Cyclic Electron Flow Alleviates the Stress of Light Fluctuation on Soybean Photosynthesis
Abstract
1. Introduction
2. Materials and Methods
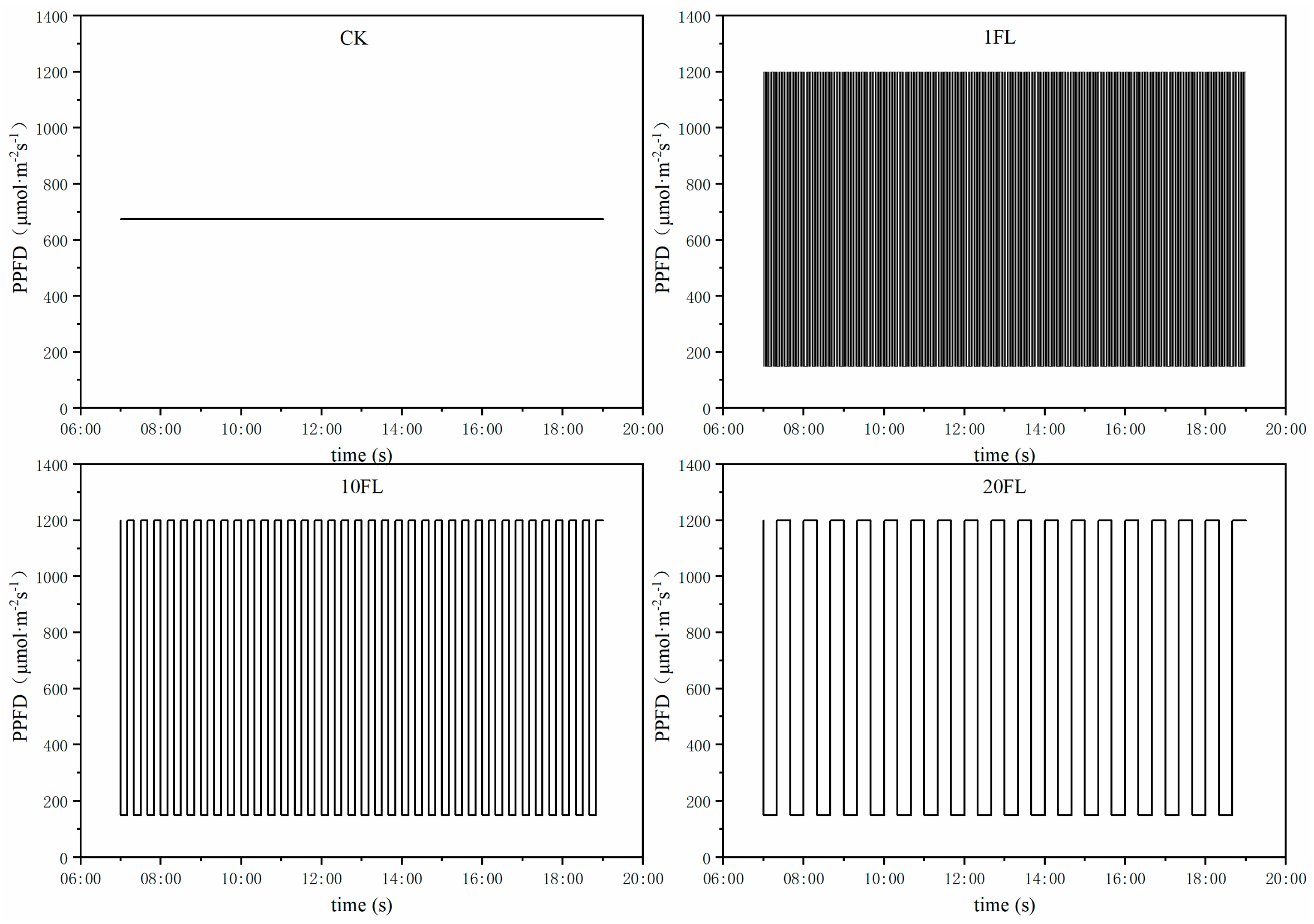
2.1. Plant Material and Growth Conditions
2.2. Measurement of Morphology and Photosynthetic Pigments
2.3. Measurement of Photosynthesis Capacity
2.4. Measurement of Chl Fluorescence Induction Kinetics
2.5. Measurement of P700 Oxidation and Plastoquinone (PQ) Size
2.6. Statistical Analysis
3. Results
3.1. Soybean Morphology and Photosynthetic Pigments
3.2. Soybean Photosynthesis and Fluorescence
3.3. Soybean Photosystem Energy Allocation
3.4. Soybean Photosynthetic Electron Transport
3.5. Soybean Cyclic Electronic Transfer
4. Discussion
4.1. Frequent Light Fluctuations Inhibit Soybean Growth and Chl Content
4.2. High-Frequency Light Fluctuation Damages the Photosynthesis System
4.3. Increasing the Fluctuation Frequency Enhances Cyclic Electron Flow and its Regulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Wang, L.; Che, Z.; Wang, R.; Cui, R.; Xu, H.; Chu, S.; Jiao, Y.; Zhang, H.; Yu, D.; et al. Novel target sites for soybean yield enhancement by photosynthesis. J. Plant Physiol. 2022, 268, 153580. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Matule, B.; Burgess, A.J.; Robson, T.M. Sunfleck properties from time series of fluctuating light. Agr. Forest Meteorol. 2021, 308–309, 108554. [Google Scholar] [CrossRef]
- Longoni, F.; Grieco, M.; Santabarbara, S.; Harbinson, J. Editorial: Photosynthesis under fluctuating light. Front. Plant Sci. 2023, 14, 1220360. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Zhang, Y.; Liu, L.; Shi, L.; Ma, Q.; Chow, W.S.; Jiang, C. Do rapid photosynthetic responses protect maize leaves against photoinhibition under fluctuating light? Photosynth. Res. 2021, 149, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Yamori, W.; Suzuki, Y.; Terashima, I. Photoprotection of PSI by Far-Red Light Against the Fluctuating Light-Induced Photoinhibition in Arabidopsis thaliana and Field-Grown Plants. Plant Cell Physiol. 2017, 58, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.Q.; Zeng, Z.L.; Yu, H.; Huang, W. Photosynthesis under fluctuating light in the CAM plant Vanilla planifolia. Plant Sci. 2022, 317, 111207. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, S.B.; Liu, T.; Huang, W. Decreased photosystem II activity facilitates acclimation to fluctuating light in the understory plant Paris polyphylla. Biochim. Biophys. Acta (BBA)–Bioenerg. 2020, 1861, 148135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gong, J.; Xiao, C.; Yang, X.; Li, X.; Zhang, Z.; Song, L.; Zhang, W.; Dong, X.; Hu, Y. Bupleurum chinense and Medicago sativa sustain their growth in agrophotovoltaic systems by regulating photosynthetic mechanisms. Renew. Sustain. Energy Rev. 2024, 189, 114024. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO 2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Shimakawa, G.; Miyake, C. Changing frequency of fluctuating light reveals the molecular mechanism for P700 oxidation in plant leaves. Plant Direct 2018, 2, e73. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological Functions of Cyclic Electron Transport Around Photosystem I in Sustaining Photosynthesis and Plant Growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron Protein Substitutes for Cyclic Electron Flow without Competing CO 2 Assimilation in Rice. Plant Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, S.; Luo, X.; Fang, L.; Liang, Z.; Yang, W. Effects of small ridge and furrow mulching degradable film on dry direct seeded rice. Sci. Rep. 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Sunoj, V.; Prasad, P.; Ciampitti, I.A.; Maswada, H.F. Narrowing Diurnal Temperature Amplitude Alters Carbon Tradeoff and Reduces Growth in C(4) Crop Sorghum. Front. Plant Sci. 2020, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, N.; Xu, H.; Maruo, T.; Guo, S. Root Zone Cooling and Exogenous Spermidine Root-Pretreatment Promoting Lactuca sativa L. Growth and Photosynthesis in the High-temperature Season. Front. Plant Sci. 2016, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta (BBA)–Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Strasserf, R.J.; Srivastava, A.; Govindjee. Polyphasic Chlorophyll a Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Díaz-Pérez, J.C. Carbon dioxide and light curves and leaf gas exchange responses to shade levels in bell pepper (Capsicum annuum L.). Plant Sci. 2023, 326, 111532. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tian, Z.; Lu, Y.; Lu, M.; Zhang, H.; Wu, H.; Hu, J. A decision-making model for light environment control of tomato seedlings aiming at the knee point of light-response curves. Comput. Electron. Agric. 2022, 198, 107103. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Zhao, S.; Song, J.; Sun, J.; Cui, N.; Chen, X.; Qu, B. Effects of light intensity on the photosynthetic characteristics of Hosta genotypes differing in the glaucousness of leaf surface. Sci. Hortic. 2024, 327, 112834. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.; Zhang, S. The role of water-water cycle in regulating the redox state of photosystem I under fluctuating light. Biochim. Biophys. Acta (BBA)–Bioenerg. 2019, 1860, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Uebayashi, N.; Ishida, S.; Ikeuchi, M.; Sato, F. Light energy allocation at PSII under field light conditions: How much energy is lost in NPQ-associated dissipation? Plant Physiol. Biochem. 