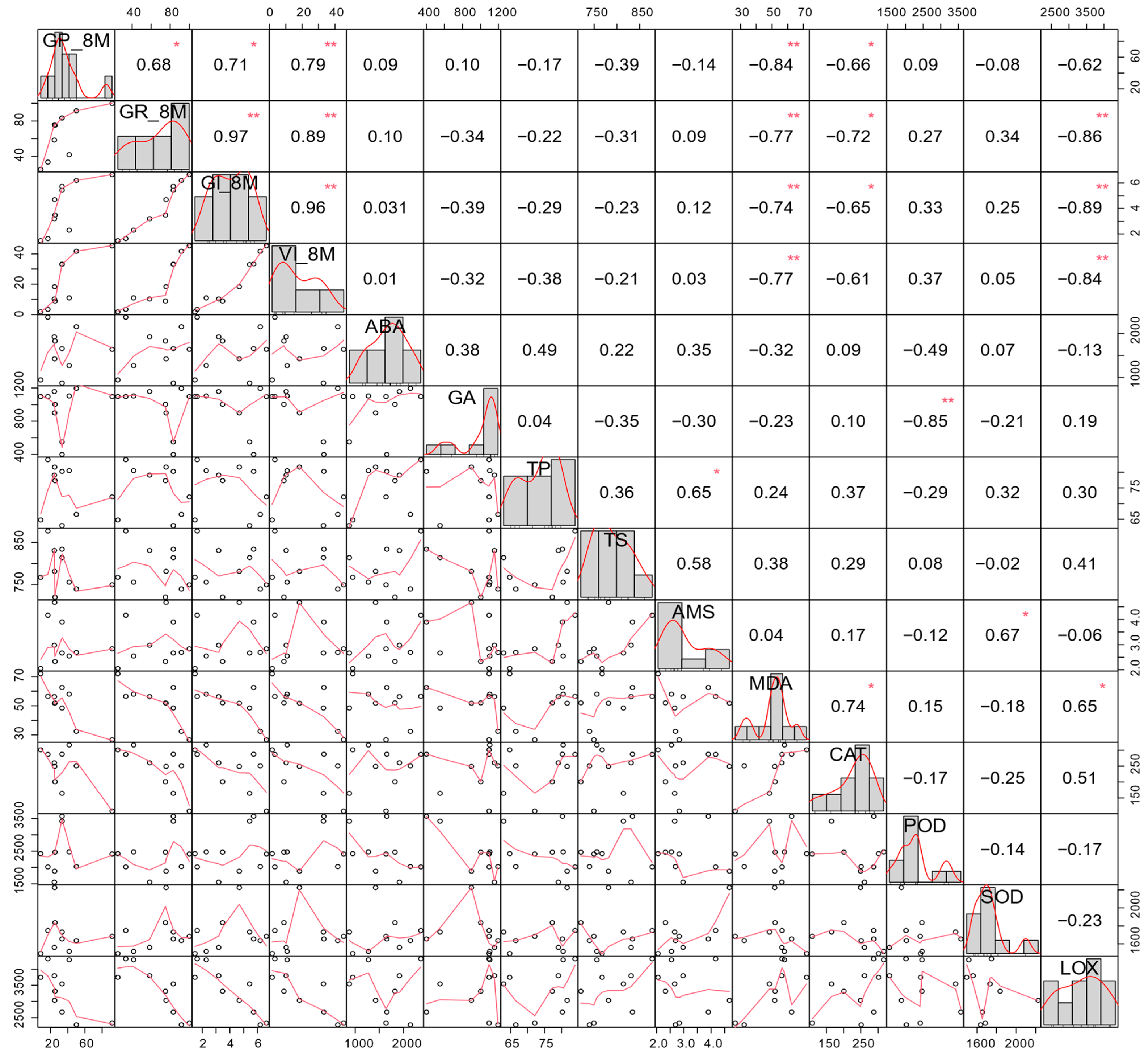
3.5. Correlation of Seed Vigor Indices and Physiological Indices in Naturally Aged Seeds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revilla, P.; Anibas, C.M.; Tracy, W.F. Sweet Corn Research around the World 2015–2020. Agronomy 2021, 11, 534. [Google Scholar] [CrossRef]
- Fan, L.; Yan, Q. Research Progress on the Reasons for Low Seed Vitality of Sweet Corn and Its Seed Treatment Techniques. Chin. Agric. Sci. Bull. 1996, 12, 24–26. [Google Scholar]
- Li, K.; Huang, C. Current Production Status, Problem and Countermeasure on Sweet Corn Industry in China. Sugar Crops China 2021, 43, 67–71. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Morscher, F.; Kranner, I.; Arc, E.; Bailly, C.; Roach, T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann. Bot. 2015, 116, 669–678. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Malecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Gerna, D.; Ballesteros, D.; Arc, E.; Stöggl, W.; Seal, C.E.; Marami-Zonouz, N.; Na, C.S.; Kranner, I.; Roach, T. Does oxygen affect ageing mechanisms of Pinus densiflora seeds? A matter of cytoplasmic physical state. J. Exp. Bot. 2022, 73, 2631–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Zhang, Y.; Sun, J.; Meng, J.S.