Altering Microbial Communities in Substrate to Stimulate the Growth of Healthy Button Mushrooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Mycopathogenic Organism and Culture Conditions
2.2. Beneficial Microorganisms and Culture Conditions
2.3. Trials in Mushroom Cultivation Chamber
2.4. Statistical Analyses
3. Results
3.1. Efficacy in Disease Control
3.2. Mushroom Productivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J. Effects of fragmentation, supplementation, and the addition of phase II compost to 2nd break compost on mushroom (Agaricus bisporus) yield. Bioresour. Technol. 2010, 101, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Hatvani, L.; Kredics, L.; Allaga, H.; Manczinger, L.; Vágvölgyi, C.; Kuti, K.; Geösel, A. First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus bisporus in Europe. Plant Dis. 2017, 101, 1052. [Google Scholar] [CrossRef]
- Potočnik, I.; Todorović, B.; Milijašević-Marčić, S.; Luković, J.; Kanižai Šarić, G.; Majić, I.; Rekanović, E. A large-scale study on the effectiveness of a Bacillus subtilis Ch-13-based biofungicide against green mould disease and mushroom yield improvement. Pestic. Phytomed. 2021, 36, 83–90. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Royse, D.J.; Thon, M.R.; Chen, X.; Romaine, C.P. Phylogenetic relationships of Trichoderma harzianum casing mushroom green mold in Europe and North America to other species of Trichoderma from world-wide sources. Mycologia 1998, 90, 76–81. [Google Scholar] [CrossRef]
- Altaf, S.; Jan, S.K.; Ahanger, S.A.; Basu, U.; Rather, R.A.; Wani, O.A.; Rasool, F.; Mushtaq, M.; Yassin, M.T.; Mostafa, A.A.-F.; et al. Management of green mold disease in white button mushroom (Agaricus bisporus) and its yield improvement. J. Fungi 2022, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.J.; Gea, F.J. Estudio de la fitotoxicidad del insecticida diflubenzuron en el cultivo de champiñón. Determinación del nivel de residuos. Boletín Asoc. Española Cultiv. Champiñón 2006, 48, 32–34. [Google Scholar]
- Grogan, H.M.; Gaze, R.H. Fungicide resistance among Cladobotryum spp.—Causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol. Res. 2000, 104, 357–364. [Google Scholar] [CrossRef]
- Romaine, C.P.; Royse, D.J.; Schlagnhaufer, C. Superpathogenic Trichoderma resistant to Topsin M found in Pennsylvania and Delaware. Mushroom News 2005, 53, 6–9. [Google Scholar]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-based biological control of plant diseases. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 273–302. [Google Scholar] [CrossRef]
