Incidence of Photosensitization in Husbandry Animals: A Meta-Study on the Effects of Feed Diversity and Feed Choice
Abstract
1. Introduction
2. Materials and Methods
2.1. Search for Case Reports
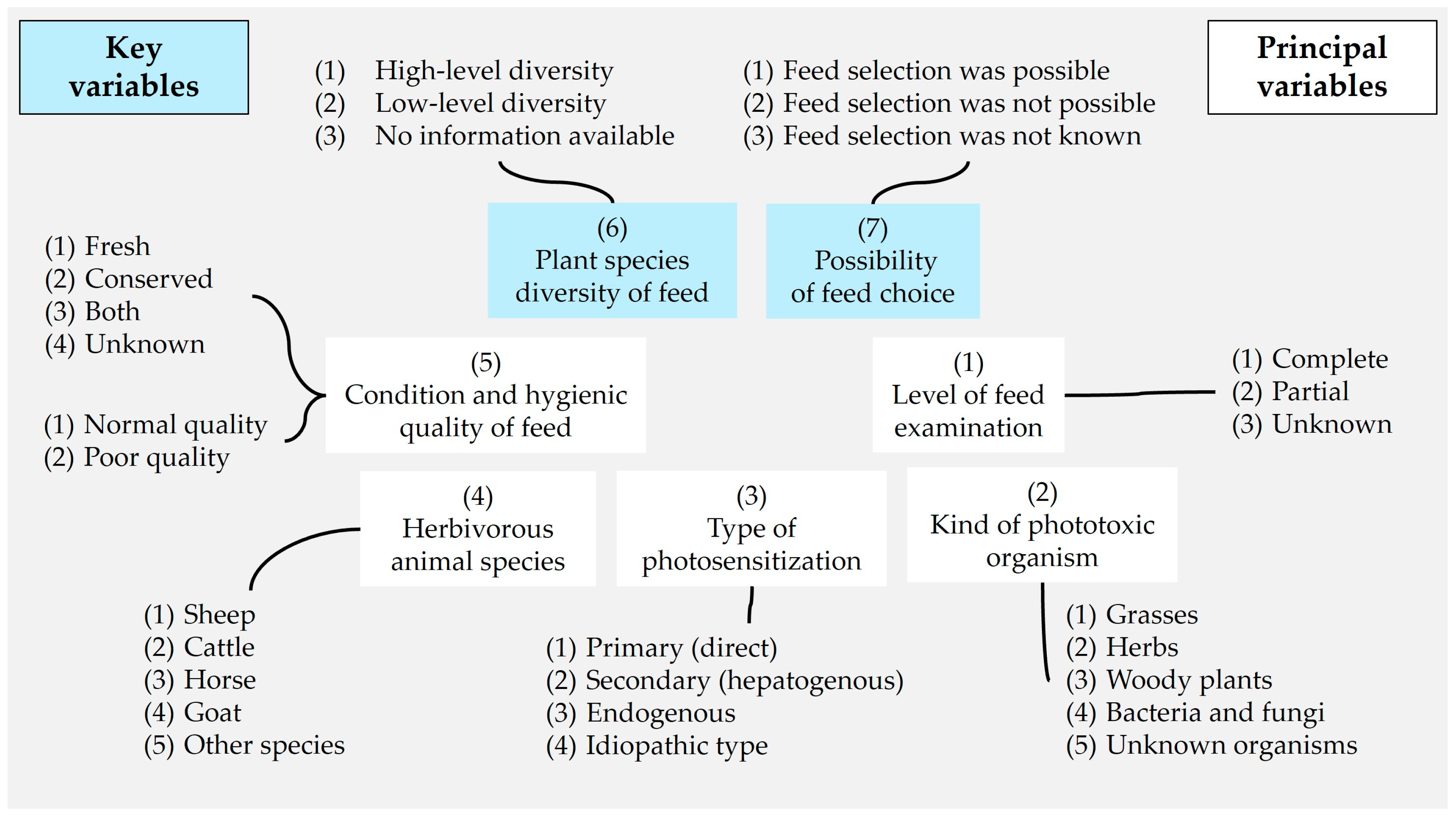
2.2. Variables of Evaluation Case Reports
2.2.1. Level of Feed Examination
2.2.2. Kind of Phototoxic Organism
2.2.3. Type of Photosensitization
2.2.4. Animal Species
2.2.5. Kind and Quality of Feed
2.2.6. Plant Species Diversity
2.2.7. Possibility of Feed Choice
2.3. Data Analysis
3. Results
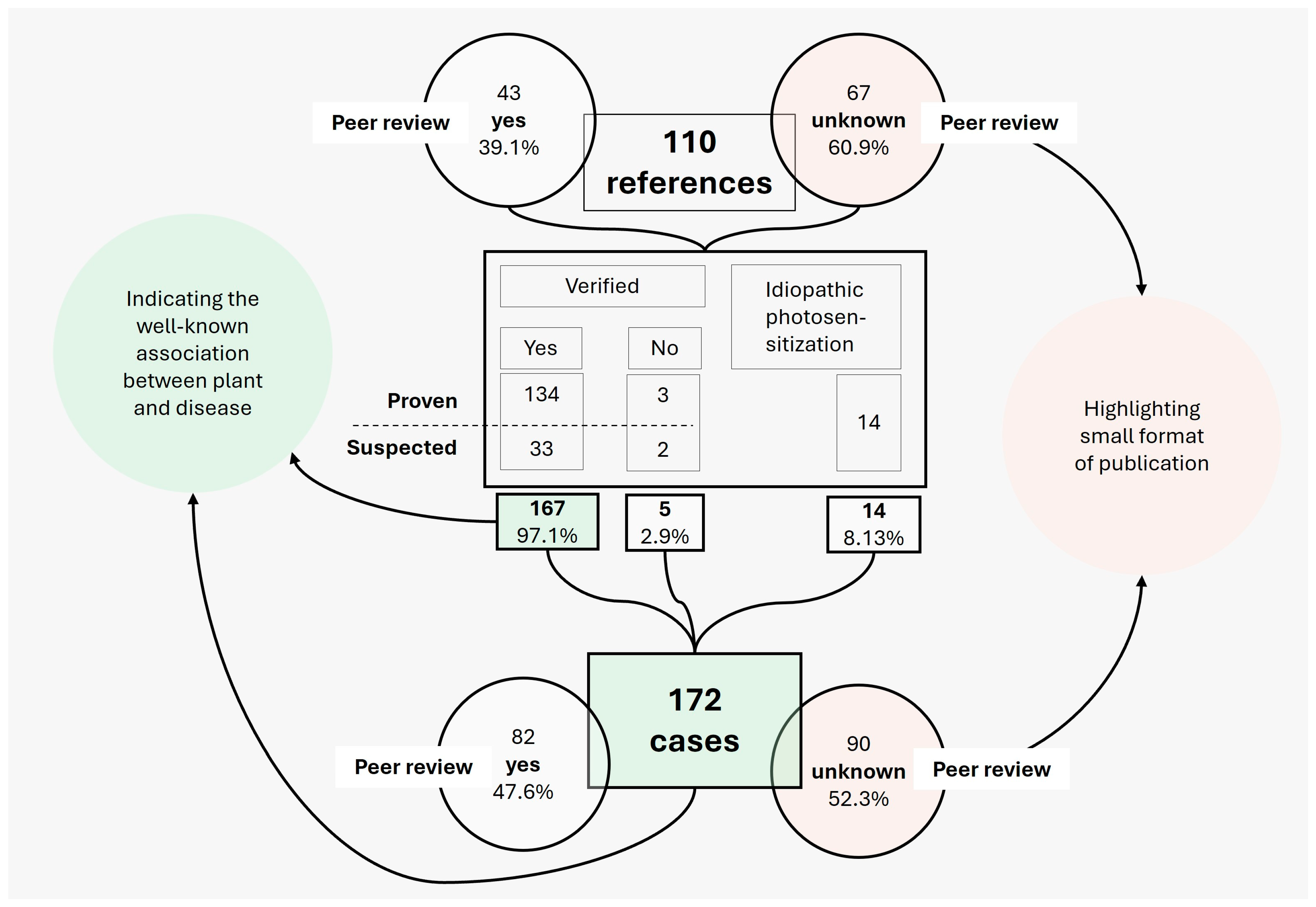
3.1. Overview of All Variables
3.2. Botanical Data Basis
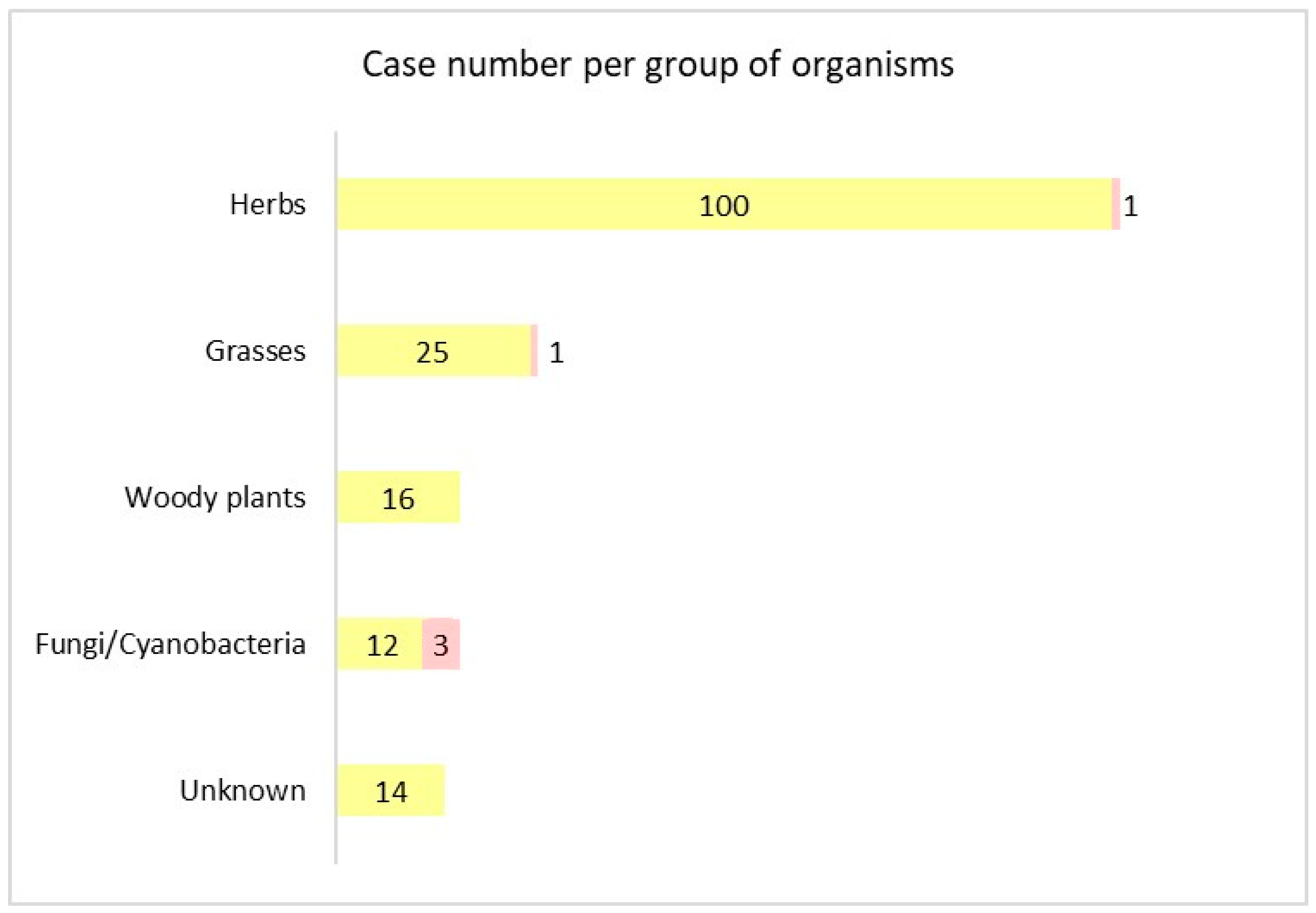
3.3. Phototoxic Organisms
3.4. Animal Species, General Feed Characteristics, and Type of Photosensitization
3.5. Feed Diversity and Feed Choice
4. Discussion
4.1. Reliability of Meta-Data
4.2. Impact of Feed Diversity and Feed Choice on the Incidence of Photosensitization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Reference List of Case Reports Sorted by Animal Species
- Cattle
- Bourke, C.A.; Rayward, D. Photosensitisation in dairy cattle grazing alligator weed (Alternanthera philoxeroides) infested pastures. Aust. Vet. J. 2003, 81, 361–362, doi:10.1111/j.1751-0813.2003.tb11515.x.
- Cardona-Álvarez, J.; Vargas-Vilória, M.; Paredes-Herbach, E. Clinical and histopathological study of the phototoxic dermatitis in Zebu calves in grazing of Brachiaria decumbens. Revista MVZ Córdoba 2016, 21, 5366–5380, doi:10.21897/rmvz.603.
- Casteel, S.W.; Weaver, A.D.; Mills, L.L.; Pace, L.W.; Rottinghaus, G.E.; Smith, K.M. Photosensitization outbreak in shorthorn calves in Missouri. J. Vet. Diagn. Invest. 1991, 3, 180–182, doi:10.1177/104063879100300218.