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Xin, X.; Song, C.; Chen, X.; Zhang, J.; Wu, S.; Li, R.; Liu, X.; Lu, X. Activity levels and expression of antioxidant enzymes in the ascorbate-glutathione cycle in artificially aged rice seed. Plant Physiol. Biochem. 2014, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Kokkinidi, D.; Kevrekidou, A.; Adamakis, I.S. The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity. Int. J. Mol. Sci. 2022, 23, 1348. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Malecka, A.; Ciereszko, I.; Staszak, A.M. Mitochondria Are Important Determinants of the Aging of Seeds. Int. J. Mol. Sci. 2019, 20, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Min, C.W.; Lee, S.H.; Cheon, Y.E.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Gupta, R.; et al. In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J. Proteom. 2017, 169, 125–135. [Google Scholar] [CrossRef]
- Sugliani, M.; Rajjou, L.; Clerkx, E.J.; Koornneef, M.; Soppe, W.J. Natural modifiers of seed longevity in the Arabidopsis mutants abscisic acid insensitive3-5 (abi3-5) and leafy cotyledon1-3 (lec1-3). New Phytol. 2009, 184, 898–908. [Google Scholar] [CrossRef]
- Clerkx, E.J.M.; Blankestijn-De Vries, H.; Ruys, G.J.; Groot, S.P.C.; Koornneef, M. Genetic differences in seed longevity of various Arabidopsis mutants. Physiol. Plant. 2004, 121, 448–461. [Google Scholar] [CrossRef]
