Cell Recycling Application in Single-Stage and Sequential-Stage Co-Production of Xylitol and Ethanol Using Corn Cob Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
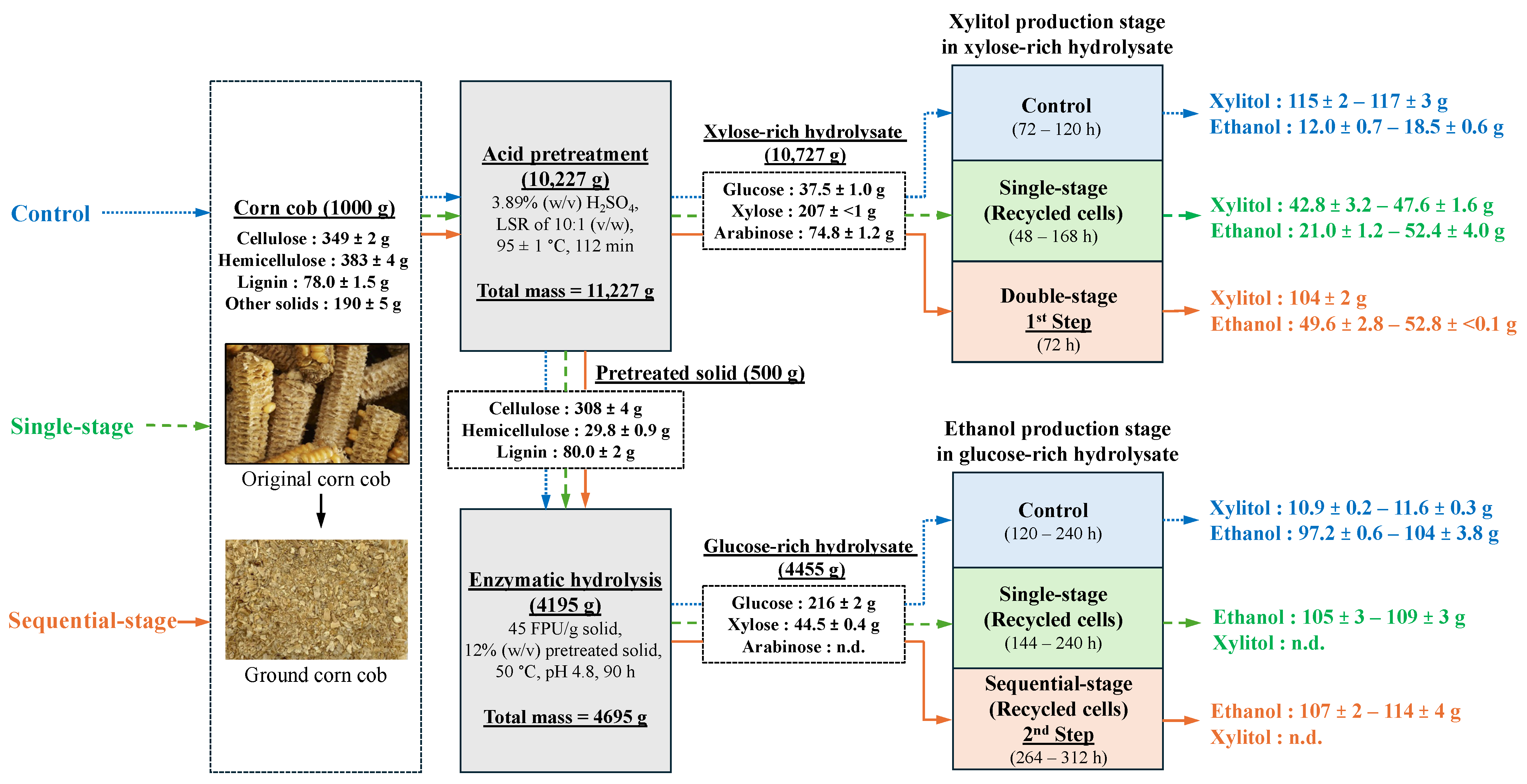
2.2. Xylose-Rich and Glucose-Rich Hydrolysates Preparation
2.3. Xylitol and Ethanol Co-Production Based on Single-Pass Nonrecycled Cell (Control) Cultivation Process
2.4. Single-Stage Co-Production of Xylitol and Ethanol Using Recycled Cells in One Substrate System
2.5. Sequential-Stage Co-Production of Xylitol and Ethanol Using Recycled Cells in Two Substrates System