- Pandin, J.A.; Védie, R.; Rousseau, T.; Le Coq, D.; Aymericha, S.; Briandeta, R. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biol. Control 2018, 127, 39–54. [Google Scholar] [CrossRef]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Abdul Khair, W.M.; Rashad, Y.M. In vitro antifungal activity of Streptomyces spororaveus RDS28 against some phytopathogenic fungi. Afr. J. Agric. Res. 2011, 6, 2835–2842. [Google Scholar]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- Wang, H.; Han, L.; Feng, J.; Thang, X. Evaluation of two Streptomyces spp. and compost for growth promotion and biocontrol potential against Rhizoctonia solani on pepper. Biocontrol Sci. Technol. 2015, 25, 852–857. [Google Scholar] [CrossRef]
- Šantrić, L.; Potočnik, I.; Radivojević, L.; Gajić Umiljendić, J.; Rekanović, E.; Duduk, B.; Milijašević-Marčić, S. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus. J. Environ. Sci. Health B 2018, 53, 677–684. [Google Scholar] [CrossRef]
- Ebrahimi-Zarandi, M.; Roohalllah, S.R.; Tarkka, M.T. Actinobacteria as effective biocontrol agents against plant pathogens, an overview on their role in eliciting plant defense. Microorganisms 2022, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef]
- McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl. Microbiol. Biotechnol. 2018, 102, 1075–1083. [Google Scholar] [CrossRef]
- Vieira, F.R.; Pecchia, J.A. An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting-phase II) for Agaricus bisporus cultivation. Microb. Ecol. 2018, 75, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, O.; Milijašević-Marčić, S.; Potočnik, I.; Stepanović, M.; Dimkić, I.; Stanković, S.; Berić, T. Isolation and identification of Bacillus spp. from compost material, compost and mushroom casing soil active against Trichoderma spp. Arch. Biol. Sci. 2016, 68, 845–852. [Google Scholar] [CrossRef]
- Kosanović, D.; Potočnik, I.; Duduk, B.; Vukojević, J.; Stajić, M.; Rekanović, E.; Milijašević-Marčić, S. Trichoderma species on Agaricus bisporus farms in Serbia and their biocontrol. Ann. Appl. Biol. 2013, 163, 218–230. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.; Navarro, M. Efficacy and effect on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Chrysay-Tokousbalides, M.; Kastanias, M.A.; Philippoussis, A.; Diamantopoulou, P. Selective fungitoxicity of famaxadone, tebuconazole and trifloxystrobin between Verticillium fungicola and Agaricus bisporus. Crop Prot. 2007, 26, 469–475. [Google Scholar] [CrossRef]
- Reeb, J.E.; Milota, M.R. Moisture content by the oven-dry method for industrial testing. In Proceedings of the 50th Annual Dubois Meeting of the Western Dry Kiln Association, Portland, OR, USA, May 1999; The Association WDK: Portland, Oregon, USA, 1999; pp. 66–74. Available online: https://ir.library.oregonstate.edu/concern/conference_proceedings_or_journals/fq977v782 (accessed on 4 May 2023).