- Zheng, Q.; Teng, Z.; Zhang, J.; Ye, N. ABA Inhibits Rice Seed Aging by Reducing H2O2 Accumulation in the Radicle of Seeds. Plants 2024, 13, 809. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Neveu, M.; Lalanne, D.; Ly Vu, B.; Kanno, Y.; Seo, M.; Leprince, O.; Buitink, J. A role for auxin signaling in the acquisition of longevity during seed maturation. New Phytol. 2020, 225, 284–296. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, K.; Wang, Y.; Tian, L.; Zhang, C.; Sun, W.; He, H.; Yu, S. OsGRETCHENHAGEN3-2 modulates rice seed storability via accumulation of abscisic acid and protective substances. Plant Physiol. 2021, 186, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Bueso, E.; Munoz-Bertomeu, J.; Campos, F.; Brunaud, V.; Martinez, L.; Sayas, E.; Ballester, P.; Yenush, L.; Serrano, R. ARABIDOPSIS THALIANA HOMEOBOX25 uncovers a role for Gibberellins in seed longevity. Plant Physiol. 2014, 164, 999–1010. [Google Scholar] [CrossRef]
- Bizouerne, E.; Buitink, J.; Vu, B.L.; Vu, J.L.; Esteban, E.; Pasha, A.; Provart, N.; Verdier, J.; Leprince, O. Gene co-expression analysis of tomato seed maturation reveals tissue-specific regulatory networks and hubs associated with the acquisition of desiccation tolerance and seed vigour. BMC Plant Biol. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Livne, S.; Lor, V.S.; Nir, I.; Eliaz, N.; Aharoni, A.; Olszewski, N.E.; Eshed, Y.; Weiss, D. Uncovering DELLA-Independent Gibberellin Responses by Characterizing New Tomato procera Mutants. Plant Cell 2015, 27, 1579–1594. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.J.; Hu, J.; Wang, Z.F.; Zhu, S.J.; Wang, J.C.; Knapp, A. Time series regression analysis between changes in kernel size and seed vigor during developmental stage of sh2 sweet corn (Zea mays L.) seeds. Sci. Hortic. 2013, 154, 25–30. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, L.; Zhu, S.; Liu, J.; Yang, R.; Wang, Q.; Li, X.; Feng, F. Changes of vigor, physiological characteristics and genetic diversities of artificially aged sweet corn seeds. J. South China Agric. Univ. 2018, 39, 25–30. [Google Scholar] [CrossRef]
- Wang, B.; Yang, R.; Ji, Z.; Zhang, H.; Zheng, W.; Zhang, H.; Feng, F. Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy 2022, 12, 1028. [Google Scholar] [CrossRef]
- Yan, L.; Yi, Q.; Xiao-Min, Z.; Yi-Fang, C.; Li, W.; Yu-Ping, L. Optimization of water-rich starch sample preparation methods for scanning electron microscopy. Plant Sci. J. 2018, 36, 119–126. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, D.J.; Krupka, M.; Kamiński, J.; Wierzbicka, M.; Floryańska, S.; Kopeć, W.; Piotrowicz-Cieślak, A.I. Physiological and Biochemical Parameters of Field Bean (Vicia faba var. minor) Seeds Stored for 33 Years. Agriculture 2023, 13, 2012. [Google Scholar] [CrossRef]