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
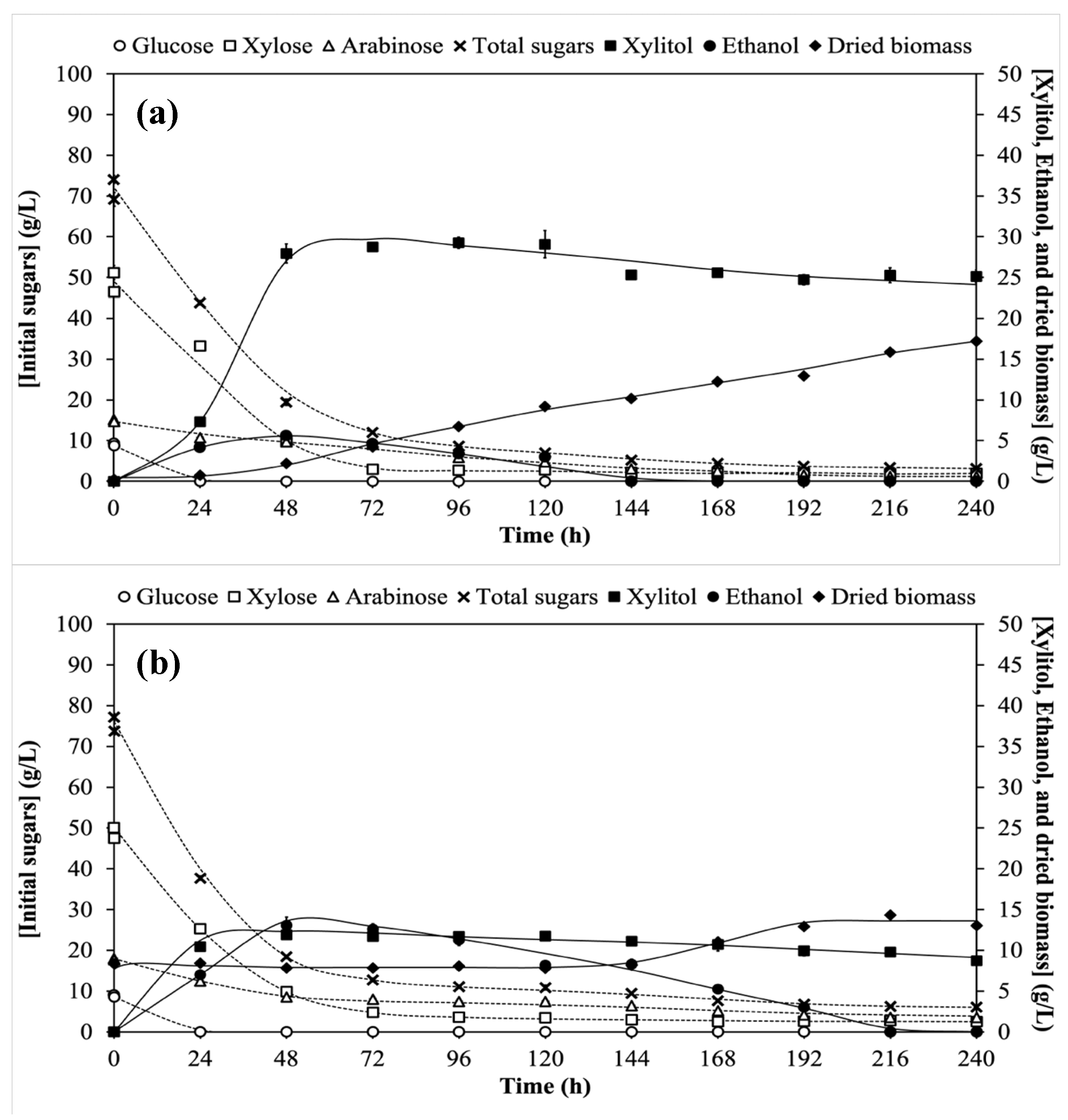
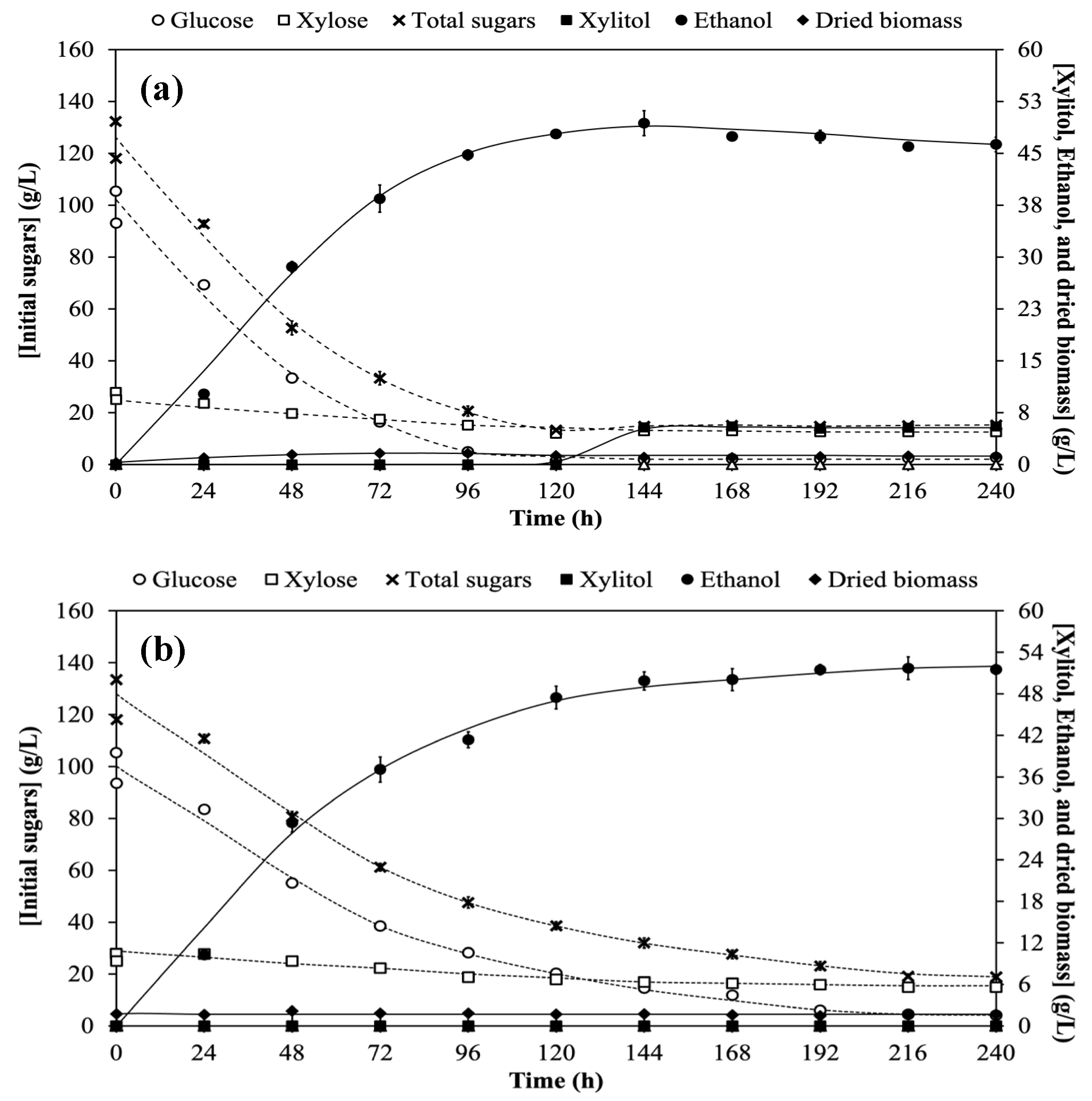
3.1. Xylitol and Ethanol Co-Production Based on Single-Pass Nonrecycled Cell (Control) Cultivation Process
3.2. Single-Stage Co-Production of Xylitol and Ethanol Using Recycled Cells in One Substrate System
3.3. Sequential-Stage Co-Production of Xylitol and Ethanol Using Recycled Cells in Two Substrates System
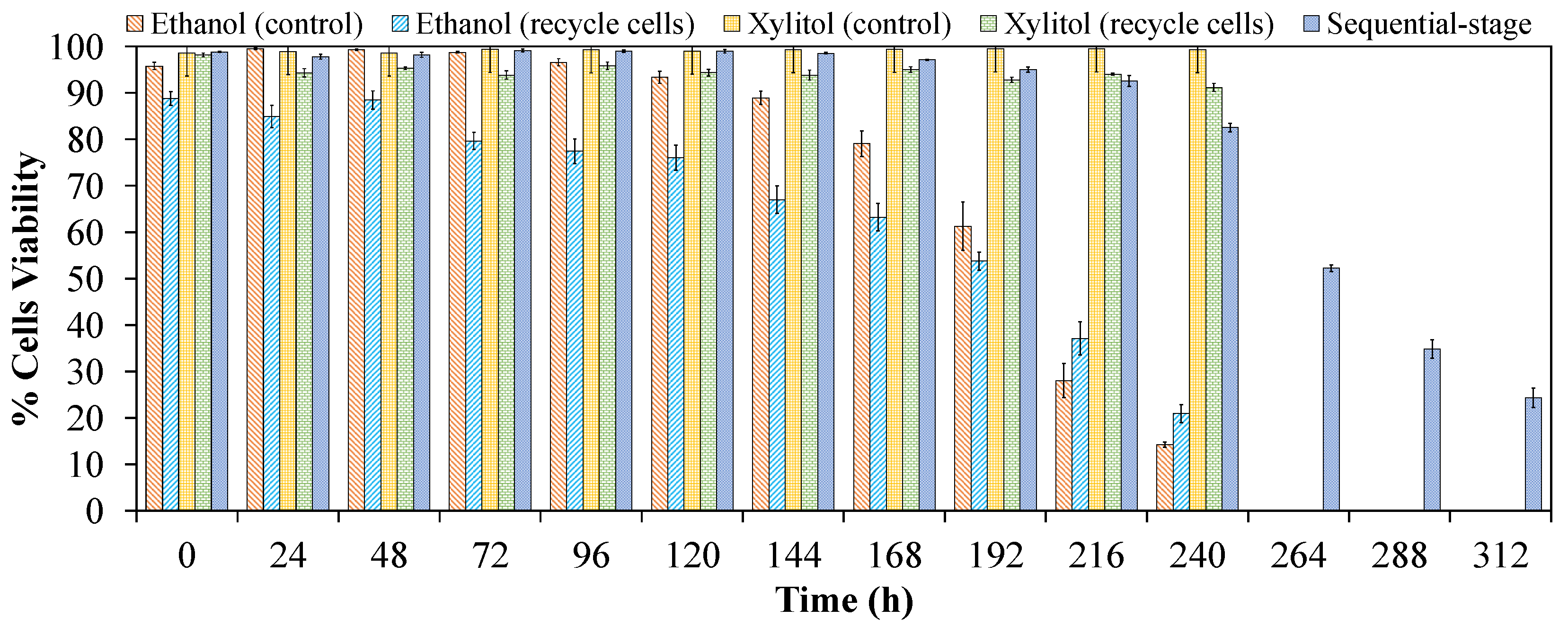
3.4. Comparative Co-Production of Xylitol and Ethanol in Single-Stage and Sequential-Stage
