Social Behaviour in Lambs (Ovis aries) Reared under an Intensive System during the Prepuberty Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Period, Measurements, and Samplings
2.3. Behavioural Observations
2.4. Plasma Sample Preparation and Cortisol Analysis
2.5. Wool Sample Preparation and Cortisol Analysis
2.6. Statistical Analysis
2.7. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Behaviours | Description |
|---|---|
| Maintenance | |
| standing | Standing still, sporadically moving the head |
| lying | Resting with eyes open or closed |
| head hanging | Standing quietly with head hanging |
| foraging | Head inside the feeder trough |
| walking | Shifting from one place to another |
| running | Going from one place to another on a faster pace |
| Agonistic | |
| clash | Two individuals facing each other move backwards and then lunge forward and hit each other head to head |
| threats | Turns towards or approaches another individual with his head down and then lunges without making contact |
| butt | Uses the front of his head to make contact with another individual |
| push | Uses other parts of the body except the head to make contact with another individual |
| Submissive | |
| avoidance | Actively moves away from another individual as a result of a previous agonistic interaction |
| Affiliative | |
| grooming | Grooms another individual’s body using the teeth |
| resting chin | Puts the chin on another individual’s rump or back |
| face contact | Places the nose near a recipient’s facial region and holds the position while smelling or rubbing the recipient’s face or head |
References
- Lawrence, A.B. Mother-daughter and peer relationships of Scottish hill sheep. Anim. Behav. 1990, 39, 481–486. [Google Scholar] [CrossRef]
- Lidfors, L.M. Living in Groups. In Encyclopedia of Evolutionary Psychological Science; Shackelford, T.K., Weekes-Shackelford, V.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 4606–4615. [Google Scholar] [CrossRef]
- Amici, F.; Widdig, A. An evolutionary perspective on the development of primate sociality. Behav. Ecol. Sociobiol. 2019, 73, 116. [Google Scholar] [CrossRef]
- Sosa, S. The influence of gender, age, matriline and hierarchical rank on individual social position, role and interactional patterns in Macaca sylvanus at ‘La Forêt des singes’: A multilevel social network approach. Front. Psychol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Verspeek, J.; Staes, N.; van Leeuwen, E.J.C.; Eens, M.; Stevens, J.M.G. Bonobo personality predicts friendship. Sci. Rep. 2019, 9, 19245. [Google Scholar] [CrossRef]
- Silk, J.B.; Beehner, J.C.; Bergman, T.J.; Crockford, C.; Engh, A.L.; Moscovice, L.R.; Wittig, R.M.; Seyfarth, R.M.; Cheney, D.L. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010, 20, 1359–1361. [Google Scholar] [CrossRef]