- Fu, Y.-B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, S.M.; Tavakkol Afshari, R.; Daneshmandi, M.S. The relationships between fatty acids and heterotrophic seedling growth in winter canola cultivars during accelerated seed aging process. S. Afr. J. Bot. 2018, 119, 353–361. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, D.; Yin, Y.; Chen, K.; He, J.; Yan, S.; Li, H.; Xiong, Y.; Zhou, W.; Li, M. Genetic dissection of Brassica napus seed vigor after aging. Theor. Appl. Genet. 2024, 137, 141. [Google Scholar] [CrossRef] [PubMed]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and Non-Enzymatic Mechanisms Contribute to Lipid Oxidation During Seed Aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef]
- Prasad, C.T.M.; Kodde, J.; Angenent, G.C.; de Vos, R.C.H.; Diez-Simon, C.; Mumm, R.; Hay, F.R.; Siricharoen, S.; Yadava, D.K.; Groot, S.P.C. Experimental rice seed aging under elevated oxygen pressure: Methodology and mechanism. Front. Plant Sci. 2022, 13, 1050411. [Google Scholar] [CrossRef]
- Du, H.; Huang, M.; Hu, J.; Li, J. Modification of the fatty acid composition in Arabidopsis and maize seeds using a stearoyl-acyl carrier protein desaturase-1 (ZmSAD1) gene. BMC Plant Biol. 2016, 16, 137. [Google Scholar] [CrossRef]