- Collett, M.G.; Thompson, K.G.; Christie, R.J. Photosensitisation, crystal-associated cholangiohepatopathy, and acute renal tubular necrosis in calves following ingestion of Phytolacca octandra (inkweed). N. Z. Vet. J. 2011, 59, 147–152, doi:10.1080/00480169.2011.567966.
- Collett, M.G. Bile duct lesions associated with turnip (Brassica rapa) photosensitization compared with those due to sporidesmin toxicosis in dairy cows. Vet. Pathol. 2014, 51, 986–991, doi:10.1177/0300985813513042.
- M. G. Collett; Z. Matthews. Photosensitivity in Cattle Grazing Brassica Crops. Institute of Veterinary, Animal, and Biomedical Sciences, Massey University, Palmerston North, New Zealand 2015.
- Davis, A.J.; Collett, M.G.; Steyl, J.C.A.; Myburgh, J.G. Hepatogenous photosensitisation in cows grazing turnips (Brassica rapa) in South Africa. J. S. Afr. Vet. Assoc. 2021, 92, e1-e6, doi:10.4102/jsava.v92i0.2106.
- Del Méndez, M.C.; Riet-Correa, F.; Schild, A.L.; Ferreira, J.L.; Pimentel, M.A. Fotossensibilizaçäo em bovinos causada por Ammi majus (Umbelliferae) no Rio Grande do Sul. Pesqui. vet. bras 1991, 17–19.
- Dorsch, M.A.; Cantón, G.J.; Odriozola, E.R. Fotosensibilización primaria por consumo de Ammi majus en bovinos: primer reporte en Argentina. Rev. vet. 2018, 29, 137, doi:10.30972/vet.2923280.
- Eyged, M.N.; Shlosberg, A.; Eilat, A.; Cohen, U.; Beemer, A. Photosensitization in dairy cattle associated with the ingestion of Ammi majus. Refuah Veterinarith 1974, 31.
- Glenn, B.L.; Monlux, A.W.; Panciera, R.J. A Hepatogenous Photosensitivity Disease of Cattle: I. Experimental Production and Clinical Aspects of the Disease. Pathologia veterinaria 1964, 1, 469–484, doi:10.1177/030098586400100601.