- Silk, J.B.; Beehner, J.C.; Bergman, T.J.; Crockford, C.; Engh, A.L.; Moscovice, L.R.; Wittig, R.M.; Seyfarth, R.M.; Cheney, D.L. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B Boil. Sci. 2009, 276, 3099–3104. [Google Scholar] [CrossRef]
- Cameron, E.Z.; Setsaas, T.H.; Linklater, W.L. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl. Acad. Sci. USA 2009, 106, 13850–13853. [Google Scholar] [CrossRef]
- Silk, J.B. The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 539–559. [Google Scholar] [CrossRef]
- House, J.S.; Umberson, D.; Landis, K.R. Structure and processes of social support. Annu. Rev. Sociol. 1988, 14, 293–318. [Google Scholar] [CrossRef]
- Seeman, T.E.; McEwen, B.S. Impact of social environment characteristics on neuroendocrine regulation. Psychosom. Med. 1996, 58, 459–471. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Alberts, S.C.; Altmann, J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch. Gen. Psychiatry 1997, 54, 1137–1143. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Häffelin, K.; Lindenwald, R.; Kaufmann, F.; Döhring, S.; Spindler, B.; Preisinger, R.; Rautenschlein, S.; Kemper, N.; Andersson, R. Corticosterone in feathers of laying hens: An assay validation for evidence-based assessment of animal welfare. Poult. Sci. 2020, 99, 4685–4694. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Reed, J.M. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Beuving, G.; Vonder, G. Effect of stressing factors on corticosterone levels in the plasma of laying hens. Gen. Comp. Endocrinol. 1978, 35, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, G.; Webster, D.; Narayan, E. Measuring wool cortisol and progesterone levels in breeding maiden Australian merino sheep (Ovis aries). PLoS ONE 2019, 14, e0214734. [Google Scholar] [CrossRef]
- Meyer, J.; Novak, M.; Hamel, A.; Rosenberg, K. Extraction and analysis of cortisol from human and monkey hair. J. Vis. Exp. 2014, 83, e50882. [Google Scholar] [CrossRef]
- Ullmann, E.; Barthel, A.; Petrowski, K.; Stalder, T.; Kirschbaum, C.; Bornstein, S.R. Pilot study of adrenal steroid hormones in hair as an indicator of chronic mental and physical stress. Sci. Rep. 2016, 6, 25842. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, K.; Laliotis, G.P.; Koutsouli, P.; Pafilis, P.; Bizelis, I. Do sheep (Ovis aries) perform third-party interventions? Ethology 2022, 128, 657–667. [Google Scholar] [CrossRef]
- Pereira, M.E.; Fairbanks, L.A. Foreword 2002: Familly, Firends, and the Evolution of Cchildhood. In Juvenile Primates: Life History, Development and Behavior, with a New Foreword; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Jensen, M.B. The role of social behavior in cattle welfare. In Advances in Cattle Welfare; Woodhead Publishing Series in Food Science, Technology and Nutrition; Tucker, C.B., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 123–155. [Google Scholar] [CrossRef]
