Fusarium and Hazelnut: A Story of Twists and Turns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Morphological Characterization
2.2. Biomolecular Markers and Phylogenetic Analysis
2.3. Antagonism against Plant Pathogenic Fungi
2.4. Bioassays on Aphids
2.5. Metabolomic Analysis
3. Results
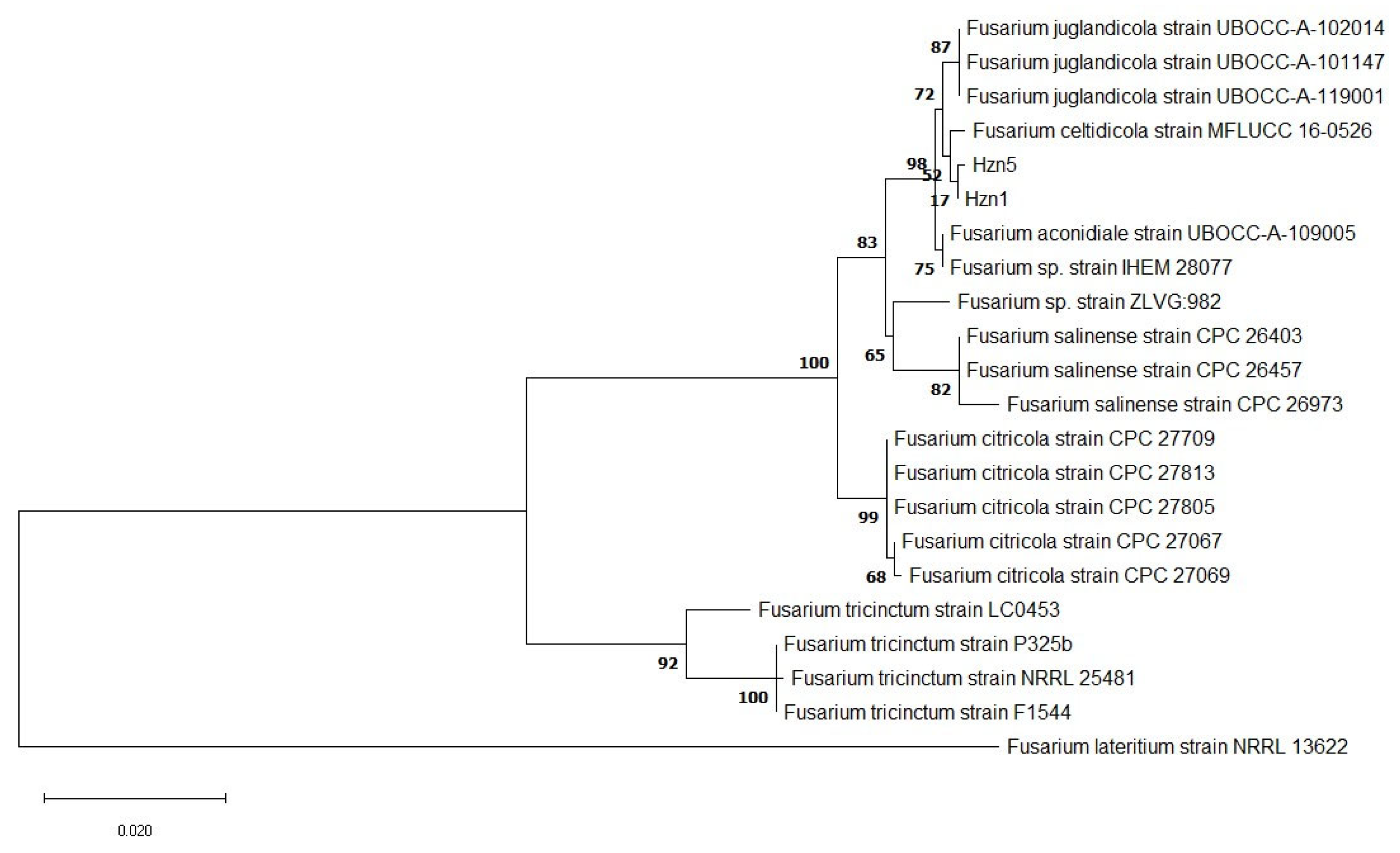
3.1. Identification and Phylogenetic Analysis
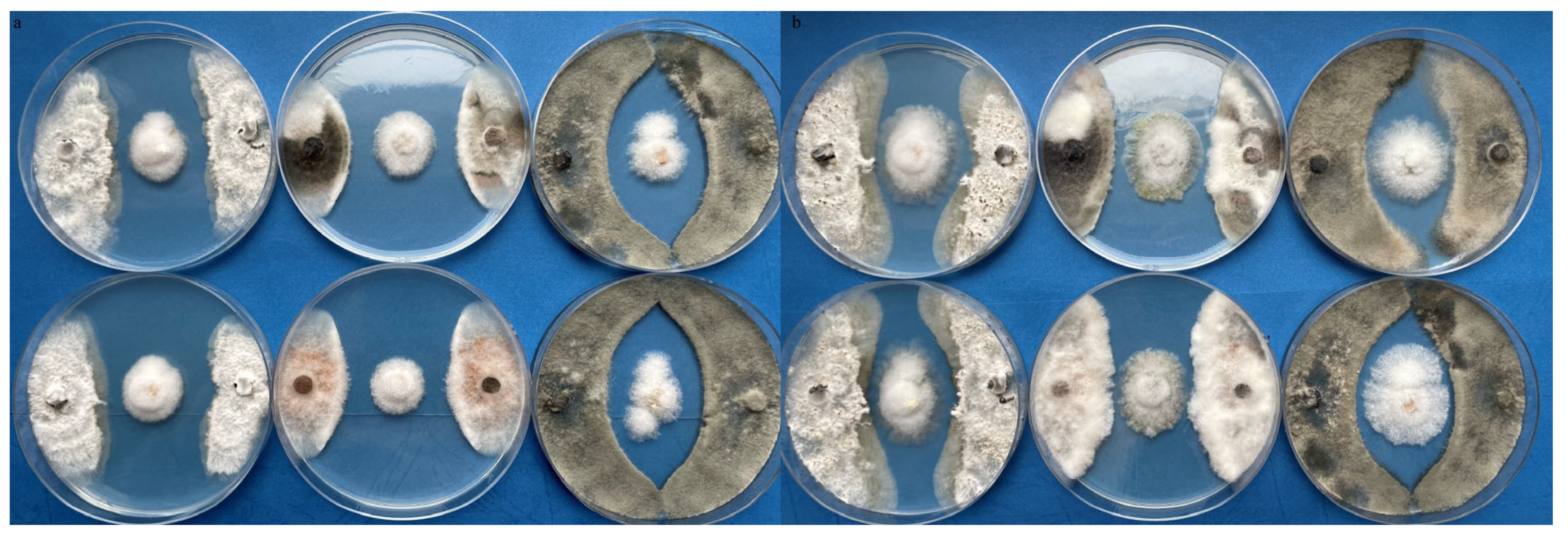
3.2. Effects against Plant Pathogenic Fungi and Aphids
3.3. Metabolomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. Mycotoxin contamination in hazelnut: Current status, analytical strategies, and future prospects. Toxins 2023, 15, 99. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Ann. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bianchini, A.; Hanna, M.A. Evaluation of mold and mycotoxin contaminations in hybrid hazelnuts grown in Nebraska. J. Food Process. Technol. 2011, 2, 119. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S. Hazelnut kernel defects and associated fungi in three provinces in Turkey. In Proceedings of the 7th International Scientific Agriculture Symposium “Agrosym 2016”, Jahorina, Bosnia and Herzegovina, 6–9 October 2016; pp. 1312–1318. [Google Scholar]
- Battilani, P.; Chiusa, G.; Arciuolo, R.; Somenzi, M.; Fontana, M.; Castello, G.; Spigolon, N. Diaporthe as the main cause of hazelnut defects in the Caucasus region. Phytopathol. Mediterr. 2018, 54, 241–252. [Google Scholar]
- Arciuolo, R.; Santos, C.; Soares, C.; Castello, G.; Spigolon, N.; Chiusa, G.; Lima, N.; Battilani, P. Molecular characterization of Diaporthe species associated with hazelnut defects. Front. Plant Sci. 2020, 11, 611655. [Google Scholar] [CrossRef] [PubMed]
- Arciuolo, R.; Chiusa, G.; Castello, G.; Camardo Leggieri, M.; Spigolon, N.; Battilani, P. Diaporthe spp. is confirmed as the main fungus associated with defective Turkish hazelnuts. Plant Health Progr. 2022, 23, 440–448. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Cai, L.; Crous, P.W. Changing the game: Resolving systematic issues in key Fusarium species complexes. Persoonia 2019, 43, i–ii. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Duran, P.; Barra, P.J.; de la Luz Mora, M.; Morina, F.; Viscardi, S.; Meriño-Gergichevich, C. First report of fungal complex causing grey necrosis of hazelnut in Chile. New Dis. Rep. 2020, 42, 7. [Google Scholar] [CrossRef]
- Torres-Cruz, T.J.; Whitaker, B.K.; Proctor, R.H.; Broders, K.; Laraba, I.; Kim, H.S.; Brown, D.W.; O’Donnell, K.; Estrada-Rodriguez, T.L.; Lee, Y.H.; et al. FUSARIUM-ID v. 3.0: An updated, downloadable resource for Fusarium species identification. Plant Dis. 2022, 106, 1610–1616. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Heckert, S.; Wiseman, M.; Jones, L. Fungi associated with and influence of moisture on development of kernel mold of hazelnut. Plant Dis. 2019, 103, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia 2018, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dias Cavalcanti, A.; da Silva Santos, A.C.; de Oliveira Ferro, L.; Bezerra, J.D.; Souza-Motta, C.M.; Magalhães, O.M.C. Fusarium massalimae sp. nov. (F. lateritium species complex) occurs endophytically in leaves of Handroanthus chrysotrichus. Mycol. Progr. 2020, 19, 1133–1142. [Google Scholar] [CrossRef]