- Giaretta, P.R.; Panziera, W.; Galiza, G.J.; Brum, J.S.; Bianchi, R.M.; Hammerschmitt, M.E.; Bazzi, T.; Barros, C.S. Seneciosis in cattle associated with photosensitization. Pesq. Vet. Bras. 2014, 34, 427–432, doi:10.1590/S0100-736X2014000500007.
- Grecco, F.B.; Dantas, A.F.M.; Riet-Correa, F.; Leite, C.G.D.; Raposo, J.B. Cattle intoxication from Enterolobium contortisiliquum pods. Vet. Hum. Toxicol. 2002, 44, 160–162.
- Horn, G.A.; Burrows, G.E. Primary photosensitization in cattle. Vet. Hum. Toxicol. 1990, 32, 331–332.
- House, J.K.; George, L.W.; Oslund, K.L.; Galey, F.D.; Stannard, A.W.; Koch, L.M. Primary photosensitization related to ingestion of alfalfa silage by cattle. J. Am. Vet. Med. Assoc. 1996, 209, 1604–1607.
- Jerrett, I.V.; Chinnock, R.J. Outbreaks of photosensitisation and deaths in cattle due to Myoporum aff. Insulare R. Br. toxicity. Aust. Vet. J. 1983, 60, 183–186, doi:10.1111/j.1751-0813.1983.tb05959.x.
- Jesse, F.; Ramanoon, S. Hepatogenous Photosensitization in Cattle- A case Report. Vet World 2012, 5, 764, doi:10.5455/vetworld.2012.764-766.
- Kelch, W.J.; Kerr, L.A.; Adair, H.S.; Boyd, G.D. Suspected buttercup (Ranunculus bulbosus) toxicosis with secondary photosensitization in a Charolais heifer. Veterinary and human toxicology (USA) 1992.
- Lazaro, N.D.; Bacha, F.B.; Pupin, R.C.; Paula, J.P.L. de; Leal, P.V.; Pott, A.; Gomes, D.C.; Lemos, R.A.A. de. Photosensitization in Cattle Caused by Spontaneous and Experimentally Ingestion of Stryphnodendron fissuratum. Acta Scientiae. Vet. 2018, 46, 8, doi:10.22456/1679-9216.81829.
- Lugton, I.W.; Woolacott, J. Liver necrosis and photosensitisation in cattle after eating Persicaria lapathifolia (pale knotweed) and Persicaria orientalis (Prince’s feather). Aust. Vet. J. 2014, 92, 62–64, doi:10.1111/avj.12148.
- McKenzie, R.A.; Dunster, P.J.; Burchill, J.C. Smartweeds (Polygonum spp) and photosensitisation of cattle. Aust. Vet. J. 1988, 65, 128, doi:10.1111/j.1751-0813.1988.tb14432.x.
- Mendonça, F.; Evencio-Neto, J.; Baratella-Evêncio, L.; Dória, R.; Freitas, S.H.; Pelegrini, L.; Cruz, R.; Ferreira, E.; Colodel, E. Natural and Experimental Poisoning of Cattle by Enterolobium contortisiliquum Pods (Fabaceae Mimosoideae) in Central-Western Brazil. Acta Veterinaria Brno - ACTA VET BRNO 2009, 78, 621–625, doi:10.2754/avb200978040621.
- Rocha E Silva, M. Photosensitization in Cattle, caused by Holocalyx glaziovii. Arq. Inst. biol. 1940, 11, 461–488.
- Scruggs, D.W.; Blue, G.K. Toxic hepatopathy and photosensitization in cattle fed moldy alfalfa hay. J. Am. Vet. Med. Assoc. 1994, 204, 264–266.
- Silva Filho, G.B.; Chaves, H.A.; Albuquerque, R.F.; Souza, P.E.; Vieira, M.E.; Nascimento, A.L.; Lima, S.C.; Mendonça, F.S. Spontaneous and experimental poisoning by Froelichia humboldtiana in cattle. Pesq. Vet. Bras. 2020, 40, 1–6, doi:10.1590/1678-5150-pvb-6351.
- Souza, P.E.C.; Oliveira, S.S.; Aguiar-Filho, C.R.; Cunha, A.L.B.; Albuquerque, R.F.; Evêncio-Neto, J.; Riet-Correa, F.; Mendonça, F.S. Primary photosensitization in cattle caused by Froelichia humboldtiana. Res. Vet. Sci. 2012, 93, 1337–1340, doi:10.1016/j.rvsc.2012.04.005.
- Thawait; Kumar, V.; Dixit; A., A.; Maiti; S.K.; Gupta; Rashmi. Berseem induced Photosensitization and its Therapeutic Management in Cattle. INTAS POLIVET 2013, 14, 228–229.
- Witte, S.T.; Curry, S.L. Hepatogenous photosensitization in cattle fed a grass hay. J. Vet. Diagn. Invest. 1993, 5, 133–136, doi:10.1177/104063879300500135.
- 2.
- Goat
- Glastonbury, J.R.; Boal, G.K. Geeldikkop in goats. Aust. Vet. J. 1985, 62, 62–63, doi:10.1111/j.1751-0813.1985.tb14238.x.
- Lemos, R.A.A. de; Nakazato, L.; Herrero Junior, G.O.; Da Silveira, A.C.; Porfírio, L.C. Fotossensibilização e colangiopatia associada a cristais em caprinos mantidos sob pastagens de Brachiaria decumbens no Mato Grosso do Sul. Cienc. Rural 1998, 28, 507–510, doi:10.1590/S0103-84781998000300026.
- Neves, J.P.L.; Silveira, Ana Eliza dos Santos; Rocha, N.S.; Crocomo, L.F.; Alves, C.E.F.; Ferreira, M.B.; Da Silva, F.G.O.; Filho, W.C.M. PHOTOSENSITIZATION IN A KID GOAT-CASE REPORT/FOTOSSENSIBILIZACAO HEPATOGENA EM CABRITO-RELATO DE CASO/FOTOSENSIBILIZACION HEPATOGENA EN CABRITO-REPORTE DE UN CASO. Veterinaria e Zootecnia 2016, 23, 588.
- Abas Mazni, O.; Sharif, H.; Mohd; Khusahry and H.N. Vance. Photosensitization in goats grazed on brachiaria decumbens. Available online: http://jtafs.mardi.gov.my/index.php/publication/issues/archive/133-1985/volume-13-no2/1103-130211 (accessed on 9 November 2022).
- Santos, D.S.; Silva, C.C.B.; Araújo, V.O.; Fátima Souza, M. de; Lacerda-Lucena, P.B.; Simões, S.V.D.; Riet-Correa, F.; Lucena, R.B. Primary photosensitization caused by ingestion of Froelichia humboldtiana by dairy goats. Toxicon 2017, 125, 65–69, doi:10.1016/j.toxicon.2016.11.258.
- 3.
- Horse
- Aboling, S. (2021). Phototoxis in white skinned horses due to roughage – a case report. Not published.
- Barbosa, J.D.; Oliveira, C.M.C. de; Tokarnia, C.H.; Peixoto, P.V. Fotossensibilização hepatógena em eqüinos pela ingestão de Brachiaria humidicola (Gramineae) no Estado do Pará. Pesq. Vet. Bras. 2006, 26, 147–153, doi:10.1590/S0100-736X2006000300003.
- Berry, J.M.; Merriam, J.G. Phototoxic dermatitis in a horse. (A case report). Vet. Med. Small Anim. Clin. 1970, 65, 251–257.
- Colon, J.L.; Jackson, C.A.; Del Piero, F. Case Presentation: Hepatic Dysfunction and Photodermatitis Secondary to Alsike Clover Poisoning. Compendium on Continuing Education for the Practicing Veterinarian [Online], 1022–1025.