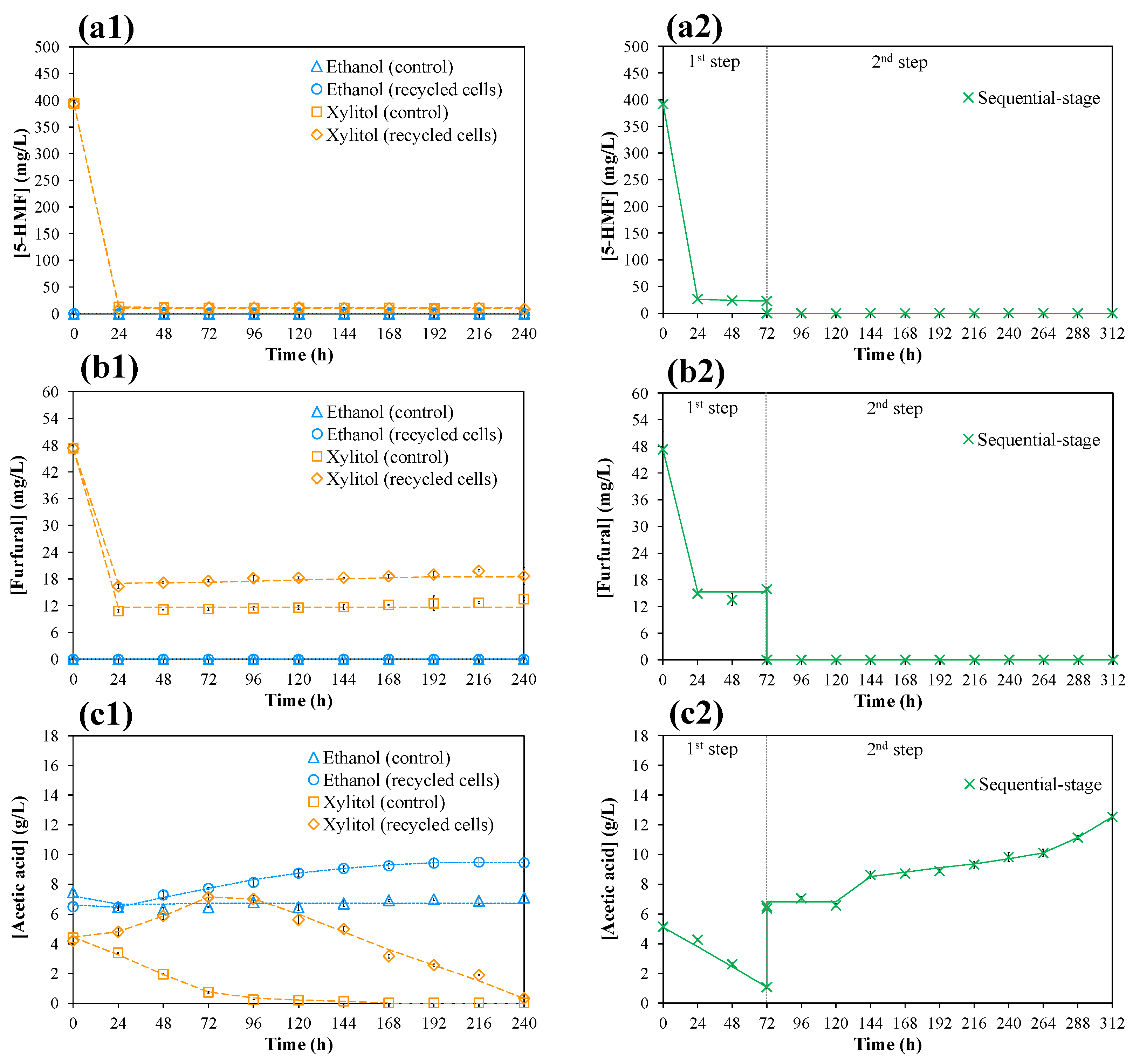
3.5. Investigation of the Inhibitors’ Degradation
3.6. Mass Balance Calculations of Xylitol and Ethanol in Various Production Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| [ ] | Concentration |
| μmax | Maximum specific growth rate (h−1) |
| Et | Ethanol |
| FPU | Filter paper units |
| Glu | Glucose |
| 5-HMF | 5-Hydroxymethylfurfural |
| n.d. | Not detected |
| QEt,max | Volumetric productivity of ethanol per liter per h (gEt/L/h) |
| QXy,max | Volumetric productivity of xylitol per liter per h (gXy/L/h) |
| qEt,max | Maximum specific ethanol production rate (gEt/gX/h) |
| qTotS,max | Maximum specific total sugars consumption rate (gTotS/gX/h) |
| qXy,max | Maximum specific xylitol production rate (gXy/gX/h) |
| TISTR | Thailand Institute of Scientific and Technological Research |
| TotS | Total sugars |
| X | Dried biomass |
| Xy | Xylitol |
| Xyl | Xylose |
| YEt/TotS | Yield of ethanol produced over total sugars consumed (gEt/gTotS) |
| YX/TotS | Yield of dried biomass produced over total sugars consumed (gX/gTotS) |
| YXy/Xyl | Yield of xylitol produced over xylose consumed (gXy/gXyl) |
| Ythe | Theoretical yields. |
References
- International Energy Agency (IEA). Share of Global Ethanol Output by Country between 2017 and 2023. Available online: https://www.iea.org/data-and-statistics/charts/share-of-global-ethanol-output-by-country-between-2017-and-2023 (accessed on 20 March 2024).
- United States Department of Energy. Global Ethanol Production by Country or Region. Available online: https://afdc.energy.gov/data/10331 (accessed on 17 March 2024).