- Santori, A.; Vitale, S.; Luongo, L.; Belisario, A. First report of Fusarium lateritium as the agent of nut gray necrosis on hazelnut in Italy. Plant Dis. 2010, 94, 484. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Santori, A.; Wajnberg, E.; Castagnone-Sereno, P.; Luongo, L.; Belisario, A. Morphological and molecular analysis of Fusarium lateritium, the cause of gray necrosis of hazelnut fruit in Italy. Phytopathology 2011, 101, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Turco, S.; Grottoli, A.; Drais, M.I.; De Spirito, C.; Faino, L.; Reverberi, M.; Cristofori, V.; Mazzaglia, A. Draft genome sequence of a new Fusarium isolate belonging to Fusarium tricinctum species complex collected from hazelnut in central Italy. Front. Plant Sci. 2021, 12, 788584. [Google Scholar] [CrossRef]
- Watanabe, M.; Yonezawa, T.; Lee, K.I.; Kumagai, S.; Sugita-Konishi, Y.; Goto, K.; Hara-Kudo, Y. Molecular phylogeny of the higher and lower taxonomy of the Fusarium genus and differences in the evolutionary histories of multiple genes. BMC Evol. Biol. 2011, 11, 322. [Google Scholar] [CrossRef]
- Nicoletti, R.; Zimowska, B. Endophytic fungi of hazelnut (Corylus avellana). Plant Prot. Sci. 2023, 59, 107–123. [Google Scholar] [CrossRef]
- Nicoletti, R. Endophytic fungi of citrus plants. Agriculture 2019, 9, 247. [Google Scholar] [CrossRef]
- Ren, F.; Dong, W.; Sun, H.; Yan, D.H. Endophytic mycobiota of jingbai pear trees in north China. Forests 2019, 10, 260. [Google Scholar] [CrossRef]
- Ren, F.; Dong, W.; Yan, D.H. Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of pinggu peach trees. Microorganisms 2019, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Di Vaio, C.; Cirillo, C. Endophytic fungi of olive tree. Microorganisms 2020, 8, 1321. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Beccaro, G.L.; Sekara, A.; Cirillo, C.; Di Vaio, C. Endophytic fungi and ecological fitness of chestnuts. Plants 2021, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, E.V.; Shumilova, L.P.; Gomzhina, M.M.; Aleksandrova, A.V.; Kokaeva, L.Y.; Pavlova, L.M. Diversity of endophytic fungi in annual shoots of Prunus mandshurica (Rosaceae) in the South of Amur region, Russia. Diversity 2022, 14, 1124. [Google Scholar] [CrossRef]
- Yabaneri, C.; Sevim, A. Endophytic fungi from the common walnut and their in vitro antagonistic activity against Ophiognomonia leptostyla. Biologia 2023, 78, 361–371. [Google Scholar] [CrossRef]
- Zimowska, B.; Nicoletti, R. Arcopilus aureus: A valuable endophytic associate of hazelnut. Acta Agrobot. 2023, 76, 175998. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for Basidiomycetes: Application to identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogen. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Stielow, J.B.; Lévesque, C.A.; Seifert, K.A.; Meyer, W.; Irinyi, L.; Smits, D.; Renfurm, R.; Verkley, G.J.M.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 2015, 35, 242–263. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two different intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Manganiello, G.; Marra, R.; Staropoli, A.; Lombardi, N.; Vinale, F.; Nicoletti, R. The shifting mycotoxin profiles of endophytic Fusarium strains: A case study. Agriculture 2019, 9, 143. [Google Scholar] [CrossRef]