- Da Trindade Nobre, V.M.; Riet-Correa, F.; Barbosa Filho, J.M.; Dantas, A.F.M.; Tabosa, I.M.; Vasconcelos, J.S. Intoxicação por Crotalaria retusa (Fabaceae) em Eqüídeos no semi-árido da Paraíba. Pesq. Vet. Bras. 2004, 24, 132–143, doi:10.1590/S0100-736X2004000300004.
- Fincher, M.G.; Fuller, H.K. Photosensitization-Trifoliosis-Light Sensitizacion. Cornell Veterinarian 1942, 32, 95–98.
- Gava, A.; Barros, C.S. Senecio spp. poisoning of horses in southern brazil. Pesq. Vet. Bras. 1997, 17, 36–40, doi:10.1590/S0100-736X1997000100006.
- Ivens, P. Hogweed suspected of causing primary photosensitisation in a horse. Vet. Rec. 2011, 169, 81–82, doi:10.1136/vr.d4472.
- Knight, A.P.; Kimberling, C. V.; Sternitz, F. R.; Roby, M. R. Cynoglossum officinale (hound’s-tongue)--a cause of pyrrolizidine alkaloid poisoning in horses. J. Am. Vet. Med. Assoc. 1984, 185, 647–650.
- Medeiros, R.M.T.; Bezerra, V.K.D.; Riet-Correa, F. Intoxicação experimental por Froelichia humboldtiana em equinos. Cienc. Rural 2014, 44, 1837–1840, doi:10.1590/0103-8478cr20131417.
- Mohamed, F.H.A.; Imbabi, S.E.; Adam, S.E.I. Hepatogenous photosensitization in horses due to Aphis craccivora on lucerne. Bulletin of Animal Health and Production in Africa 1977, 25, 184–187.
- Puschner, B.; Chen, X.; Read, D.; Affolter, V.K. Alfalfa hay induced primary photosensitization in horses. Vet. J. 2016, 211, 32–38, doi:10.1016/j.tvjl.2016.03.004.
- Renner, J.E.; Gallo, G.G.; Montesinos Ramos, I.G.; Baschlar, H.O. Contact photodermatitis and keratitis in horses due to parsnip. Vet. Arg. 1991, 450–454.
- Singh, R.P. A case of photosensitisation in horse. Indian Vet. J. 1970, 47, 450–451.
- Traub, J.L.; Potter, K.A.; Bayly, W.M.; Reed, S.M. Alsike clover poisoning [in a horse]. Modern Veterinary Practice 1982, 63, 307–309.
- Vind R. A case of hypericin poisoning in a horse. Nordisk Veterinaermedicin 1957, 322–328.
- Winter, J.C.; Thieme, K.; Eule, J.C.; Saliu, E.-M.; Kershaw, O.; Gehlen, H. Photodermatitis and ocular changes in nine horses after ingestion of wild parsnip (pastinaca sativa). BMC veterinary research 2022, 18, 80, doi:10.1186/s12917-022-03162-2.
- 4.
- Sheep
- Al-Dujaily, A.H.; Abeed, S.A.; Hatem, A.A. Photosensitation in sheep associated with ingestion of Tribulus Terrestris in Al-Najaf Desert, Iraq. Plant Archives 2019, 19, 280–283.
- Amjadi, A.R.; Ahourai, P.; Baharsefat, M. First report of Geeldikkop in sheep in Iran. Archives of Razi Institute 1977, 29, doi:10.22092/ari.1977.108807.
- Araújo, V.O. de; Oliveira Neto, T.S.; Simões, S.V.D.; Da Silva, T.K.F.; Riet-Correa, F.; Lucena, R.B. Primary photosensitization and contact dermatitis caused by Malachra fasciata Jacq. N.V. (Malvaceae) in sheep. Toxicon 2017, 138, 184–187, doi:10.1016/j.toxicon.2017.09.009.
- Badiei, K.; Mostaghni, K.; Nazifi, S.; Khodakaram Tafti, A.; Ghane, M.; Momeni, S.A. Experimental Panicum miliaceum poisoning in sheep. Small Ruminant Research 2009, 82, 99–104, doi:10.1016/j.smallrumres.2009.02.002.
- Bourke, C.A. Sunlight associated hyperthermia as a consistent and rapidly developing clinical sign in sheep intoxicated by St John’s wort (Hypericum perforatum). Aust. Vet. J. 2000, 78, 483–488, doi:10.1111/j.1751-0813.2000.tb11868.x.
- Bridges, C.H.; Camp, B.J.; Livingston, C.W.; Bailey, E.M. Kleingrass (Panicum coloratum L.) poisoning in sheep. Vet. Pathol. 1987, 24, 525–531, doi:10.1177/030098588702400609.
- Brook, P.J.; Mutch, G.V. Field control of facial eczema of sheep. New Zealand Journal of Agricultural Research 1964, 7, 138–145, doi:10.1080/00288233.1964.10418146.
- Brum, K.B.; Haraguchi, M.; Lemos, R.A.; Riet-Correa, F.; Fioravanti, M.C.S. Crystal-associated cholangiopathy in sheep grazing Brachiaria decumbens containing the saponin protodioscin. Pesq. Vet. Bras. 2007, 27, 39–42, doi:10.1590/S0100-736X2007000100007.
- Bull, L.B.; Macindoe, R.H.F. Photosensitization in Sheep: Trefoil Dermatitis. Aust. Vet. J. 1926, 2, 85–91, doi:10.1111/j.1751-0813.1926.tb05332.x.
- Button, C.; Paynter, D.I.; Shiel, M.J.; Colson, A.R.; Paterson, P.J.; Lyford, R.L. Crystal-associated cholangiohepatopathy and photosensitisation in lambs. Aust. Vet. J. 1987, 64, 176–180, doi:10.1111/j.1751-0813.1987.tb09677.x.
- Choez A., K.; Galarza Z., P. Brote de jacapo en ovinos en Junín, Perú. Rev. investig. vet. Perú 2018, 29, 1060–1064, doi:10.15381/rivep.v29i3.14839.
- DENT, C.H.R.; Kater), J.C.R.(A CONDITION RESEMBLING FACIAL ECZEMA IN SHEEP IN NEW SOUTH WALES. Aust. Vet. J. 1967, 43, 71, doi:10.1111/j.1751-0813.1967.tb15072.x.
- Diamantino, G.M.L.; Pierezan, F.; Ferreira, M.I.C.; Rocha, W.S.D.; Veiga, V.M.O.; Martins, C.E.; Veiga, M.O.; Soto-Blanco, B. Photosensitization by Brachiaria ruziziensis in a sheep herd. Toxicon 2020, 185, 1–4, doi:10.1016/j.toxicon.2020.06.022.
- Faccin, T.C.; Riet-Correa, F.; Rodrigues, F.S.; Santos, A.C.; Melo, G.K.A.; Silva, J.A.; Ferreira, R.; Itavo, C.C.B.F.; Lemos, R.A.A. Poisoning by Brachiaria brizantha in flocks of naïve and experienced sheep. Toxicon 2014, 82, 1–8, doi:10.1016/j.toxicon.2014.02.008.
- Flåøyen, A. A difference in susceptibility of two breeds of sheep to the ’alveld toxin’. Vet. Res. Commun. 1991, 15, 455–457, doi:10.1007/BF00346543.
- Ferrer, L.M.; Ortín, A.; Loste, A.; Fernández, A.; Verde, M.T.; Ramos, J.J. Photosensitisation in sheep grazing alfalfa infested with aphids and ladybirds. Vet. Rec. 2007, 161, 312–313, doi:10.1136/vr.161.9.312.