- van Schaik, C.; de Visser, J. Fragile sons or harassed daughters? Sex differences in mortality among juvenile primates. Folia Primatol. 1990, 55, 10–23. [Google Scholar] [CrossRef]
- Estevez, I.; Andersen, I.-L.; Nævdal, E. Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 2007, 103, 185–204. [Google Scholar] [CrossRef]
- Veissier, I.; Boissy, A.; Nowak, R.; Orgeur, P.; Poindron, P. Ontogeny of social awareness in domestic herbivores. Appl. Anim. Behav. Sci. 1998, 57, 233–245. [Google Scholar] [CrossRef]
- Fisher, A.; Matthews, L. The social behaviour of sheep. In Social Behaviour in Farm Animals; CAB International: Wallingford, UK, 2001; pp. 211–245. [Google Scholar]
- Lawrence, A.B.; Wood-Gush, D.G.M. Home-range behaviour and social organization of Scottish blackface sheep. J. Appl. Ecol. 1988, 25, 25–40. [Google Scholar] [CrossRef]
- Keller, M.; Cornilleau, F.; Archer, E.; Lévy, F. Development of social familiarity in ewes. Physiol. Behav. 2011, 104, 392–397. [Google Scholar] [CrossRef]
- Norton, E.; Benaben, S.; Mbotha, D.; Schley, D. Seasonal variations in physical contact amongst domestic sheep and the implications for disease transmission. Livest. Sci. 2012, 145, 34–43. [Google Scholar] [CrossRef]
- Doyle, R.E.; Broster, J.C.; Barnes, K.; Browne, W.J. Temperament, age and weather predict social interaction in the sheep flock. Behav. Process. 2016, 131, 53–58. [Google Scholar] [CrossRef]
- Michelena, P.; Sibbald, A.M.; Erhard, H.W.; McLeod, J.E. Effects of group size and personality on social foraging: The distribution of sheep across patches. Behav. Ecol. 2008, 20, 145–152. [Google Scholar] [CrossRef]
- Jørgensen, G.H.M.; Andersen, I.L.; Berg, S.; Bøe, K.E. Feeding, resting and social behaviour in ewes housed in two different group sizes. Appl. Anim. Behav. Sci. 2009, 116, 198–203. [Google Scholar] [CrossRef]
- Broster, J.C.; Dehaan, R.L.; Swain, D.L.; Friend, M.A. Ewe and lamb contact at lambing is influenced by both shelter type and birth number. Animal 2010, 4, 796–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miranda-de la Lama, G.M.; Villarroel, M.; María, G. Behavioural and physiological profiles following exposure to novel environment and social mixing in lambs. Small Rumin. Res. 2012, 103, 158–163. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.M.; Mattiello, S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Kiełtyka-Kurc, A.; Górecki, M.T. Social behavior in preweaning lambs and their preferences in social interactions. Anim. Sci. J. 2015, 86, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Berger, J. The ecology, structure and functions of social play in Bighorn sheep (Ovis canadensis). J. Zool. 1980, 192, 531–542. [Google Scholar] [CrossRef]