- Dahman, Y.; Dignan, C.; Fiayaz, A.; Chaudhry, A. An introduction to biofuels, foods, livestock, and the environment. In Biopolymer-Based Materials, and Bioenergy; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2019; pp. 241–276. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Koloor, S.S.R.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass: Challenges and future directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef]
- Ale, S.; Femeena, P.V.; Mehan, S.; Cibin, R. Environmental impacts of bioenergy crop production and benefits of multifunctional bioenergy systems. In Bioenergy with Carbon Capture and Storage; Academic Press: New York, NY, USA, 2019; pp. 195–217. [Google Scholar] [CrossRef]
- Mathur, S.; Kumar, D.; Kumar, V.; Dantas, A.; Verma, R.; Kuca, K. Xylitol: Production strategies with emphasis on biotechnological approach, scale up, and market trends. Sustain. Chem. Pharm. 2023, 35, 101203. [Google Scholar] [CrossRef]
- ReportLinker. Prospective Insights into Xylitol Sector: 2023 Global Review. Available online: https://www.reportlinker.com/p06284602/Xylitol-Global-Market-Report.html?utm_source=GNW (accessed on 20 March 2024).
- Porninta, K.; Khemacheewakul, J.; Techapun, C.; Phimolsiripol, Y.; Jantanasakulwong, K.; Sommanee, S.; Mahakuntha, C.; Feng, J.; Htike, S.L.; Kumar, A.; et al. Pretreatment and Enzymatic Hydrolysis Optimization of Lignocellulosic Biomass for Ethanol, Xylitol, and Phenylacetylcarbinol Co-Production using Candida magnoliae. Front. Bioeng. Biotechnol. 2024, 11, 2023. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, J.; Lin, Z.; Zhang, J. Aerobic and sequential anaerobic fermentation to produce xylitol and ethanol using non-detoxified acid pretreated corncob. Biotechnol. Biofuels 2014, 7, 166. [Google Scholar] [CrossRef]
- Eknikom, S.; Nasuno, R.; Takagi, H. Molecular mechanism of ethanol fermentation inhibition via protein tyrosine nitration of pyruvate decarboxylase by reactive nitrogen species in yeast. Sci. Rep. 2022, 12, 4664. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Morley, A. An evolutionary perspective on the Crabtree effect. Front. Mol. Biosci. 2014, 1, 17. [Google Scholar] [CrossRef]
- Baptista, S.L.; Cunha, J.T.; Romaní, A.; Domingues, L. Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2. Bioresour. Technol. 2018, 267, 481–491. [Google Scholar] [CrossRef]
- Mareczky, Z.; Anikó, F.; Anikó, F.; Fehér, C.; Réczey, K. Effects of pH and aeration conditionson xylitol production by Candida and Hansenula yeasts. Period. Polytech. Chem. Eng. 2016, 60, 54–59. [Google Scholar] [CrossRef]
- Nimbalkar, P.R.; Khedkar, M.A.; Chavan, P.V.; Bankar, S.B. Biobutanol from agricultural residues: Technology and economics. In Advances and Developments in Biobutanol Production; Segovia-Hernandez, J.G., Behera, S., Sanchez-Ramirez, E., Eds.; Woodhead Publishing: Cambridge, UK, 2023; Volume Advances in Pollution Research, pp. 139–169. [Google Scholar]
- Trilokesh, C.; Uppuluri, K.B. Biobutanol from lignocellulosic biomass and microalgae: Scope, technology, and economics. In Sustainable Biofuels; Ray, R.C., Ed.; Academic Press: New York, NY, USA, 2021; Volume Applied Biotechnology Reviews, pp. 163–223. [Google Scholar]
- Xue, C.; Cheng, C. Butanol production by Clostridium. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 35–77. [Google Scholar]
- Silva, V.F.; Nakanishi, S.C.; Dionísio, S.R.; Rossell, C.E.; Ienczak, J.L.; Gonçalves, A.R.; Rocha, G.J. Using cell recycling batch fermentations to validate a setup for cellulosic ethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 1853–1859. [Google Scholar] [CrossRef]