- Shang, Q.J.; Phookamsak, R.; Camporesi, E.; Khan, S.; Lumyong, S.; Hyde, K.D. The holomorph of Fusarium celtidicola sp. nov. from Celtis australis. Phytotaxa 2018, 361, 251–265. [Google Scholar] [CrossRef]
- Lombard, L.; Crous, P.W.; Cobo-Diaz, J.F.; Le Floch, G.; Nodet, P. Fungal Planet 1282. Persoonia 2021, 46, 520–521. [Google Scholar]
- Lombard, L.; Crous, P.W.; Weill, A.; Le Floch, G.; Nodet, P. Fungal Planet 1283. Persoonia 2021, 46, 522–523. [Google Scholar]
- Pyszko, P.; Šigutová, H.; Kolařík, M.; Kostovčík, M.; Ševčík, J.; Šigut, M.; Višňovská, D.; Drozd, P. Mycobiomes of two distinct clades of ambrosia gall midges (Diptera: Cecidomyiidae) are species-specific in larvae but similar in nutritive mycelia. Microbiol. Spectr. 2024, 12, e02830-23. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P.; Zając, J.; Jobczyk, A.; Taerum, S.J. The culturable leaf mycobiome of Viscum album subsp. austriacum. For. Pathol. 2023, 53, e12821. [Google Scholar] [CrossRef]
- Brglez, A.; Piškur, B.; Ogris, N. Eutypella parasitica and other frequently isolated fungi in wood of dead branches of young sycamore maple (Acer pseudoplatanus) in Slovenia. Forests 2020, 11, 467. [Google Scholar] [CrossRef]
- Markakis, E.A.; Roditakis, E.N.; Kalantzakis, G.S.; Chatzaki, A.; Soultatos, S.K.; Stavrakaki, M.; Tavlaki, G.I.; Koubouris, G.C.; Bagkis, N.; Goumas, D.E. Characterization of fungi associated with olive fruit rot and olive oil degradation in Crete, southern Greece. Plant Dis. 2021, 105, 3623–3635. [Google Scholar] [CrossRef] [PubMed]
- Patejuk, K.; Baturo-Cieśniewska, A.; Najberek, K.; Pusz, W. First report of Fusarium lateritium causing shoot dieback of Acer negundo in Europe. Plant Dis. 2022, 106, 1519. [Google Scholar] [CrossRef] [PubMed]
- Laraba, I.; Busman, M.; Geiser, D.M.; O’Donnell, K. Phylogenetic diversity and mycotoxin potential of emergent phytopathogens within the Fusarium tricinctum species complex. Phytopathology 2022, 112, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Senatore, M.T.; Ward, T.J.; Cappelletti, E.; Beccari, G.; McCormick, S.P.; Busman, M.; Laraba, I.; O’Donnell, K.; Prodi, A. Species diversity and mycotoxin production by members of the Fusarium tricinctum species complex associated with Fusarium head blight of wheat and barley in Italy. Int. J. Food Microbiol. 2021, 358, 109298. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A review of the mycotoxin enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium cyclodepsipeptide mycotoxins: Chemistry, biosynthesis, and occurrence. Toxins 2020, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Spicer, L.J.; Caloni, F. Enniatin B1: Emerging mycotoxin and emerging issues. Toxins 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Valenti, I.; Tini, F.; Sevarika, M.; Agazzi, A.; Beccari, G.; Bellezza, I.; Ederli, L.; Grottelli, S.; Pasquali, M.; Romani, R.; et al. Impact of enniatin and deoxynivalenol co-occurrence on plant, microbial, insect, animal and human systems: Current knowledge and future perspectives. Toxins 2023, 15, 271. [Google Scholar] [CrossRef]
- Herrmann, M.; Zocher, R.; Haese, A. Enniatin production by Fusarium strains and its effect on potato tuber tissue. Appl. Environ. Microbiol. 1996, 62, 393–398. [Google Scholar] [CrossRef]