- Napolitano, F.; Braghieri, A.; Cifuni, G.; Pacelli, C.; Girolami, A. Behaviour and meat production of organically farmed unweaned lambs. Small Rumin. Res. 2002, 43, 179–184. [Google Scholar] [CrossRef]
- Andanson, S.; Boissy, A.; Veissier, I. Conditions for assessing cortisol in sheep: The total form in blood v. the free form in saliva. Animal 2020, 14, 1916–1922. [Google Scholar] [CrossRef]
- Papadaki, K.; Laliotis, G.P.; Koutsouli, P.; Bizelis, I. Association of personality traits with maintenance and social behaviour of rams (Ovis aries). Small Rumin. Res. 2023, 220, 106928. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Pascual-Alonso, M.; Aguayo-Ulloa, L.; Sepúlveda, W.S.; Villarroel, M.; María, G.A. Social personality in sheep: Can social strategies predict individual differences in cognitive abilities, morphology features, and reproductive success? J. Vet. Behav. 2019, 31, 82–91. [Google Scholar] [CrossRef]
- Done-Currie, J.; Hecker, J.; Wodzicka-Tomaszewska, M. Behaviour of sheep transferred from pasture to an animal house. Appl. Anim. Behav. Sci. 1984, 12, 121–130. [Google Scholar] [CrossRef]
- Hass, C.C. Social status in female bighorn sheep (Ovis canadensis): Expression, development and reproductive correlates. J. Zool. 1991, 225, 509–523. [Google Scholar] [CrossRef]
- Odagiri, K.; Matsuzawa, Y.; Yoshikawa, Y. Analysis of sexual behavior in rams (Ovis aries). Exp. Anim. 1995, 44, 187–192. [Google Scholar] [CrossRef]
- Powell, D.; Speeg, B.; Li, S.; Blumer, E.; McShea, W. An ethogram and activity budget of captive Sichuan takin (Budorcas taxicolor tibetana) with comparisons to other Bovidae. Mammalia 2013, 77, 391–401. [Google Scholar] [CrossRef]
- Papadaki, K.; Laliotis, G.P.; Bizelis, I. Acoustic variables of high-pitched vocalizations in dairy sheep breeds. Appl. Anim. Behav. Sci. 2021, 241, 105398. [Google Scholar] [CrossRef]
- Nejad, J.G.; Lohakare, J.; Son, J.; Kwon, E.; West, J.; Sung, K. Wool cortisol is a better indicator of stress than blood cortisol in ewes exposed to heat stress and water restriction. Animal 2014, 8, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Gardela, J.; Carbajal, A.; Tallo-Parra, O.; Olvera-Maneu, S.; Álvarez-Rodríguez, M.; Jose-Cunilleras, E.; López-Béjar, M. Temporary relocation during rest periods: Relocation stress and other factors influence hair cortisol concentrations in horses. Animals 2020, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019.
- Bracke, M.; Spruijt, B.; Metz, J. Overall animal welfare assessment reviewed. Part 1: Is it possible? Neth. J. Agric. Sci. 1999, 47, 279–291. [Google Scholar] [CrossRef]