- García Barrachina, M.; González Arto, M.; Miguel Ferrer, L. Intoxication with Hypericum perforatum. Study of drowsiness and photosensitivity in sheep. Albéitar 2007, 30–32.
- Giaretta, P.R.; Panziera, W.; Hammerschmitt, M.E.; Bianchi, R.M.; Galiza, G.J.; Wiethan, I.S.; Bazzi, T.; Barros, C.S. Clinical and pathological aspects of chronic Senecio spp. poisoning in sheep. Pesq. Vet. Bras. 2014, 34, 967–973, doi:10.1590/S0100-736X2014001000008.
- Glastonbury, J.R.; Doughty, F.R.; Whitaker, S.J.; Sergeant, E. A syndrome of hepatogenous photosensitisation, resembling geeldikkop, in sheep grazing Tribulus terrestris. Aust. Vet. J. 1984, 61, 314–316, doi:10.1111/j.1751-0813.1984.tb07135.x.
- Golder, H.M.; Moss, N.; Rogers, G.; Jackson, B.; Gannon, N.; Wong, P.; Lean, I.J. Acute photosensitisation and mortality in a herd of dairy cattle in Tasmania. N. Z. Vet. J. 2017, 65, 39–45, doi:10.1080/00480169.2016.1232181.
- Greenwood, P.E.; Williamson, G.N. An outbreak of facial eczema in sheep. Aust. Vet. J. 1985, 62, 65–66, doi:10.1111/j.1751-0813.1985.tb14241.x.
- Hansen, D.E.; McCoy, R.D.; Hedstrom, O.R.; Snyder, S.P.; Ballerstedt, P.B. Photosensitization associated with exposure to Pithomyces chartarum in lambs. J. Am. Vet. Med. Assoc. 1994, 204, 1668–1671.
- İçen, H.; Sekİn, S.; Karataș, A.; Çakmak, F.; Vural, M.E. Hypericum perforatum toxication in Awassi sheep. Yüzüncü yıl Üniversitesi Veteriner Fakültesi Dergisi 2012, 23, 51–53.
- Kako, M.D.N.; Al-Sultan, I.I.; Saleem, A.N. Studies of sheep experimentally poisoned with Hypericum perforatum. Vet. Hum. Toxicol. 1993, 35, 298–300.
- Kessell, A.E.; Ladmore, G.E.; Quinn, J.C. An outbreak of primary photosensitisation in lambs secondary to consumption of Biserrula pelecinus (biserrula). Aust. Vet. J. 2015, 93, 174–178, doi:10.1111/avj.12318.
- Kümper, H. Hypericum perforatum poisoning in sheep. In Tagung der Fachgruppe “Krankheiten der kleinen Wiederkauer”, Giessen, 10. Juni 1988, Giessen, German Federal Republic; Deutsche Veterinärmedizinische Gesellschaft: Giessen, German Federal Republic, 1988; pp 86–89.
- Lancaster, M.J.; Vit, I.; Lyford, R.L. Analysis of bile crystals from sheep grazing Panicum schinzii (sweet grass). Aust. Vet. J. 1991, 68, 281, doi:10.1111/j.1751-0813.1991.tb03246.x.
- Marasas, W.F.; Adelaar, T.F.; Kellerman, T.S.; Minné, J.A.; van Rensburg, I.B.; Burroughs, G.W. First report of facial eczema in sheep in South Africa. Onderstepoort J. Vet. Res. 1972, 39, 107–112.
- Micheloud, J.F.; Colque-Caro, L.A.; Comini, L.R.; Cabrera, J.L.; Núñez-Montoya, S.; Martinez, O.G.; Gimeno, E.J. Spontaneous photosensitization by Heterophyllaea pustulata Hook. f. (Rubiaceae), in sheep from Northwestern Argentina. Trop Anim Health Prod 2017, 49, 1553–1556, doi:10.1007/s11250-017-1354-0.
- Naci; HAYDARDEDEOĞLU, O. Hepatogenous photosensitization in Akkaraman lambs: special emphasis to oxidative stress and thrombocytopenia. Veteriner Fakültesi dergisi 2013, 60, 116–122, doi:10.1501/Vetfak_0000002564.
- Nazifi, S.; Ghane, M.; Fazeli, M.; Ghafari, N.; Azizi, S.; Mansourian, M. Proso millet (Panicum miliaceum) poisoning in Iranian fat-tailed sheep. Comp Clin Pathol 2009, 18, 249–253, doi:10.1007/s00580-008-0784-5.
- Oertli, E.H.; Rowe, L.D.; Lovering, S.L.; Ivie, G.W.; Bailey, E.M. Phototoxic effect of Thamnosma texana (Dutchman’s breeches) in sheep. American Journal of Veterinary Research 1983, 44, 1126–1129.
- Ozmen, O.; Sahinduran, S.; Haligur, M.; Albay, M.K. Clinicopathological studies on facial eczema outbreak in sheep in Southwest Turkey. Trop Anim Health Prod 2008, 40, 545–551, doi:10.1007/s11250-008-9132-7.
- Petazzi, F.; Rubino, G.; Pieragostini, E.; Giordano, G. Photosensitization caused by Hypericum species. Changes in the blood proteins of Ionica goats. Summa 2002, 19, 25–27.
- Peterson, J.E.; Payne, A.; Culvenor, C.C. Heliotropium europaeum poisoning of sheep with low liver copper concentrations and the preventive efficacy of cobalt and antimethanogen. Aust. Vet. J. 1992, 69, 51–56, doi:10.1111/j.1751-0813.1992.tb07448.x.
- Puoli, J.R.; Reid, R.L.; Belesky, D.P. Photosensitization in Lambs Grazing Switchgrass. Agronomy Journal 1992, 84, 1077–1080, doi:10.2134/agronj1992.00021962008400060033x.
- Quinn, J.C.; Chen, Y.; Hackney, B.; Tufail, M.S.; Weston, L.A.; Loukopoulos, P. Acute-onset high-morbidity primary photosensitisation in sheep associated with consumption of the Casbah and Mauro cultivars of the pasture legume Biserrula. BMC veterinary research 2018, 14, 11, doi:10.1186/s12917-017-1318-7.
- Regnault, T.R. Secondary photosensitisation of sheep grazing bambatsi grass (Panicum coloratum var makarikariense). Aust. Vet. J. 1990, 67, 419, doi:10.1111/j.1751-0813.1990.tb03040.x.
- Riet-Correa, F.; Haraguchi, M.; Dantas, A.F.M.; Burakovas, R.G.; Yokosuka, A.; Mimaki, Y.; Medeiros, R.M.; Matos, P.F. de. Sheep poisoning by Panicum dichotomiflorum in northeastern Brazil. Pesq. Vet. Bras. 2009, 29, 94–98, doi:10.1590/S0100-736X2009000100015.
- Saturnino, K.C.; Mariani, T.M.; Barbosa-Ferreira, M.; Brum, K.B.; Fernandes, C.E.d.S.; Lemos, R.A. Intoxicação experimental por Brachiaria decumbens em ovinos confinados. Pesq. Vet. Bras. 2010, 30, 195–202, doi:10.1590/S0100-736X2010000300002.
- Sinclair, D.P. Pithomyces chartarum spores on pasture and their relation to facial eczema in sheep. New Zealand Journal of Agricultural Research 1961, 4, 492–503, doi:10.1080/00288233.1961.10431607.
- Stafford, K.J.; West, D.M.; Alley, M.R.; Waghorn, G.C. Suspected photosensitisation in lambs grazing birdsfoot trefoil (Lotus corniculatus). N. Z. Vet. J. 1995, 43, 114–117, doi:10.1080/00480169.1995.35866.
- Stroebel, J.C. Induction of photosensitivity in sheep with Erodium moschatum (L.) L’Hérit. J. S. Afr. Vet. Assoc. 2002, 73, 57–61, doi:10.4102/jsava.v73i2.556.