- Fanelli, F.; Ferracane, R.; Ritieni, A.; Logrieco, A.F.; Mulè, G. Transcriptional regulation of enniatins production by Fusarium avenaceum. J. Appl. Microbiol. 2014, 116, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, V.C.; Mirabelli, V.; Cimmarusti, M.T.; Haidukowski, M.; Leslie, J.F.; Logrieco, A.F.; Caliandro, R.; Fanelli, F.; Mulè, G. Enniatin and beauvericin biosynthesis in Fusarium species: Production profiles and structural determinant prediction. Toxins 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.M.; Makboul, M.A.; Moharram, A.M.; Tekwani, B.L.; Calderón, A.I. A new enniatin antibiotic from the endophyte Fusarium tricinctum Corda. J. Antib. 2015, 68, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Wang, H.; Liu, T.; Xin, Z. Fusartricin, a sesquiterpenoid ether produced by an endophytic fungus Fusarium tricinctum Salicorn 19. Eur. Food Res. Technol. 2015, 240, 805–814. [Google Scholar] [CrossRef]
- Zocher, R.; Keller, U.; Kleinkauf, H. Enniatin synthetase, a novel type of multifunctional enzyme catalyzing depsipeptide synthesis in Fusarium oxysporum. Biochemistry 1982, 21, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Haese, A.; Schubert, M.; Herrmann, M.; Zocher, R. Molecular characterization of the enniatin synthetase gene encoding a multifunctional enzyme catalysing N-methyldepsipeptide formation in Fusarium scirpi. Mol. Microbiol. 1993, 7, 905–914. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Wilson, A.H.; Chandler, E.; Thomsett, M. Detection and differentiation of trichothecene and enniatin-producing Fusarium species on small-grain cereals. Eur. J. Plant Pathol. 2004, 110, 503–514. [Google Scholar] [CrossRef]
- Gautier, C.; Pinson-Gadais, L.; Richard-Forget, F. Fusarium mycotoxins enniatins: An updated review of their occurrence, the producing Fusarium species, and the abiotic determinants of their accumulation in crop harvests. J. Agric. Food Chem. 2020, 68, 4788–4798. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Faeth, S.H.; Gunatilaka, A.L. 13α–Hydroxylucilactaene and other metabolites of an endophytic strain of Fusarium acuminatum. Nat. Prod. Commun. 2007, 2, 547–550. [Google Scholar] [CrossRef]
- Clark, T.N.; Carroll, M.; Ellsworth, K.; Guerrette, R.; Robichaud, G.A.; Johnson, J.A.; Gray, C.A. Antibiotic mycotoxins from an endophytic Fusarium acuminatum isolated from the medicinal plant Geum macophyllum. Nat. Prod. Commun. 2018, 13, 1301–1304. [Google Scholar]
- Firakova, S.; Šturdíková, M.; Liptaj, T.; Prónayová, N.; Bezáková, L.; Proksa, B. Enniatins produced by Fusarium dimerum, an endophytic fungal strain. Die Pharmazie 2008, 63, 539–541. [Google Scholar] [PubMed]
- Wätjen, W.; Debbab, A.; Hohlfeld, A.; Chovolou, Y.; Kampkötter, A.; Edrada, R.A.; Ebel, R.; Hakiki, A.; Mosaddak, M.; Totzke, F.; et al. Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation. Mol. Nutr. Food Res. 2009, 53, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.T.; Dong, X.Y.; Li, Z.H.; Yan, H.; He, J.; Liu, J.K.; Feng, T. Antibacterial metabolites from kiwi endophytic fungus Fusarium tricinctum, a potential biocontrol strain for kiwi canker disease. J. Agric. Food Chem. 2023, 71, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, X.; Iacovelli, R.; Hackl, T.; Haslinger, K. Genomic and metabolomic analysis of the endophytic fungus Fusarium sp. VM-40 isolated from the medicinal plant Vinca minor. J. Fungi 2023, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, Y.; Ming, Y.; Li, C.; Li, Z.; Chen, J.; Luo, M. Biological activity and chemical composition of the endophytic fungus Fusarium sp. TP-G1 obtained from the root of Dendrobium officinale Kimura et Migo. Rec. Nat. Prod. 2018, 12, 549–556. [Google Scholar] [CrossRef]