- Bizelis, J.; Deligeorgis, S.; Rogdakis, E. Puberty attainment and reproductive characteristics in ewe lambs of Chios and Karagouniki breeds raised on two planes of nutrition. Anim. Reprod. Sci. 1990, 23, 197–212. [Google Scholar] [CrossRef]
- Oliveira, A.F.S.; Rossi, A.O.; Silva, L.F.R.; Lau, M.C.; Barreto, R.E. Play behaviour in nonhuman animals and the animal welfare issue. J. Ethol. 2010, 28, 1–5. [Google Scholar] [CrossRef]
- Vieira, M.L.; Sartorio, R. Motivational, causal and functional analysis of play behavior in two rodent species. Estud. Psicol. 2002, 7, 189–196. [Google Scholar] [CrossRef]
- Lickliter, R.E. Activity patterns and companion preferences of domestic goat kids. Appl. Anim. Behav. Sci. 1987, 19, 137–145. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Lawrence, A.B. A review of the behavioural and physiological adaptations of hill and lowland breeds of sheep that favour lamb survival. Appl. Anim. Behav. Sci. Int. Soc. Appl. Ethonolgy Spec. Issue 2005, 92, 235–260. [Google Scholar] [CrossRef]
- Hall, S.J.G.; Kirkpatrick, S.M.; Broom, D.M. Behavioural and physiological responses of sheep of different breeds to supplementary feeding, social mixing and taming, in the context of transport. Anim. Sci. 1998, 67, 475–483. [Google Scholar] [CrossRef]
- Briefer, E.F.; McElligott, A.G. Social effects on vocal ontogeny in an ungulate, the goat, Capra hircus. Anim. Behav. 2012, 83, 991–1000. [Google Scholar] [CrossRef]
| Variables | Period A (2.5–4 Months of Age) n = 21 | Period B (4–5.5 Months of Age) n = 21 | Period C (5.5–7 Months of Age) n = 21 | Significance (p Value) |
|---|---|---|---|---|
| Social behaviour | ||||
| Actor agonistic (frequency) | 11 × 10−4 ± 2 × 10−4 a | 5 × 10−4 ± 1 × 10−4 b | 8 × 10−4± 1 × 10−4 b | p = 0.006 |
| Actor affiliative (frequency) | 21 × 10−4 ± 3 × 10−4 a | 8 × 10−4 ± 1 × 10−4 b | 5 × 10−4 ± 1 × 10−4 b | p < 0.001 |
| Actor submissive (frequency) | 6 × 10−5 ± 2 × 10−5 | 0 × 10−5 ± 0 × 10−5 | 2 × 10−5 ± 1 × 10−5 | NS |
| Receiver agonistic (frequency) | 836 × 10−4 ± 20 × 10−4 a | 5 × 10−4 ± 0 × 10−4 b | 9 × 10−4 ± 1 × 10−4 b | p < 0.001 |
| Receiver affiliative (frequency) | 150 × 10−4 ± 22 × 10−4 a | 7 × 10−4 ± 1 × 10−4 b | 5 × 10−4 ± 1 × 10−4 b | p < 0.001 |
| Receiver submissive (frequency) | 0 × 10−5 ± 0 × 10−5 | 0 × 10−5 ± 0 × 10−5 | 1 × 10−5 ± 1 × 10−5 | NS |
| Growth rates | ||||
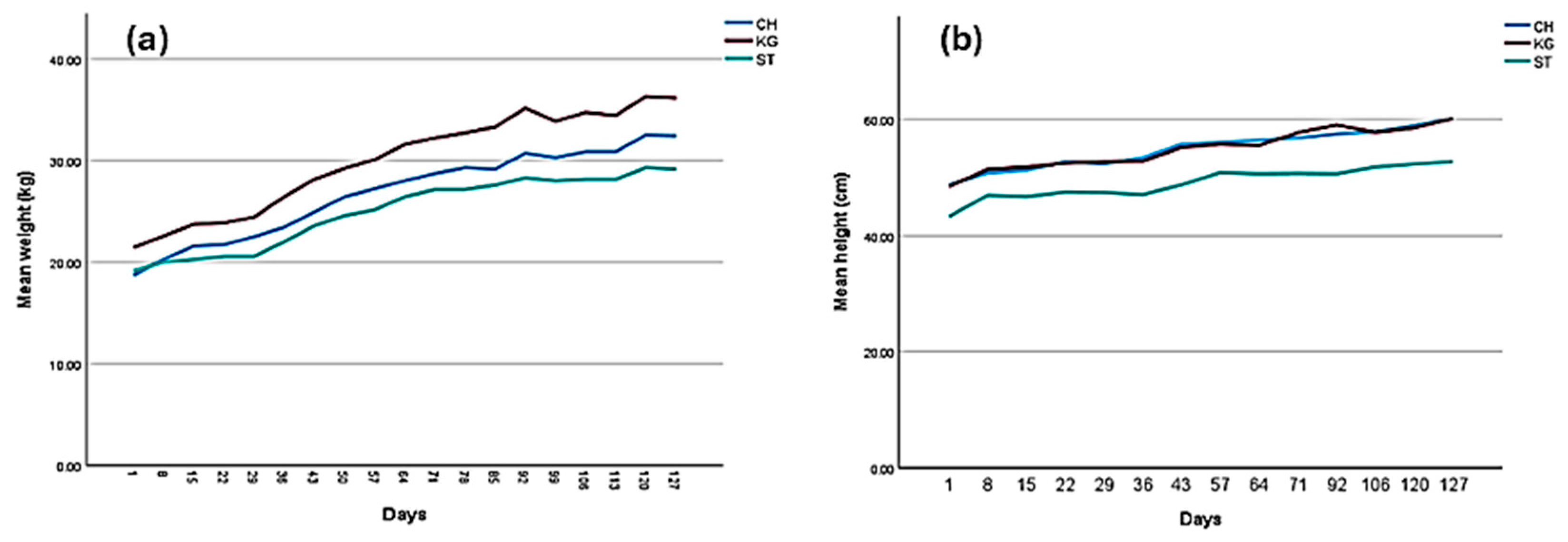
| Average Daily Gain (ADG; kg/day) | 0.137 ± 0.009 a | 0.106 ± 0.008 a | 0.064 ± 0.007 b | p = 0.001 |
| Height growth/day (cm/day) | 0.151 ± 0.010 a | 0.051 ± 0.008 b | 0.055 ± 0.010 b | p < 0.001 |
| Cortisol levels | ||||
| Blood cortisol (ng/mL) | 3.391 ± 0.517 | 2.966 ± 0.626 | 3.089 ± 0.892 | NS |
| Wool cortisol (pg/mg) | 7.899 ± 1.195 a | 15.831 ± 2.350 b | 23.398 ± 5.344 b | p = 0.002 |