- Wisløff, H.; Wilkins, A.L.; Scheie, E.; Flåøyen, A. Accumulation of sapogenin conjugates and histological changes in the liver and kidneys of lambs suffering from alveld, a hepatogenous photosensitization disease of sheep grazing Narthecium ossifragum. Vet. Res. Commun. 2002, 26, 381–396, doi:10.1023/a:1016298929610.
- 5.
- Other animals
- Birgel Junior, E.H.; dos Santos, M.C.; Ramos, J.A.C. de; Pogliani, F.C.; Birgel, D.B.; Della Libera, A.M.M.P.; Gregory, L.; Araujo, W.P. de; Benesi, F.J. Secondary hepatogenous photosensitization in a llama (Lama glama) bred in the state of Sáo Paulo, Brazil. Can. Vet. J. 2007, 48, 323–324, doi:10.4141/cjas68-044.
- Byrne, K.V. Dermatitis in White Pigs Due to Photosensitization. Aust. Vet. J. 1937, 13, 74–75, doi:10.1111/j.1751-0813.1937.tb01152.x.
- Griffiths, I.B.; Douglas, R.G. Phytophotodermatitis in pigs exposed to parsley (Petroselinum crispum). Vet. Rec. 2000, 146, 73–74, doi:10.1136/vr.146.3.73.
- Johnson, J.H.; Jensen, J.M. Hepatotoxicity and Secondary Photosensitization in a Red Kangaroo (Megaleia rufus) Due to Ingestion of Lantana camara. Journal of Zoo and Wildlife Medicine 1998, 29, 203–207.
- Oliveira, C.H.S. de; Barbosa, J.D.; Oliveira, C.M.C.; Bastianetto, E.; Melo, M.M.; Haraguchi, M.; Freitas, L.G.L.; Silva, M.X.; Leite, R.C. Hepatic photosensitization in buffaloes intoxicated by Brachiaria decumbens in Minas Gerais state, Brazil. Toxicon 2013, 73, 121–129, doi:10.1016/j.toxicon.2013.07.001.
- Smith, B.L.; Asher, G.W.; Thompson, K.G.; Hoggard, G.K. Hepatogenous photosensitisation in fallow deer (Dama dama) in New Zealand. N. Z. Vet. J. 1997, 45, 88–92, doi:10.1080/00480169.1997.36001.
- Steventon, C.A.; Raidal, S.R.; Quinn, J.C.; Peters, A. Steroidal Saponin Toxicity In Eastern Grey Kangaroos (Macropus Giganteus): A Novel Clinicopathologic Presentation Of Hepatogenous Photosensitization. J. Wildl. Dis. 2018, 54, 491–502, doi:10.7589/2017-03-066.
- Woolford, L.; Fletcher, M.T.; Boardman, W.S.J. Suspected pyrrolizidine alkaloid hepatotoxicosis in wild southern hairy-nosed wombats (Lasiorhinus latifrons). J. Agric. Food Chem. 2014, 62, 7413–7418, doi:10.1021/jf405811n.
- 6.
- Goat + sheep
- Opasina, B.A. Photosensitization jaundice syndrome in West African dwarf sheep and goats grazed on Brachiaria decumbens. Tropical Grasslands 1985, 19, 120–123.
- Jacob, R.H.; Peet, R.L. Poisoning of sheep and goats by Tribulis terrestris (caltrop). Aust. Vet. J. 1987, 64, 288–289, doi:10.1111/j.1751-0813.1987.tb15966.x.
- Cattle + Sheep
- van Halderen, A.; Harding, W.R.; Wessels, J.C.; Schneider, D.J.; Heine, E.W.; van der Merwe, J.; Fourie, J.M. Cyanobacterial (blue-green algae) poisoning of livestock in the western Cape Province of South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 260–264.
- Dollahite, J.W.; Younger, R.L.; Hoffman, G.O. Photosensitization in cattle and sheep caused by feeding Ammi majus (Greater Ammi; bishop’s weed). American Journal of Veterinary Research 1978, 39, 193–197.
- 7.
- Horse + other animals
- Knupp, S.N.R.; Borburema, C.C.; Oliveira Neto, T.S.; Medeiros, R. de; Knupp, L.S.; Riet-Correa, F.; Lucena, R.B. Surtos de fotossensibilização primária em equídeos causados por Froelichia humboldtiana. Pesq. Vet. Bras. 2014, 34, 1191–1195, doi:10.1590/S0100-736X2014001200008.
- 8.
- Sheep + horse
- Pimentel, L.A.; Riet-Correa, F.; Guedes, K.M.; Macêdo, J.T.; Medeiros, R.M.; Dantas, A.F. Fotossensibilização primária em eqüídeos e ruminantes no semi-árido causada por Froelichia humboldtiana (Amaranthaceae). Pesq. Vet. Bras. 2007, 27, 23–28, doi:10.1590/S0100-736X2007000100005.
- 9.
- Goat + Sheep + Cattle
- Bale, S. Poisoning of sheep, goats and cows by the weed Hypericum triquetrifolium. Refuah Veterinarith 1978, 35, 36–37.
- 10.
- Goat + Horse
- Stegelmeier, B.L.; Colegate, S.M.; Knoppel, E.L.; Rood K. A.; Collert, M.G. Wild parsnip (Pastinaca sativa)-induced photosensitization. Toxicon 2019, 167, 60–66, doi:10.1016/j.toxicon.2019.06.007.
- 11.
- Cattle + Sheep + Horse
- Mendonça, M.F.F.; Pimentel, L.A.; Leal, P.V.; Oliveira Filho, J.C.; Caymmi, L.G.; Silva, A.W.O.; Jesus, R.S.; Peixoto, T.C. Hepatogenous photosensitization in ruminants and horses caused by the ingestion of Chamaecrista serpens in Brazil. Toxicon 2021, 193, 13–20, doi:10.1016/j.toxicon.2021.01.013.