- Thani, A.; Laopaiboon, P.; Laopaiboon, L. Batch and continuous ethanol fermentation by Saccharomyces cerevisiae NP 01 using cell recycling technique. Asia-Pac. J. Sci. Technol. 2017, 19, 1–8. [Google Scholar]
- Dasgupta, D.; Ghosh, D.; Bandhu, S.; Adhikari, D.K. Lignocellulosic sugar management for xylitol and ethanol fermentation with multiple cell recycling by Kluyveromyces marxianus IIPE453. Microbiol. Res. 2017, 200, 64–72. [Google Scholar] [CrossRef]
- Nunta, R.; Techapun, C.; Sommanee, S.; Mahakuntha, C.; Porninta, K.; Punyodom, W.; Phimolsiripol, Y.; Rachtanapun, P.; Wang, W.; Zhuang, X.; et al. Valorization of rice straw, sugarcane bagasse and sweet sorghum bagasse for the production of bioethanol and phenylacetylcarbinol. Sci. Rep. 2023, 13, 727. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Shen, F.; Su, Y.; Wan, Y. Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind. Eng. Chem. Res. 2009, 48, 7346–7353. [Google Scholar] [CrossRef]
- Mejias-Ortiz, M.; Mencher, A.; Morales, P.; Tronchoni, J.; Gonzalez, R. Saccharomyces cerevisiae responds similarly to co-culture or to a fraction enriched in Metschnikowia pulcherrima extracellular vesicles. Microb. Biotechnol. 2023, 16, 1027–1040. [Google Scholar] [CrossRef]
- Leksawasdi, N.; Chow, Y.Y.S.; Breuer, M.; Hauer, B.; Rosche, B.; Rogers, P.L. Kinetic analysis and modelling of enzymatic (R)-phenylacetylcarbinol batch biotransformation process. J. Biotechnol. 2004, 111, 179–189. [Google Scholar] [CrossRef]
- Arshad, M.; Khan, Z.M.; Khalil-ur-Rehman; Shah, F.A.; Rajoka, M.I. Optimization of process variables for minimization of byproduct formation during fermentation of blackstrap molasses to ethanol at industrial scale. Lett. Appl. Microbiol. 2008, 47, 410–414. [Google Scholar] [CrossRef]
- Tangtua, J.; Techapun, C.; Pratanaphon, R.; Kuntiya, A.; Chaiyaso, T.; Hanmoungjai, P.; Seesuriyachan, P.; Leksawasdi, N. Screening of 50 microbial strains for production of ethanol and (R)-phenylacetylcarbinol. Chiang Mai J. Sci. 2013, 40, 299–304. [Google Scholar]
- Mahakuntha, C.; Reungsang, A.; Nunta, R.; Leksawasdi, N. Kinetics of whole cells and ethanol production from Candida tropicalis TISTR 5306 cultivation in batch and fed-batch modes using assorted grade fresh longan juice. An. Acad. Bras. Cienc. 2021, 93, e20200220. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Costa, S.; Marchetti, M.G.; Pedrini, P. Optimized production of xylitol from xylose using a hyper-acidophilic Candida tropicalis. Biomolecules 2013, 5, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, R.; Jiang, X.; Chen, X.; Yuan, X.; Liu, Z.; Jin, M. Boosting ethanol productivity of Zymomonas mobilis 8b in enzymatic hydrolysate of dilute acid and ammonia pretreated corn stover through medium optimization, high cell density fermentation and cell recycling. Front. Microbiol. 2019, 10, 2316. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Sunga, R. The Complete Aqueous Sulfuric Acid Solutions Density-Concentration Calculator. Available online: https://www.handymath.com/cgi-bin/sulfurictble11.cgi?submit=Back+to+Calculator&tmptr=25&spcfgrv=&conc=3.89 (accessed on 13 May 2024).
- Croft, T.; Venkatakrishnan, P.; Lin, S.-J. NAD+ Metabolism and Regulation: Lessons from Yeast. Biomolecules 2020, 10, 330. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, M.; Oliver, D.J.; Schnable, P.S. The roles of aldehyde dehydrogenases (ALDHs) in the PDH bypass of Arabidopsis. BMC Biochem. 2009, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, K.-S.; Chen, C.-Y.; Hsu, C.-L.; Chang, T.-C.; Jang, H.-D. Enhancement of the efficiency of bioethanol production by Saccharomyces cerevisiae via gradually batch-wise and fed-batch increasing the glucose concentration. Fermentation 2018, 4, 45. [Google Scholar] [CrossRef]
- Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Jacob, S.; Kumar, D.; Awasthi, M.K.; Pant, K.K.; Parameswaran, B.; Kumar, V. High level xylitol production by Pichia fermentans using non-detoxified xylose-rich sugarcane bagasse and olive pits hydrolysates. Bioresour. Technol. 2021, 342, 126005. [Google Scholar] [CrossRef] [PubMed]
- Qazizada, M.E. Design of a batch stirred fermenter for ethanol production. Procedia Eng. 2016, 149, 389–403. [Google Scholar] [CrossRef]
- Du, C.; Li, Y.; Zong, H.; Yuan, T.; Yuan, W.; Jiang, Y. Production of bioethanol and xylitol from non-detoxified corn cob via a two-stage fermentation strategy. Bioresour. Technol. 2020, 310, 123427. [Google Scholar] [CrossRef]
- Ji, H.; Xu, K.; Dong, X.; Sun, D.; Jin, L. Sequential Production of d-xylonate and Ethanol from Non-Detoxified Corncob at Low-pH by Pichia kudriavzevii via a Two-Stage Fermentation Strategy. J. Fungi 2021, 7, 1038. [Google Scholar] [CrossRef]

| [Product], Kinetic Parameter, Cells Viability | Xylitol Production Stage in Xylose-Rich Hydrolysate | Ethanol Production Stage in Glucose-Rich Hydrolysate | Sequential-Stage Co-Production | |||
|---|---|---|---|---|---|---|
| Control | Single-Stage (Recycled Cells) | Control | Single-Stage (Recycled Cells) | 1st Step: Xylose-Rich Hydrolysate | 2nd Step: Glucose-Rich Hydrolysate (Recycled Cells) | |
| [Xy]max (g/L) | 28.7–29.3 A | 10.7–11.9 C | 5.19–5.51 D | n.d. | 25.9 B | n.d. |
| [Et]max (g/L) | 5.59 D | 12.6–13.1 C | 46.3–49.3 B | 49.9–51.7 A | 12.4–13.2 C | 50.9–54.1 A |
| [X]max (g/L) | 17.2 A | 14.3 B | 1.41–1.61 E | 2.16 E | 6.10 C | 4.67–4.94 D |
| YXy/Xyl (gXy/gXyl) | 0.661–0.669 A | 0.238–0.316 D | 0.417–0.456 C | n.d. | 0.534 B | n.d. |
| YEt/TotS (gEt/gTotS) | 0.120 E | 0.208–0.236 D | 0.450–0.465 B | 0.472–0.487 A | 0.194–0.198 D | 0.394–0.420 C |
| YX/TotS (gX/gTotS) | 0.235 A | 0.205 B | 0.014–0.018 E | 0.040 D | 0.088 C | 0.036–0.037 DE |
| μmax (h−1) | 0.027 B | 0.012 C | 0.028 B | 0.011 C | 0.033 A | 0.003 D |
| qTotS,max (gTotS/gX/h) | −0.86 C | −0.18 D | −1.63 B | −0.66 C | −2.18 A | −0.30 D |
| qXy,max (gXy/gX/h) | 0.59 A | 0.10 D | 0.19 C | – | 0.26 B | – |
| qEt,max (gEt/gX/h) | 0.37 C | 0.07 D | 0.85 A | 0.57 B | 0.84 A | 0.10 D |
| QXy,max (gXy/L/h) | 0.40–0.41 A | 0.22–0.25 C | 0.036–0.038 D | – | 0.36 B | – |
| QEt,max (gEt/L/h) | 0.12 D | 0.26–0.27 C | 0.38–0.41 A | 0.35–0.36 B | 0.26–0.27 C | 0.27–0.28 C |
| Cell viability * (%) | 99.3 A | 91.2 B | 14.2 D | 20.9 C | 99.1 A | 19.6 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porninta, K.; Mahakuntha, C.; Khemacheewakul, J.; Techapun, C.; Phimolsiripol, Y.; Rachtanapun, P.; Jantanasakulwong, K.; Feng, J.; Htike, S.L.; Nunta, R.; et al. Cell Recycling Application in Single-Stage and Sequential-Stage Co-Production of Xylitol and Ethanol Using Corn Cob Hydrolysates. Agriculture 2024, 14, 1062. https://doi.org/10.3390/agriculture14071062
Porninta K, Mahakuntha C, Khemacheewakul J, Techapun C, Phimolsiripol Y, Rachtanapun P, Jantanasakulwong K, Feng J, Htike SL, Nunta R, et al. Cell Recycling Application in Single-Stage and Sequential-Stage Co-Production of Xylitol and Ethanol Using Corn Cob Hydrolysates. Agriculture. 2024; 14(7):1062. https://doi.org/10.3390/agriculture14071062
Chicago/Turabian StylePorninta, Kritsadaporn, Chatchadaporn Mahakuntha, Julaluk Khemacheewakul, Charin Techapun, Yuthana Phimolsiripol, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Juan Feng, Su Lwin Htike, Rojarej Nunta, and et al. 2024. "Cell Recycling Application in Single-Stage and Sequential-Stage Co-Production of Xylitol and Ethanol Using Corn Cob Hydrolysates" Agriculture 14, no. 7: 1062. https://doi.org/10.3390/agriculture14071062