| Variables | CH n =21 | KG n =21 | ST n =21 | Significance (p Value) |
|---|---|---|---|---|
| Maintenance behaviours | ||||
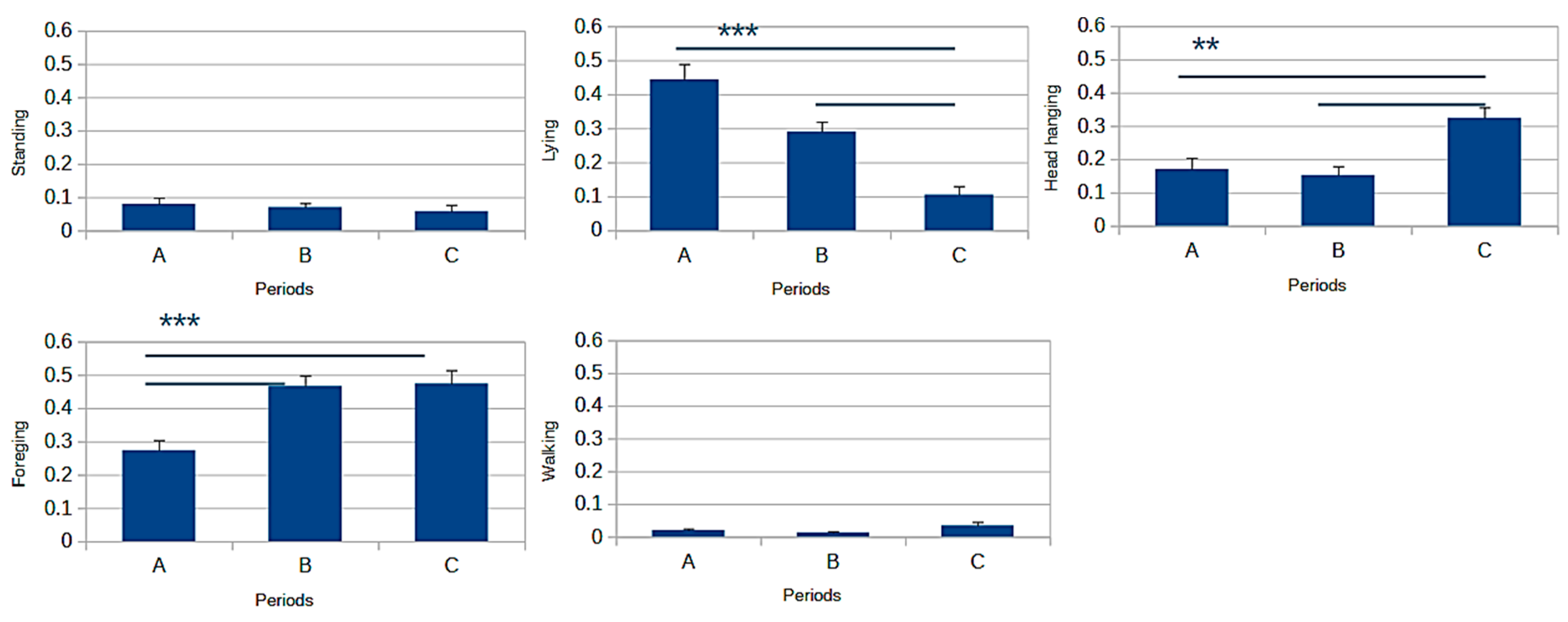
| Standing (frequency) | 0.031 ± 0.012 a | 0.104 ± 0.018 b | 0.072 ± 0.015 a,b | 0.001 |
| Lying (frequency) | 0.260 ± 0.033 | 0.319 ± 0.059 | 0.261 ± 0.040 | NS |
| Head hanging (frequency) | 0.255 ± 0.035 | 0.162 ± 0.037 | 0.231 ± 0.029 | NS |
| Foraging (frequency) | 0.420 ± 0.029 | 0.378 ± 0.047 | 0.418 ± 0.041 | NS |
| Walking (frequency) | 0.022 ± 0.007 | 0.031 ± 0.009 | 0.014 ± 0.005 | NS |
| Running (frequency) | N/A | N/A | N/A | N/A |
| Social behaviour | ||||
| Actor agonistic (frequency) | 9 × 10−4 ± 1 × 10−4 | 10 × 10−4 ± 2 × 10−4 | 6 × 10−4 ± 1 × 10−4 | NS |
| Actor affiliative (frequency) | 12 × 10−4 ± 1 × 10−4 a,b | 16 × 10−4 ± 3 × 10−4 a | 6 × 10−4 ± 1 × 10−4 b | 0.011 |
| Actor submissive (frequency) | 2 × 10−5 ± 1 × 10−5 | 2 × 10−5 ± 1 × 10−5 | 3 × 10−5 ± 1 × 10−5 | NS |
| Receiver agonistic (frequency) | 457 × 10−4 ± 210 × 10−4 | 148 × 10−4 ± 76 × 10−4 | 246 × 10−4 ± 107 × 10−4 | NS |
| Receiver affiliative (frequency) | 61 × 10−4 ± 22 × 10−4 | 54 × 10−4 ± 20 × 10−4 | 47 × 10−4 ± 17 × 10−4 | NS |
| Receiver submissive (frequency) | 0 × 10−5 ± 0 × 10−5 | 0.6 × 10−5 ± 1 × 10−5 | 0.8 × 10−5 ± 1 × 10−5 | NS |
| Growth rates | ||||
| Average Daily Gain (ADG; kg/day) | 0.108 ± 0.009 a | 0.119 ± 0.011 a | 0.080 ± 0.009 b | 0.037 |
| Height growth/day (cm/day) | 0.092 ± 0.014 | 0.091 ± 0.015 | 0.074 ± 0.011 | NS |
| Cortisol levels | ||||
| Blood cortisol (ng/mL) | 1.805 ± 0.417 a | 2.851 ± 0.442 a,b | 4.789 ± 0.928 b | 0.029 |
| Wool cortisol (pg/mg) | 10.861 ± 2.385 | 17.328 ± 3.056 | 18.939 ± 4.966 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, K.; Samaras, A.; Pavlidis, M.; Bizelis, I.; Laliotis, G.P. Social Behaviour in Lambs (Ovis aries) Reared under an Intensive System during the Prepuberty Period. Agriculture 2024, 14, 1089. https://doi.org/10.3390/agriculture14071089
Papadaki K, Samaras A, Pavlidis M, Bizelis I, Laliotis GP. Social Behaviour in Lambs (Ovis aries) Reared under an Intensive System during the Prepuberty Period. Agriculture. 2024; 14(7):1089. https://doi.org/10.3390/agriculture14071089
Chicago/Turabian StylePapadaki, Kallirroi, Athanasios Samaras, Michail Pavlidis, Iosif Bizelis, and George P. Laliotis. 2024. "Social Behaviour in Lambs (Ovis aries) Reared under an Intensive System during the Prepuberty Period" Agriculture 14, no. 7: 1089. https://doi.org/10.3390/agriculture14071089
APA StylePapadaki, K., Samaras, A., Pavlidis, M., Bizelis, I., & Laliotis, G. P. (2024). Social Behaviour in Lambs (Ovis aries) Reared under an Intensive System during the Prepuberty Period. Agriculture, 14(7), 1089. https://doi.org/10.3390/agriculture14071089