- Huang, X.Y.; Liu, P.Q.; Peng, Y.Z.; Xiong, X.F.; Cai, J.G.; Lu, G.Y. Seed Vigor Comparisons of the High Oil Corn Hybrids and the Common Corn Hybrids Under Different Accelerated Aging Conditions. J. Maize Sci. 2003, 11, 36–38. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Zhang, L.; Shi, Y.; Song, Y.; Wang, X.; Cai, Q.; He, W.; Xie, H.; Zhang, J. The lipoxygenase OsLOX10 affects seed longevity and resistance to saline-alkaline stress during rice seedlings. Plant Mol. Biol. 2023, 111, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Glass formation in plant anhydrobiotes: Survival in the dry state. Cryobiology 2004, 48, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Intracellular glasses and seed survival in the dry state. C. R. Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, X.Q.; Jia, S.G.; Li, M.L.; Mao, P.S. Transcriptomic analysis reveals the changes of energy production and AsA-GSH cycle in oat embryos during seed ageing. Plant Physiol. Biochem. 2020, 153, 40–52. [Google Scholar] [CrossRef]
- Wang, B.; Yang, R.; Zhang, Z.; Huang, S.; Ji, Z.; Zheng, W.; Zhang, H.; Zhang, Y.; Feng, F. Integration of miRNA and mRNA analysis reveals the role of ribosome in to anti-artificial aging in sweetcorn. Int. J. Biol. Macromol. 2023, 240, 124434. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Baek, K.-H.; Lee, H.-S.; Kwak, S.-S.; Bang, J.-W.; Kwon, S.-Y. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Yin, G.L.; Whelan, J.; Zhang, Z.S.; Xin, X.; He, J.J.; Chen, X.L.; Zhang, J.M.; Zhou, Y.C.; Lu, X.X. Composition of Mitochondrial Complex I during the Critical Node of Seed Aging in Oryza sativa. J. Plant Physiol. 2019, 236, 7–14. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Kozaki, A.; Aoyanagi, T. Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids. Int. J. Mol. Sci. 2022, 23, 1876. [Google Scholar] [CrossRef]
- Yin, G.K.; Whelan, J.; Wu, S.H.; Zhou, J.; Chen, B.Y.; Chen, X.L.; Zhang, J.M.; He, J.J.; Xin, X.; Lu, X.X. Comprehensive Mitochondrial Metabolic Shift during the Critical Node of Seed Ageing in Rice. PLoS ONE 2016, 11, e0148013. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.C.; Takaki, M. Biochemical and physiological changes of Tabebuia roseoalba (Ridl.) Sandwith (Bignoniaceae) seeds under storage. J. Seed Sci. 2014, 36, 100–107. [Google Scholar] [CrossRef]
- Li, B.B.; Zhang, S.B.; Lv, Y.Y.; Wei, S.; Hu, Y.S. Reactive oxygen species-induced protein carbonylation promotes deterioration of physiological activity of wheat seeds. PLoS ONE 2022, 17, e0263553. [Google Scholar] [CrossRef] [PubMed]
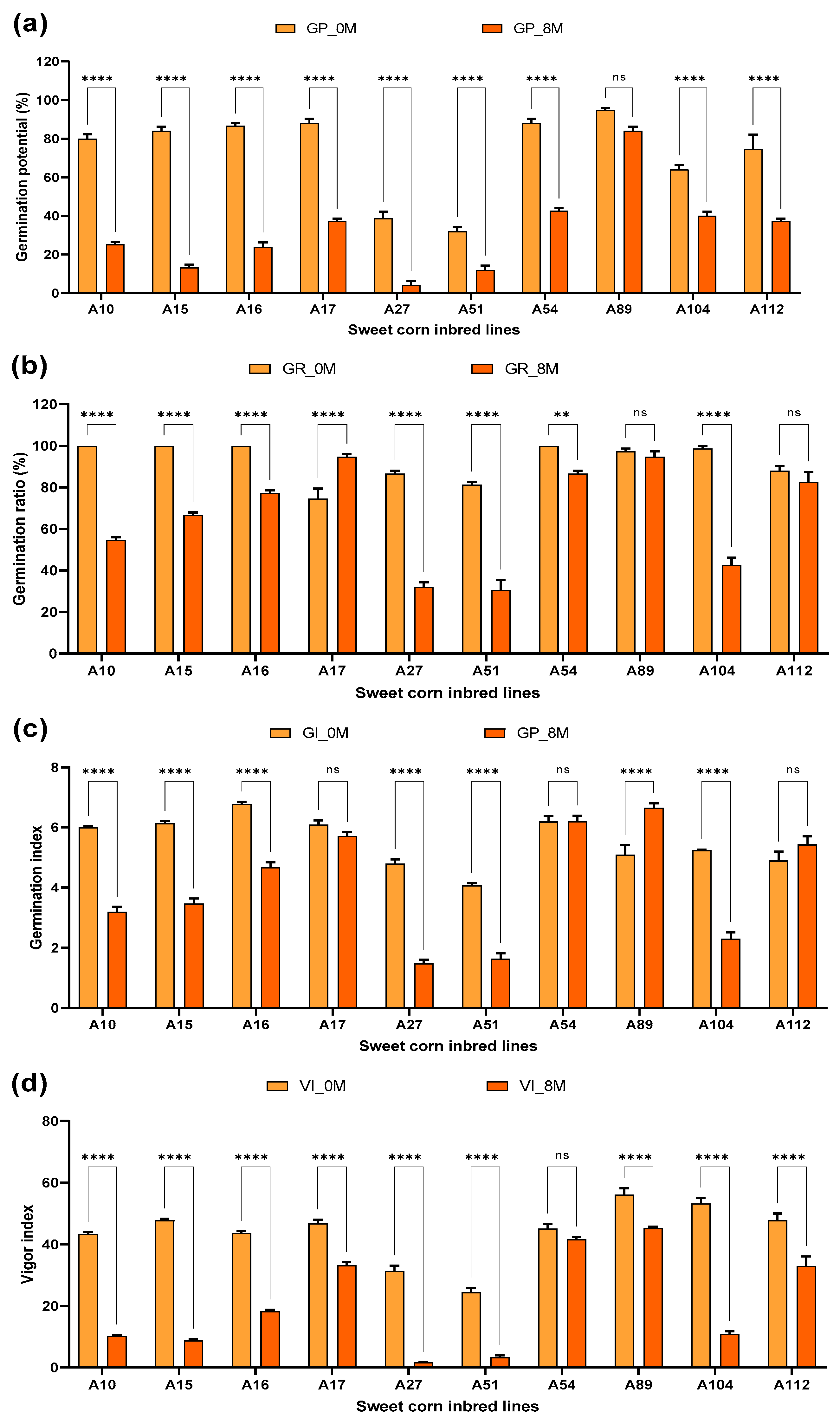