References
- Gupta, R.C. Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Plumlee, K.H. Veterinary Toxicology; Mosby (Elsevier): Amsterdam, The Netherlands, 2004; Volume 1, p. 477. [Google Scholar]
- Aboling, S.; Scharmann, F.; Bunzel, D. Equine atypical myopathy: Selection of sycamore maple seedlings (Acer pseudoplatanus L.) in pasture horses is driven by seedling maturity and might be associated with phenolic compounds. Vet. Rec. 2020, 187, e116. [Google Scholar] [CrossRef] [PubMed]
- Aboling, S.; Rottmann, S.; Wolf, P.; Jahn-Falk, D.; Kamphues1, J. Case Report: Complex Plant Poisoning in Heavily Pregnant Heifers in Germany. J. Vet. Sci. Technol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Petit, R.; Izambart, J.; Guillou, M.; da Silva Almeida, J.R.G.; de Oliveira Junior, R.G.; Sol, V.; Ouk, T.-S.; Grougnet, R.; Quintans-Júnior, L.J.; Sitarek, P.; et al. A Review of Phototoxic Plants, Their Phototoxic Metabolites, and Possible Developments as Photosensitizers. Chem. Biodivers. 2024, 21, e202300494. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.E.; McCoy, R.D.; Hedstrom, O.R.; Snyder, S.P.; Ballerstedt, P.B. Photosensitization associated with exposure to Pithomyces chartarum in lambs. J. Am. Vet. Med. Assoc. 1994, 204, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Clare, N.T. Photosensitization in Diseases of Domestic Animals: A Review; Commonwealth Agricultural Bureaux: Wallingford, UK, 1952. [Google Scholar]
- Collett, M.G. Photosensitisation diseases of animals: Classification and a weight of evidence approach to primary causes. Toxicon X 2019, 3, 100012. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.P. Pithomyces chartarum spores on pasture and their relation to facial eczema in sheep. N. Z. J. Agric. Res. 1961, 4, 492–503. [Google Scholar] [CrossRef][Green Version]
- Kümper, H. Hypericum perforatum poisoning in sheep. In Tagung der Fachgruppe “Krankheiten der kleinen Wiederkauer”, Giessen, 10 Juni 1988; Deutsche Veterinärmedizinische Gesellschaft: Giessen, Germany, 1988; pp. 86–89. [Google Scholar]
- Chen, Y.; Quinn, J.C.; Weston, L.A.; Loukopoulos, P. The aetiology, prevalence and morbidity of outbreaks of photosensitisation in livestock: A review. PLoS ONE 2019, 14, e0211625. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Barnes, P.W.; Robson, P.R.H.; Robbins, S.A.; Jansen, M.A.K.; Carlos, L. Linkages between stratopheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Phytochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Aboling, S. Do Poisonous Plants in Pastures Communicate Their Toxicity?—Metastudy and Evaluation of Pasture Poisoning Cases in Central Europe. Animals 2023, 13, 3795. [Google Scholar] [CrossRef]
- Thukral, A.K. A review on measurement of Alpha diversity in biology. Agric. Res. J. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Forbes, J.M. Voluntary Food Intake and Diet Selection in Farm Animals, 2nd ed.; CABI: King’s Lynn, UK, 2007. [Google Scholar]
- Cuchillo-Hilario, M.; Wrage-Mönnig, N.; Isselstein, J. Forage selectivity by cattle and sheep co-grazing swards differing in plant species diversity. Grass Forage Sci. 2018, 73, 10. [Google Scholar] [CrossRef]
- Pfister, J.A.; Cheney, C.D.; Provenza, F.D. Behavioral toxicology of livestock inngesting plant toxins. J. Range Manag. 1992, 45, 30–36. [Google Scholar] [CrossRef]
- Miller, S.M.; Thompson, R.P. Seasonal patterns of diet composition, herbage intake and digestibility limit the performance of cattle grazing native pasture in the Falkland Islands. Grass Forage Sci. 2007, 62, 135–144. [Google Scholar] [CrossRef]
- Provenza, F.D.; Villalba, J.J.; Bryant, J.P. Foraging by herbivores: Linking the biochemical diversity of plants to herbivore culture and landscape diversity. In Landscape Ecology and Resource Management. Linking Theory with Practice; Bissonette, J.A., Storch, I., Eds.; Island Press: Washington, DC, USA, 2003; pp. 387–421. [Google Scholar]
- Smith, B.L.; Asher, G.W.; Thompson, K.G.; Hoggard, G.K. Hepatogenous photosensitisation in fallow deer (Dama dama) in New Zealand. N. Z. Vet. J. 1997, 45, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sargison, N.D.; Baird, G.J.; Sotiraki, S.; Gilleard, J.S.; Busin, V. Hepatogenous photosensitisation in Scottish sheep casued by Dicrocoelium dendriticum. Vet. Parasitol. 2012, 189, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Diamantino, G.M.L.; Pierezan, F.; Ferreira, M.I.C.; Rocha, W.S.D.; Veiga, V.M.O.; Martins, C.E.; Veiga, M.O.; Soto-Blanco, B. Photosensitization by Brachiaria ruziziensis in a sheep herd. Toxicon 2020, 185, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E. Toxicologic Aspects of Photosensitization in Livestock. JNCI J. Natl. Cancer Inst. 1982, 69, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.J. Outbreak of ragwort (Senecio jacobea) poisoning in horses. Equine Vet. J. 1983, 15, 248–250. [Google Scholar] [CrossRef]
- Vesonder, R.; Haliburton, J.; Stubblefield, R.; Gilmore, W.; Peterson, S. Aspergillus flavus and aflatoxins B1, B2, and M1 in corn associated with equine death. Arch. Environ. Contam. Toxicol. 1991, 20, 151–153. [Google Scholar] [CrossRef]
- Gehlen, H.; May, A.; Venner, M. Lebererkrankungen beim Pferd. Pferdeheilkunde 2010, 26, 668–679. [Google Scholar] [CrossRef]
- Smith, B.L.; O’Hara, P.J. Bovine photosensitization in New Zealand. N. Z. Vet. J. 1978, 26, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Stickdorn, T.; Ellis, A.D.; Kienzle, E. Horse feed hygiene evaluation with microbial and sensory examination. In Forages and Grazing in Horse Nutrition; Saastamoinen, M., Fradinho, M.J., Santos, A.S., Miraglia, N., Eds.; Wageningen Academic Publisher: Wageningen, The Netherlands, 2012; Volume 132. [Google Scholar]
- Ellenberg, H. Indicator Values of Plants in Central Europe (Zeigerwerte von Pflanzen in Mitteleuropa); E. Goltze: Göttingen, Germany, 1991; Volume 18, p. 248. [Google Scholar]
- Dierschke, H.; Briemle, G. Kulturgrasland; Ulmer: Stuttgart, Germany, 2002. [Google Scholar]
- Cann, A.J. Mathe für Biologen; Wiley VCH-Verlag: Mörlenbach, Germany, 2004. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: London, UK, 2021. [Google Scholar]
- Piruzyan, L.; Ostrovskii, M.; Landau, M. “ Sensory” safety of drugs: Photosensitized injury to structures of the eye. Biol. Bull. Acad. Sci. USSR 1991, 18, 29. [Google Scholar]
- Frohne, D.; Jensen, U. Systematik des Pflanzenreichs unter Besonderer Berücksichtigung Chemischer Merkmale und Pflanzlicher Drogen, 5th ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1998; p. 371. [Google Scholar]
- Opasina, B.A. Photosensitization jaundice syndrome in West African dwarf sheep and goats grazed on Brachiaria decumbens. Trop. Grassl. 1985, 19, 120–123. [Google Scholar]
- Knupp, S.N.R.; Borburema, C.C.; Oliveira Neto, T.S.; Medeiros, R.d.; Knupp, L.S.; Riet-Correa, F.; Lucena, R.B. Surtos de fotossensibilização primária em equídeos causados por Froelichia humboldtiana. Pesqui. Veterinária Bras. 2014, 34, 1191–1195. [Google Scholar] [CrossRef]
- Bale, S. Poisoning of sheep, goats and cows by the weed Hypericum triquetrifolium. Refuah Vet. 1978, 35, 36–37. [Google Scholar]
- Colon, J.L.; Jackson, C.A.; Del Piero, F. Case Presentation: Hepatic Dysfunction and Photodermatitis Secondary to Alsike Clover Poisoning. Compend. Equine Rounds 1996, 18, 1022–1026. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19972202735 (accessed on 27 May 2024).