2014, 81, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, T.; Zhang, S.; Huang, W. Balancing light use efficiency and photoprotection in tobacco leaves grown at different light regimes. Environ. Exp. Bot. 2020, 175, 104046. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plantarum 2011, 142, 56–64. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Wang, J.; Huang, W. Photosynthetic regulation under fluctuating light in field-grown Cerasus cerasoides: A comparison of young and mature leaves. Biochim. Biophys. Acta (BBA)–Bioenerg. 2019, 1860, 148073. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Yang, X.; Wang, F.; Qi, M.; Li, T.; Liu, Y. Cyclic electron flow protects photosystem I donor side under low night temperature in tomato. Environ. Exp. Bot. 2020, 177, 104151. [Google Scholar] [CrossRef]






| Treatment | Plant Height (cm) | Leaf Area Per Plant (dm2) | Specific Leaf Weight (mg/cm2) | Chl a + b (mg/cm2) | Chl a/b | Car (mg/cm2) |
|---|---|---|---|---|---|---|
| Ck | 22.42 ± 1.67 a | 4.16 ± 0.41 a | 4.22 ± 0.66 a | 14.23 ± 3.27 a | 2.4 ± 0.23 b | 2.58 ± 0.43 a |
| 1FL | 17.13 ± 0.71 c | 2.85 ± 0.44 b | 4.46 ± 0.43 a | 10.03 ± 1.05 b | 2.62 ± 0.23 ab | 2.47 ± 0.21 a |
| 10FL | 19.4 ± 1.3 c | 3.38 ± 1.08 ab | 3.85 ± 0.58 a | 11.25 ± 1.93 ab | 2.42 ± 0.14 b | 2.69 ± 0.47 a |
| 20FL | 21.63 ± 0.99 b | 4.12 ± 1.05 a | 3.67 ± 0.58 a | 11.49 ± 1.77 ab | 2.93 ± 0.25 a | 3.08 ± 0.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Gao, J.; Wang, Q.; Zeng, W.; Diwakar, D.; Zhang, Y.; Tan, X.; Sun, Z.; Yang, F.; Yang, W. Cyclic Electron Flow Alleviates the Stress of Light Fluctuation on Soybean Photosynthesis. Agriculture 2024, 14, 1056. https://doi.org/10.3390/agriculture14071056
Lei Y, Gao J, Wang Q, Zeng W, Diwakar D, Zhang Y, Tan X, Sun Z, Yang F, Yang W. Cyclic Electron Flow Alleviates the Stress of Light Fluctuation on Soybean Photosynthesis. Agriculture. 2024; 14(7):1056. https://doi.org/10.3390/agriculture14071056
Chicago/Turabian StyleLei, Yi, Jing Gao, Qi Wang, Weiying Zeng, Dhungana Diwakar, Yaodan Zhang, Xianming Tan, Zudong Sun, Feng Yang, and Wenyu Yang. 2024. "Cyclic Electron Flow Alleviates the Stress of Light Fluctuation on Soybean Photosynthesis" Agriculture 14, no. 7: 1056. https://doi.org/10.3390/agriculture14071056
APA StyleLei, Y., Gao, J., Wang, Q., Zeng, W., Diwakar, D., Zhang, Y., Tan, X., Sun, Z., Yang, F., & Yang, W. (2024). Cyclic Electron Flow Alleviates the Stress of Light Fluctuation on Soybean Photosynthesis. Agriculture, 14(7), 1056. https://doi.org/10.3390/agriculture14071056