- Grove, J.F.; Pople, M. The insecticidal activity of beauvericin and the enniatin complex. Mycopathologia 1980, 70, 103–105. [Google Scholar] [CrossRef]
- Strongman, D.B.; Strunz, G.M.; Giguère, P.; Yu, C.-M.; Calhoun, L. Enniatins from Fusarium avenaceum isolated from balsam fir foliage and their toxicity to spruce budworm larvae, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). J. Chem. Ecol. 1988, 14, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Meca, G.; Soriano, J.M.; Gaspari, A.; Ritieni, A.; Moretti, A.; Mañes, J. Antifungal effects of the bioactive compounds enniatins A, A1, B, B1. Toxicon 2010, 56, 480–485. [Google Scholar] [CrossRef]
- Meca, G.; Sospedra, I.; Valero, M.A.; Mañes, J.; Font, G.; Ruiz, M.J. Antibacterial activity of the enniatin B, produced by Fusarium tricinctum in liquid culture, and cytotoxic effects on Caco-2 cells. Toxicol. Mech. Methods 2011, 21, 503–512. [Google Scholar] [CrossRef]
- Hikino, H.; Nabetani, S.; Takemoto, T. Structure and biosynthesis of chrysogine, a metabolite of Penicillium chrysogenum. Yakugaku Zasshi 1973, 93, 619–623. [Google Scholar] [CrossRef]
- Niederer, D.; Tamm, C.; Zürcher, W. Nitrogen containing metabolites of Fusarium sambucinum. Tetr. Lett. 1992, 33, 3997–4000. [Google Scholar] [CrossRef]
- Hestbjerg, H.; Nielsen, K.F.; Thrane, U.; Elmholt, S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: An ecological interpretation. J. Agric. Food Chem. 2002, 50, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.L.; Phipps, R.K.; Nielsen, K.F.; Schroers, H.J.; Frank, J.; Thrane, U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 2009, 57, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, R.D.; Saei, W.; Westphal, K.R.; Klitgaard, C.S.; Nielsen, K.L.; Lysøe, E.; Gardiner, D.M.; Wimmer, R.; Sondergaard, T.E.; Sørensen, J.L. Chrysogine biosynthesis is mediated by a two-module nonribosomal peptide synthetase. J. Nat. Prod. 2017, 80, 2131–2135. [Google Scholar] [CrossRef]
- Beccari, G.; Senatore, M.T.; Tini, F.; Sulyok, M.; Covarelli, L. Fungal community, Fusarium head blight complex and secondary metabolites associated with malting barley grains harvested in Umbria, central Italy. Int. J. Food Microbiol. 2018, 273, 33–42. [Google Scholar] [CrossRef]
- Palacios, S.A.; Del Canto, A.; Erazo, J.; Torres, A.M. Fusarium cerealis causing Fusarium head blight of durum wheat and its associated mycotoxins. Int. J. Food Microbiol. 2021, 346, 109161. [Google Scholar] [CrossRef] [PubMed]
- Ayada, H.; Dhioui, B.; Mazouz, H.; El Harrak, A.; Jaiti, F.; Ouhmidou, B.; Diouri, M.; Moumni, M. In silico comparative genomic analysis unravels a new candidate protein arsenal specifically associated with Fusarium oxysporum f. sp. albedinis pathogenesis. Sci. Rep. 2022, 12, 19098. [Google Scholar] [CrossRef]