- Chen, Y.; Loukopoulos, P.; Xie, G.; Quinn, J.C. Relative perceptions of prevalence, impact and importance of photosensitisation in Australian livestock: A survey of veterinarians, livestock traders and livestock producers. Aust. Vet. J. 2022, 100, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Araya, O.S.; Ford, E.J.H. An investigation of the type of photosensitization caused by the ingestion of St John’s Wort (Hypericum perforatum) by calves. J. Comp. Pathol. 1981, 91, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.C.; Thieme, K.; Eule, J.C.; Saliu, E.-M.; Kershaw, O.; Gehlen, H. Photodermatitis and ocular changes in nine horses after ingestion of wild parsnip (pastinaca sativa). BMC Vet. Res. 2022, 18, 80. [Google Scholar] [CrossRef]
- Puschner, B.; Chen, X.; Read, D.; Affolter, V. Alfalfa hay induced primary photosensitization in horses. Vet. J. 2016, 211, 32–38. [Google Scholar] [CrossRef]
- Iason, G.R.; Villalba, J.J. Behavioral Strategies of Mammal Herbivores Against Plant Secondary Metabolites: The Avoidance-Tolerance Continuum. J. Chem. Ecol. 2006, 32, 115–1132. [Google Scholar] [CrossRef] [PubMed]
- Leiber, F.; Walkenhorst, M.; Holinger, M. The relevance of feed diversity and choice in nutrition of ruminant livestock. Landbauforschung 2020, 70, 35. [Google Scholar] [CrossRef]
- Opitz von Boberfeld, W. Grünlandlehre; UTB: Stuttgart, Germany, 1994. [Google Scholar]
- Provenza, F.D.; Pfister, J.A.; Cheney, C.D. Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Manag. 1992, 45, 36–45. [Google Scholar] [CrossRef]
- Crawley, M.J.; Johnston, A.E.; Silvertown, J.; Dodd, M.; Mazancourt, C.D.; Heard, M.S.; Henman, D.F.; Edwards, G.R. Determinants of Species Richness in the Park Grass Experiment. Am. Nat. 2005, 165, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Oruc, H.H.; Akkoc, A.; Uzunoglu, I.; Kennerman, E. Nitrate Poisoning in Horses Associated With Ingestion of Forage and Alfalfa. J. Equine Vet. Sci. 2010, 30, 159–162. [Google Scholar] [CrossRef]
- Vondran, S.E.A. Effects of alfalfa chaff on the gastric mucosa in adult horses. Pferdeheilkunde 2017, 33, 66–71. [Google Scholar] [CrossRef]
- Chizzola, R.; Tollemache, D.O. Skin lesions of horses- provoked by poisonous plants? Wien. Tieraerztliche Monatsschrift 1992, 79, 120–121. [Google Scholar]
- Calapai, G.; Miroddi, M.; Minciullo, P.L.; Caputi, A.P.; Gangemi, S.; Schmidt, R.J. Contact dermatitis as an adverse reaction to some topically used European herbal medicinal products—Part 1: Achillea millefolium-Curcuma longa. Contact Dermat. 2014, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vogt-Andersen, U.; Calov, B. Long-term effects of sheep grazing on giant hogweed (Heracleum mantegazzianum). Hydrobiologia 1996, 340, 277–284. [Google Scholar] [CrossRef]
- Rosa García, R.; Celaya, R.; García, U.; Osoro, K. Goat grazing, its interactions with other herbivores and biodiversity conservation issues. Small Rumin. Res. 2012, 107, 49–64. [Google Scholar] [CrossRef]
| Continent | Cases (n) | Portion (%) | Feed Examination by Authors | ||
|---|---|---|---|---|---|
| Completely | Partly | Unknown | |||
| South America | 54 | 31.4 | 31 | 13 | 10 |
| Australia | 38 | 22.1 | 14 | 20 | 4 |
| North America | 33 | 19.2 | 18 | 10 | 5 |
| Europe | 14 | 8.1 | 2 | 9 | 3 |
| Asia | 12 | 7.0 | 3 | 5 | 4 |
| New Zealand | 11 | 6.4 | 3 | 4 | 4 |
| Africa | 8 | 4.7 | 1 | 6 | 1 |
| India | 2 | 1.2 | 0 | 0 | 2 |
| Sum (n) | 172 | - | 72 | 67 | 33 |
| Portion (%) | - | 100 | 41.9 | 39.0 | 19.2 |
| Animal Species | Normal Feed Quality | Poor Feed Quality | Total |
|---|---|---|---|
| Sheep | 60 | 9 | 69 |
| Cattle | 45 | 10 | 55 |
| Horse | 30 | 1 | 31 |
| Goat | 11 | 0 | 11 |
| Other animals 2 | 10 | 1 | 11 |
| Sum (n) | 156 | 21 | 177 1 |
| Portion (%) | 88.1 | 11.9 | 100 |
| Animal Species | Primary Photosensitization | Secondary Photosensitization | Unknown Kind of Photosensitization | Total |
|---|---|---|---|---|
| Sheep | 22 | 44 | 3 | 69 |
| Cattle | 13 | 29 | 13 | 55 |
| Horse | 18 | 10 | 3 | 31 |
| Goat | 4 | 7 | 0 | 11 |
| Other animals | 2 | 8 | 1 | 11 |
| Sum (n) | 59 | 98 | 20 | 177 |
| Portion (%) | 33.3 | 55.4 | 11.3 | 100 |
| Animal Species | Kind of Feed | Sum (n) | |||
|---|---|---|---|---|---|
| Fresh | Conserved | Both | Unknown | ||
| Sheep | 59 | 4 | 3 | 3 | 69 |
| Cattle | 39 | 7 | 5 | 4 | 55 |
| Horse | 13 | 13 | 3 | 2 | 31 |
| Goat | 9 | 0 | 0 | 2 | 11 |
| Other animals | 7 | 1 | 3 | 0 | 11 |
| Sum (n) | 127 | 25 | 14 | 11 | 177 |
| Portion (%) | 71.8 | 14.1 | 7.9 | 6.2 | 100 |
| Feedstuff | High Diversity | Low Diversity | Unknown Level of Diversity | Sum of Animals (n) | Feed Choice | No Feed Choice | Unknown Possibility of Feed Choice | Sum of Animals (n) |
|---|---|---|---|---|---|---|---|---|
| Fresh feed | 21 | 56 | 50 | 127 | 8 | 83 | 36 | 127 |
| Conserved feed | 5 | 12 | 8 | 25 | 3 | 22 | 0 | 25 |
| Fresh and conserved feed | 6 | 5 | 3 | 14 | 4 | 6 | 4 | 14 |
| Unknown feed condition | 0 | 4 | 7 | 11 | 0 | 2 | 9 | 11 |
| Sum (n) | 32 | 77 | 68 | 177 | 15 | 113 | 49 | 177 |
| (%) | 18.1 | 43.5 | 38.4 | 100 | 8.5 | 63.8 | 27.7 | 100 |
| Animal Species | High Diversity | Low Diversity | Unknown Level of Diversity | Sum | Feed Choice | No feed Choice | Unknown Possibility of Feed Choice | Sum |
|---|---|---|---|---|---|---|---|---|
| Sheep | 8 | 35 | 26 | 69 | 8 | 43 | 18 | 69 |
| Cattle | 12 | 21 | 22 | 55 | 4 | 36 | 15 | 55 |
| Horse | 8 | 12 | 11 | 31 | 2 | 21 | 8 | 31 |
| Goat | 2 | 4 | 5 | 11 | 0 | 8 | 3 | 11 |
| Other animals | 2 | 5 | 4 | 11 | 1 | 5 | 5 | 11 |
| Sum (n) | 32 | 77 | 68 | 177 | 15 | 113 | 49 | 177 |
| Portion (%) | 18.1 | 43.5 | 38.4 | 100 | 8.5 | 63.8 | 27.7 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, R.; Aboling, S. Incidence of Photosensitization in Husbandry Animals: A Meta-Study on the Effects of Feed Diversity and Feed Choice. Agriculture 2024, 14, 1137. https://doi.org/10.3390/agriculture14071137
Moritz R, Aboling S. Incidence of Photosensitization in Husbandry Animals: A Meta-Study on the Effects of Feed Diversity and Feed Choice. Agriculture. 2024; 14(7):1137. https://doi.org/10.3390/agriculture14071137
Chicago/Turabian StyleMoritz, Rieke, and Sabine Aboling. 2024. "Incidence of Photosensitization in Husbandry Animals: A Meta-Study on the Effects of Feed Diversity and Feed Choice" Agriculture 14, no. 7: 1137. https://doi.org/10.3390/agriculture14071137
APA StyleMoritz, R., & Aboling, S. (2024). Incidence of Photosensitization in Husbandry Animals: A Meta-Study on the Effects of Feed Diversity and Feed Choice. Agriculture, 14(7), 1137. https://doi.org/10.3390/agriculture14071137