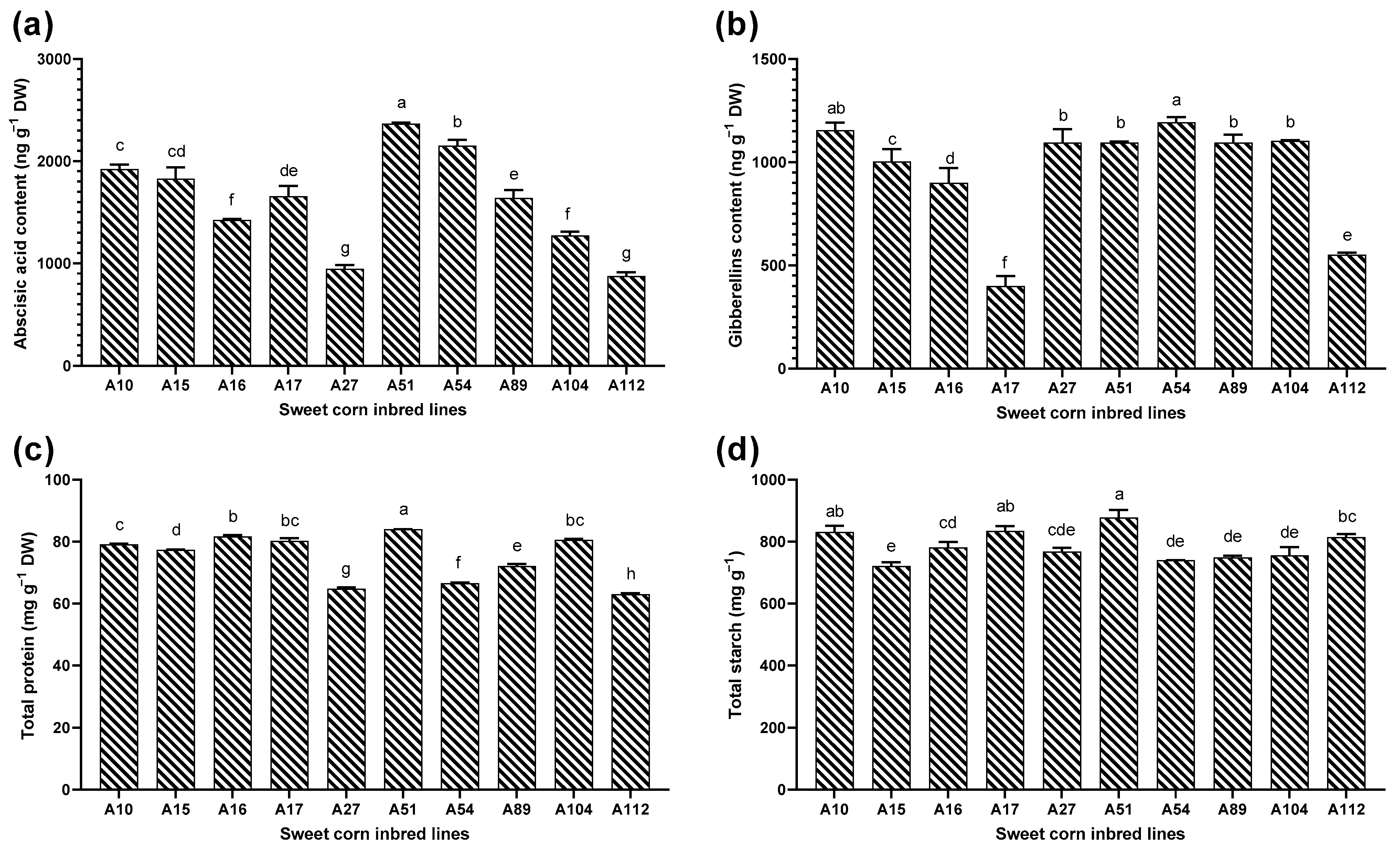

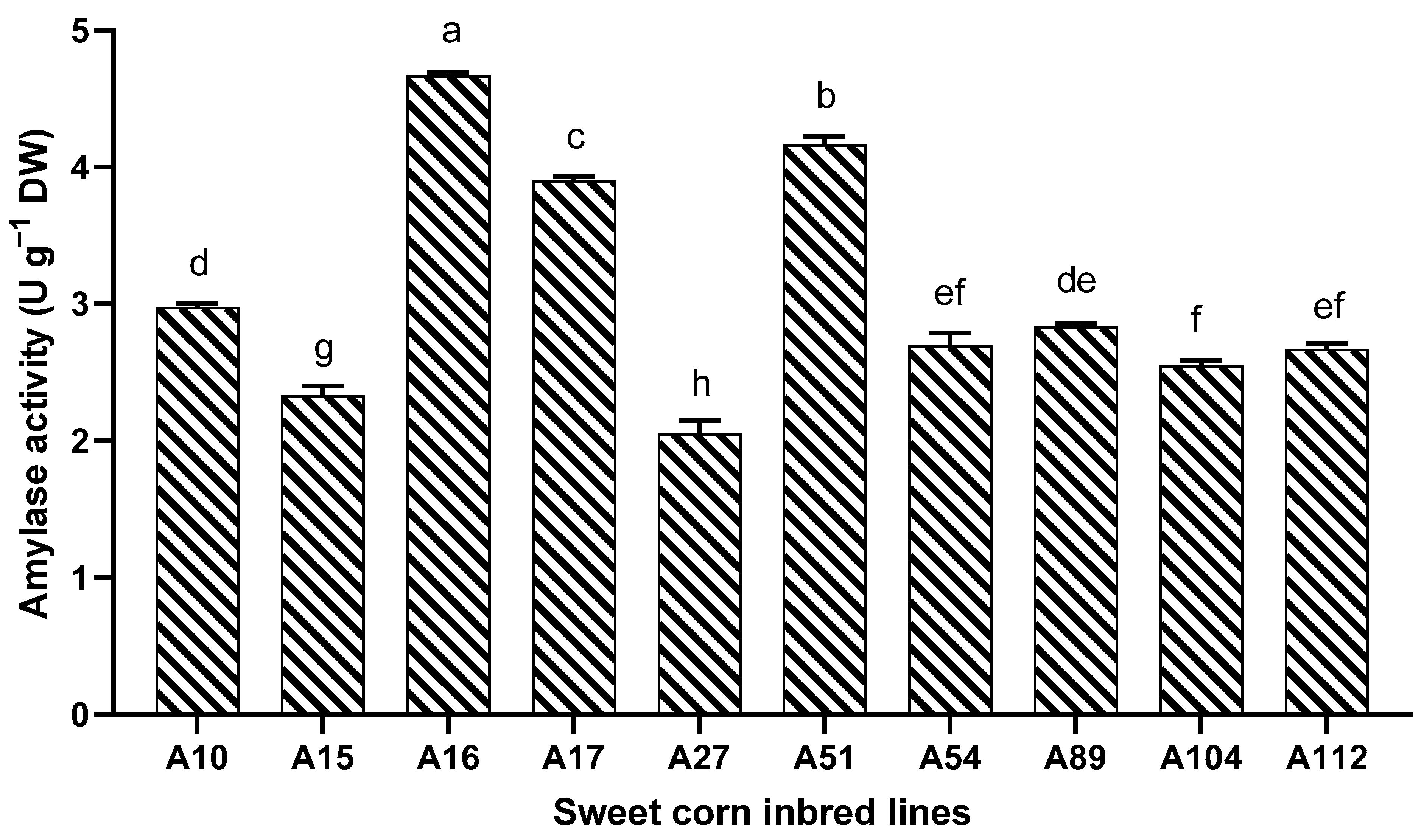
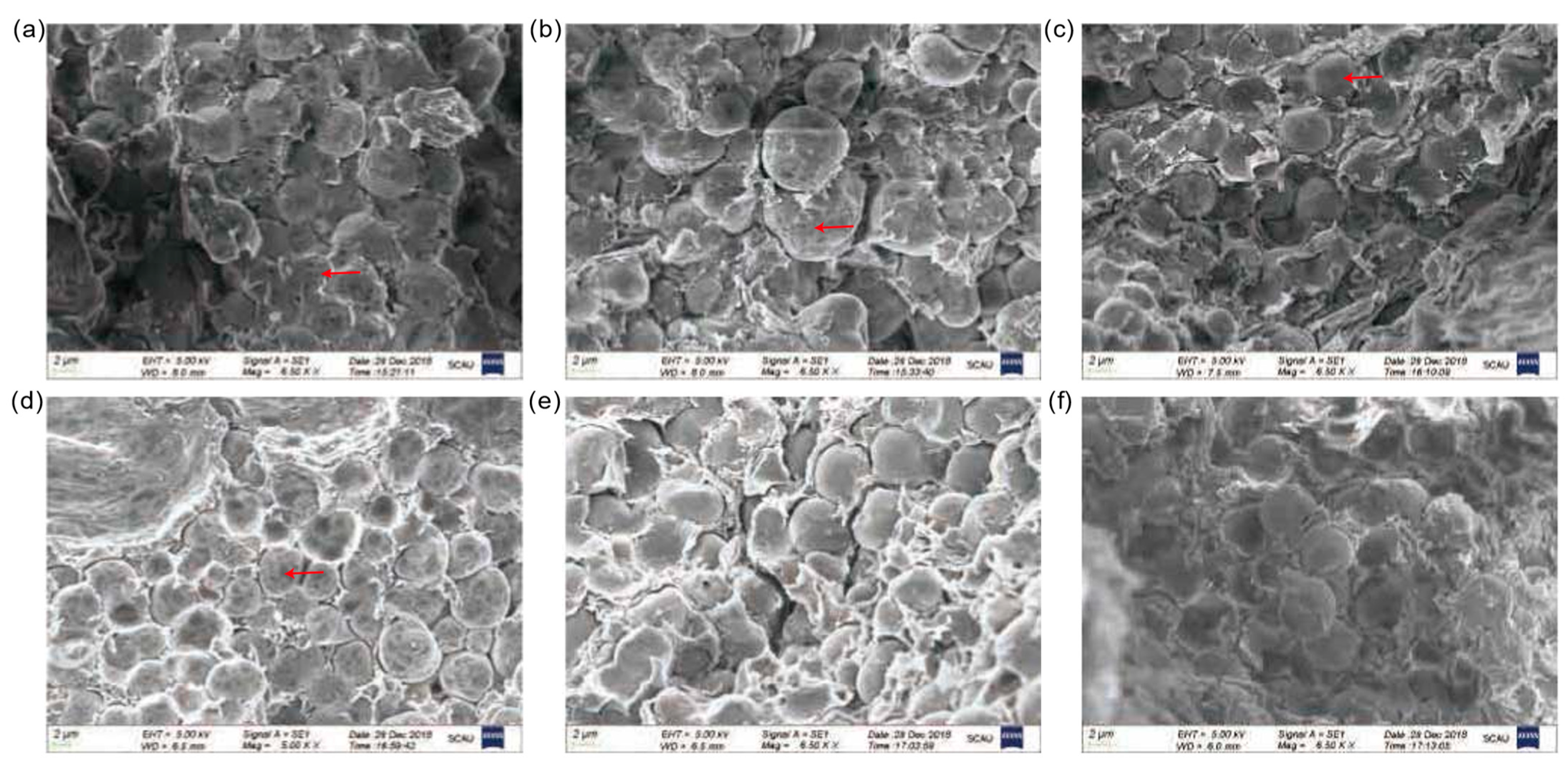
| Soruce of Variation | Germination Potential | Germination Ratio | Germination Index | Vigor Index | ||||
|---|---|---|---|---|---|---|---|---|
| MS | p Value | MS | p Value | MS | p Value | MS | p Value | |
| natural aging | 25,174.00 | <0.01 | 11,985.00 | <0.01 | 41.22 | <0.01 | 8141.00 | <0.01 |
| lines | 2524.00 | <0.01 | 1397.00 | <0.01 | 10.38 | <0.01 | 813.60 | <0.01 |
| natural aging × lines | 598.00 | <0.01 | 792.50 | <0.01 | 2.98 | <0.01 | 244.90 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, G.; Yang, R.; Lei, D.; Du, Y.; Li, Y.; Feng, F. Physiological, Biochemical, and Ultrastructural Changes in Naturally Aged Sweet Corn Seeds. Agriculture 2024, 14, 1039. https://doi.org/10.3390/agriculture14071039
Yue G, Yang R, Lei D, Du Y, Li Y, Feng F. Physiological, Biochemical, and Ultrastructural Changes in Naturally Aged Sweet Corn Seeds. Agriculture. 2024; 14(7):1039. https://doi.org/10.3390/agriculture14071039
Chicago/Turabian StyleYue, Gaohong, Ruichun Yang, Dan Lei, Yanchao Du, Yuliang Li, and Faqiang Feng. 2024. "Physiological, Biochemical, and Ultrastructural Changes in Naturally Aged Sweet Corn Seeds" Agriculture 14, no. 7: 1039. https://doi.org/10.3390/agriculture14071039