- Shah, S.P.; Chunduri, J.R. Genome-wide analysis and in silico screening of secondary metabolite potential of endophytic fungi Fusarium multiceps BPAL1 obtained in Mumbai, India. Egypt. J. Basic Appl. Sci. 2023, 10, 812–823. [Google Scholar] [CrossRef]
- Rana, S.; Singh, S.K. Insights into the genomic architecture of a newly discovered endophytic Fusarium species belonging to the Fusarium concolor complex from India. Front. Microbiol. 2023, 14, 1266620. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Akk, E.; Thrane, U.; Giese, H.; Sondergaard, T.E. Production of fusarielins by Fusarium. Int. J. Food Microbiol. 2013, 160, 206–211. [Google Scholar] [CrossRef]
- Hemphill, C.F.P.; Sureechatchaiyan, P.; Kassack, M.U.; Orfali, R.S.; Lin, W.; Daletos, G.; Proksch, P. OSMAC approach leads to new fusarielin metabolites from Fusarium tricinctum. J. Antib. 2017, 70, 726–732. [Google Scholar] [CrossRef]
- Chen, D.; Liu, L.; Lu, Y.; Chen, S. Identification of fusarielin M as a novel inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB). Bioorg. Chem. 2021, 106, 104495. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.A.U.; Sarotti, A.M.; Wu, X.; DeVine, L.; Cao, S. Polyketides, diketopiperazines and an isochromanone from the marine-derived fungal strain Fusarium graminearum FM1010 from Hawaii. Phytochemistry 2022, 198, 113138. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sunaga, R.; Furihata, K.; Morisaki, N.; Iwasaki, S. Isolation and structures of an antifungal antibiotic, fusarielin A, and related compounds produced by a Fusarium sp. J. Antib. 1995, 48, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Zhao, L.L.; Hu, C.Q.; Zhang, H.P. Fusarielin E, a new antifungal antibiotic from Fusarium sp. Chin. Chem. Lett. 2007, 18, 954–956. [Google Scholar] [CrossRef]
- Tchoukoua, A.; Hasegawa, R.; Hendracipta, K.A.; Sato, S.; Koseki, T.; Shiono, Y. Structure elucidation of new fusarielins from Fusarium sp. and their antimicrobial activity. Magn. Reson. Chem. 2018, 56, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.A.; Witte, T.; Robinson, K.A.; Sproule, A.; Foote, S.J.; Johnston, A.; Harris, L.J.; Overy, D.P.; Loewen, M.C. Transcriptomic and exometabolomic profiling reveals antagonistic and defensive modes of Clonostachys rosea action against Fusarium graminearum. Mol. Plant-Microbe Interact. 2020, 33, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Aoyama, H.; Noguchi-Yachide, T.; Hashimoto, Y.; Kobayashi, H. Fusarielin A as an anti-angiogenic and anti-proliferative agent: Basic biological characterization. Chem. Pharm. Bull. 2008, 56, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, T.E.; Klitgaard, L.G.; Purup, S.; Kobayashi, H.; Giese, H.; Sørensen, J.L. Estrogenic effects of fusarielins in human breast cancer cell lines. Toxicol. Lett. 2012, 214, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.N.; Hobbs, B.C.; Raistrick, H. Studies in the biochemistry of micro-organisms. LIII. The crystalline colouring matters of Fusarium culmorum (WG Smith) Sacc and related forms. Biochem. J. 1937, 31, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Bahadoor, A.; Schneiderman, D.; Gemmill, L.; Bosnich, W.; Blackwell, B.; Melanson, J.E.; McRae, G.; Harris, L.J. Hydroxylation of longiborneol by a Clm2-encoded CYP450 monooxygenase to produce culmorin in Fusarium graminearum. J. Nat. Prod. 2016, 79, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, R.; McCormick, S.P.; Proctor, R.H.; Teresi, J.M.; Hao, G.; Ward, T.J.; Alexander, N.; Vaughan, M.M. Synergistic phytotoxic effects of culmorin and trichothecene mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.B.; Miller, J.D. The fungal metabolite culmorin and related compounds. Nat. Toxins 1999, 7, 305–309. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Roscoe, M.; Pleskach, K. A multi-year survey of mycotoxins and ergosterol in Canadian oats. Mycotoxin Res. 2020, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Vaclavikova, M.; Wiesenberger, G.; Haider, M.; Hametner, C.; Fröhlich, J.; Berthiller, F.; Adam, G.; Mikula, H.; Fruhmann, P. Chemical synthesis of culmorin metabolites and their biologic role in culmorin and acetyl-culmorin treated wheat cells. Org. Biomol. Chem. 2018, 16, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Strongman, D.B.; Miller, J.D.; Calhoun, L.; Findlay, J.A.; Whitney, N.J. The biochemical basis for interference competition among some lignicolous marine fungi. Bot. Mar. 1987, 30, 21–26. [Google Scholar] [CrossRef]
- Dowd, P.F.; Miller, J.D.; Greenhalgh, R. Toxicity and interactions of some Fusarium graminearum metabolites to caterpillars. Mycologia 1989, 81, 646–650. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple fungal metabolites including mycotoxins in naturally infected and Fusarium-inoculated wheat samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef]
- Spanic, V.; Maricevic, M.; Ikic, I.; Sulyok, M.; Sarcevic, H. Three-year survey of Fusarium multi-metabolites/mycotoxins contamination in wheat samples in potentially epidemic FHB conditions. Agronomy 2023, 13, 805. [Google Scholar] [CrossRef]
- Lehner, S.M.; Neumann, N.K.N.; Sulyok, M.; Lemmens, M.; Krska, R.; Schuhmacher, R. Evaluation of LC-high-resolution FT-Orbitrap MS for the quantification of selected mycotoxins and the simultaneous screening of fungal metabolites in food. Food Addit. Contam. Part A 2011, 28, 1457–1468. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence 3′–5′ | Reference |
|---|---|---|
| ITS1-F | CTTGGTCATTTAGAGGAAGTAA | [29] |
| ITS4 | TCCTCCGCTTATTGATATGC | [30] |
| bRPB2-6F | TGGGGYATGGTNTGYCCYGC | [31] |
| bRPB2-7R | GAYTGRTTRTGRTCRGGGAAVGG | |
| EF1-1018F | GAYTTCATCAAGAACATGAT | [32] |
| EF1-1620R | GACGTTGAADCCRACRTTGTC | |
| T1 | AACATGCGTGAGATTGTAAGT | [33] |
| Bt2b | ACCCTCAGTGTAGTGACCCTTGGC | [34] |
| Isolate | Species | Origin | tef-1 | rpb2 |
|---|---|---|---|---|
| UBOCC-A-109005 | F. aconidiale | Triticum aestivum, France | MZ078246 | MZ078218 |
| MFLUCC 16-0526 | F. celtidicola | Celtis australis, Italy | ON745620 | ON759296 |
| CPC 27067 | F. citricola | Citrus limon, Italy | LT746194 | LT746307 |
| CPC 27069 | F. citricola | Citrus sinensis, Italy | LT746195 | LT746308 |
| CPC 27709 | F. citricola | C. sinensis, Italy | LT746196 | LT746309 |
| CPC 27805 | F. citricola | Citrus reticulata, Italy | LT746197 | LT746310 |
| CPC 27813 | F. citricola | C. reticulata, Italy | LT746198 | LT746311 |
| UBOCC-A-101147 | F. juglandicola | Juglans regia, France | MZ078244 | MZ078216 |
| UBOCC-A-102014 | F. juglandicola | J. regia, France | MZ078245 | MZ078217 |
| UBOCC-A-119001 | F. juglandicola | J. regia, France | MZ078243 | MZ078215 |
| NRRL 13622 | F. lateritium | Ulmus americana, USA | AY707173 | JX171571 |
| CPC 26403 | F. salinense | C. sinensis, Italy | LT746191 | LT746304 |
| CPC 26457 | F. salinense | C. sinensis, Italy | LT746192 | LT746305 |
| CPC 26973 | F. salinense | C. sinensis, Italy | LT746193 | LT746306 |
| F1544 | F. tricinctum | Triticum turgidum, Italy | OL964791 | OL658768 |
| LC0453 | F. tricinctum | Hosta sp., China | MW620151 | MW474676 |
| NRRL 25481 | F. tricinctum | T. aestivum, Germany | OL772833 | MH582357 |
| P325b | F. tricinctum | T. turgidum, Italy | OL658799 | OL658796 |
| IHEM 28077 | Fusarium sp. | bat, Belgium | OU641411 | OU641410 |
| ZLVG.982 | Fusarium sp. | Pinus sylvestris, Slovenia | OR105858 | OR098304 |
| ITS | tef-1 | rpb2 | Tub | |
|---|---|---|---|---|
| Hzn1 | OP699807 F. juglandicola id 99.82, qc 99% | OP715604 F. juglandicola MZ191070 F. lateritium * id 99.24, qc 99% | ON759296 F. celtidicola id 99.73, qc 99% | |
| Hzn5 | OP699807 F. juglandicola id 99.82, qc 99% | OP715604 F. juglandicola MZ191070 F. lateritium * id 99.24, qc 99% | ON759296 F. celtidicola OL690434 F. juglandicola MZ078218 F. aconidiale id 99.86, qc 99% | MZ191071 F. lateritium * id 100, qc 96% |
| Compound | Formula | Monoisotopic Mass (MW) | Hzn1 | Hzn5 | ||
|---|---|---|---|---|---|---|
| CDB | MEB | CDB | MEB | |||
| Enniatin A/C/F * | C36H63N3O9 | 681.456430 | + | + | + | + |
| Enniatin A1/E/G/I/O1/O2/O3 * | C35H61N3O9 | 667.440780 | + | + | + | + |
| Enniatin B | C33H57N3O9 | 639.409480 | + | + | + | + |
| Enniatin B1/B4/D/H * | C34H59N3O9 | 653.425130 | + | + | + | + |
| Enniatin B2/B3/J2/J3/K1 * | C32H55N3O9 | 625.393830 | + | + | + | + |
| Enniatin J1 | C31H53N3O9 | 611.378180 | + | + | + | + |
| Enniatin L/P1 * | C34H59N3O10 | 669.420045 | + | + | + | + |
| Enniatin P2 | C33H57N3O10 | 655.404395 | + | + | + | + |
| Chrysogine | C10H10N2O2 | 190.074227 | + | − | + | − |
| Fusarielin A | C25H38O4 | 402.277009 | + | + | + | + |
| Fusarielin B | C25H40O5 | 420.287574 | + | − | + | + |
| Fusarielin D/G * | C25H36O4 | 400.261359 | − | − | + | − |
| Fusarielin E | C25H39ClO4 | 438.253687 | + | − | − | − |
| Fusarielin F | C25H36O5 | 416.256274 | − | + | + | + |
| Fusarielin M | C25H36O3 | 384.266445 | + | + | + | + |
| Culmorin | C15H26O2 | 238.193280 | + | + | + | + |
| Longiborneol | C15H26O | 222.198365 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimowska, B.; Ludwiczuk, A.; Manganiello, G.; Wojtanowski, K.; Kot, I.; Staropoli, A.; Vinale, F.; Nicoletti, R. Fusarium and Hazelnut: A Story of Twists and Turns. Agriculture 2024, 14, 1080. https://doi.org/10.3390/agriculture14071080
Zimowska B, Ludwiczuk A, Manganiello G, Wojtanowski K, Kot I, Staropoli A, Vinale F, Nicoletti R. Fusarium and Hazelnut: A Story of Twists and Turns. Agriculture. 2024; 14(7):1080. https://doi.org/10.3390/agriculture14071080
Chicago/Turabian StyleZimowska, Beata, Agnieszka Ludwiczuk, Gelsomina Manganiello, Krzysztof Wojtanowski, Izabela Kot, Alessia Staropoli, Francesco Vinale, and Rosario Nicoletti. 2024. "Fusarium and Hazelnut: A Story of Twists and Turns" Agriculture 14, no. 7: 1080. https://doi.org/10.3390/agriculture14071080