- Richer, D.L. Synergism—A patent view. Pestic. Sci. 1987, 19, 309–315. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman & Company: New York, NY, USA, 2013; pp. 1–915. ISBN 978-0-7167-8604-4. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 7. Available online: www.statsoft.com (accessed on 12 May 2024).
- Carrasco, J.; García-Delgado, C.; Lavega, R.; Tello, M.L.; De Toro, M.; Barba-Vicente, V.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Pérez, M.; Preston, G.M. Holistic assessment of the microbiome dynamics in the substrates used for commercial champignon (Agaricus bisporus) cultivation. Microb. Biotechnol. 2020, 13, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Tautorus, T.E.; Townsley, P.M. Biological control of olive green mold in Agaricus bisporus cultivation. Appl. Environ. Microbiol. 1983, 45, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, Y.; Yang, S.; Zhang, W.; Xu, M.; Ma, A.; Zhuang, G.; Chen, G.; Liu, W. Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour. Technol. 2014, 170, 183–195. [Google Scholar] [CrossRef]
- Nagy, A.; Manczinger, L.; Tombácz, D.; Hatvani, L.; Gyõrfi, J.; Antal, Z.; Sajben, E.; Vágvõllgyi, C.; Kredics, L. Biological control of oyster mushroom green mould disease by antagonistic Bacillus species. In Biological Control of Fungal and Bacterial Plant Pathogens IOBC-WPRS Bulletin, Proceedings of the Meeting of the IOBC-WPRS, Graz, Austria, 7–10 June 2010; Pertot, I., Elad, Y., Gessler, C., Cini, A., Eds.; IOBC-Global: Zürich, Switzerland, 2012; Volume 78, pp. 289–293. [Google Scholar]
- Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Potočnik, I.; Rekanović, E.; Todorović, B.; Luković, J.; Paunović, D.; Stanojević, O.; Milijašević-Marčić, S. The effects of casing soil treatment with Bacillus subtilis Ch-13 biofungicide on green mould control and mushroom yield. Pestic. Phytomed. 2019, 34, 53–60. [Google Scholar] [CrossRef]
- Potočnik, I.; Luković, J.; Todorović, B.; Stepanović, M.; Šantrić, L.; Milijašević-Marčić, S.; Rekanović, E. Improvement of procedure for casing treatment with a Bacillus subtilis Ch-13-based biofungicide to control green mould disease of mushrooms. Pestic. Phytomed. 2022, 37, 95–102. [Google Scholar] [CrossRef]
- Kim, W.G.; Weon, H.Y.; Seok, S.J.; Lee, K.H. In vitro antagonistic characteristics of Bacilli isolates against Trichoderma spp. and three species of mushrooms. Mycobiology 2008, 36, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.D.W.; Frostick, L.E. Investigating microbial activities in compost using mushroom (Agaricus bisporus) cultivation as an experimental system. Bioresour. Technol. 2008, 99, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
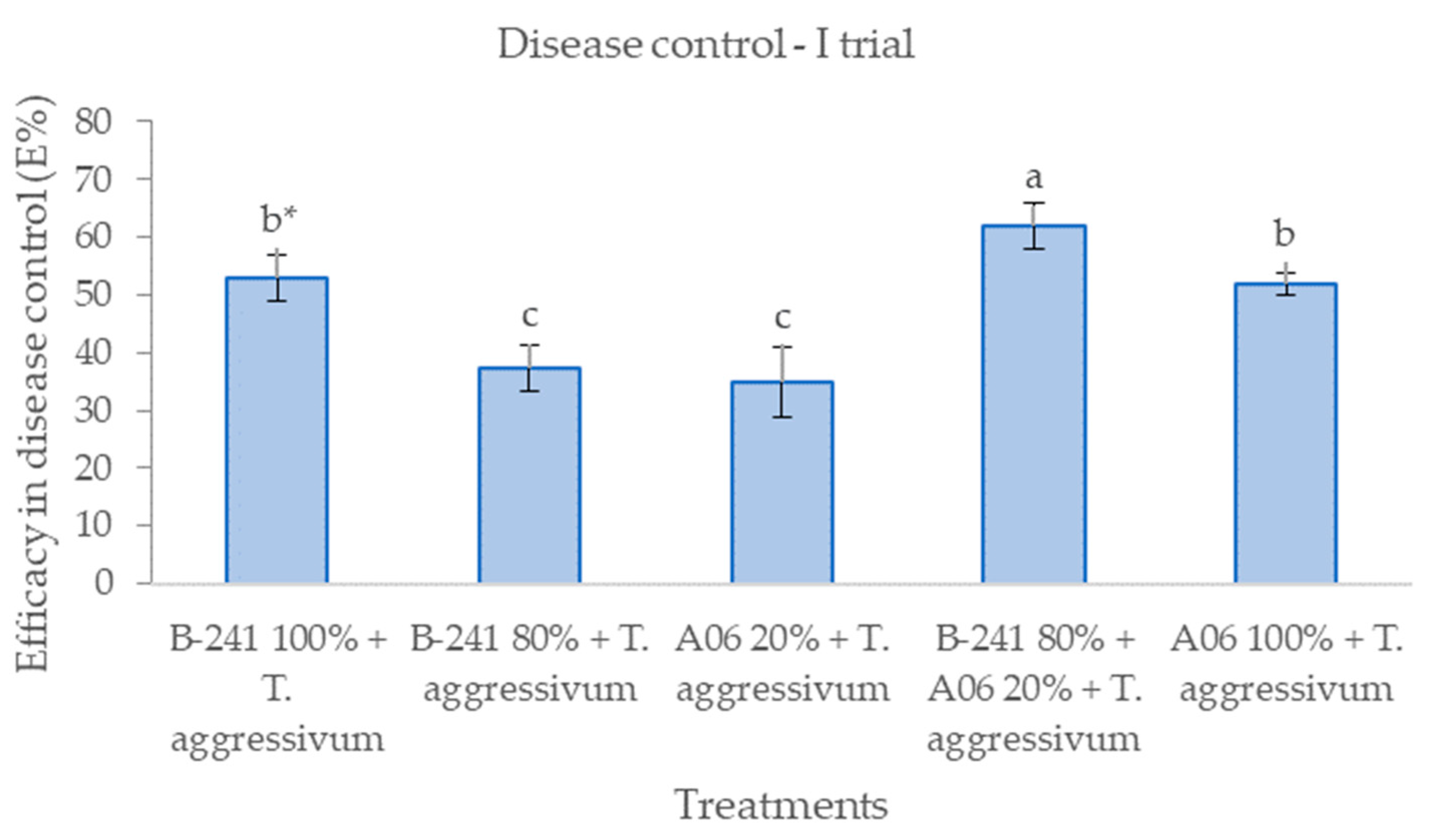

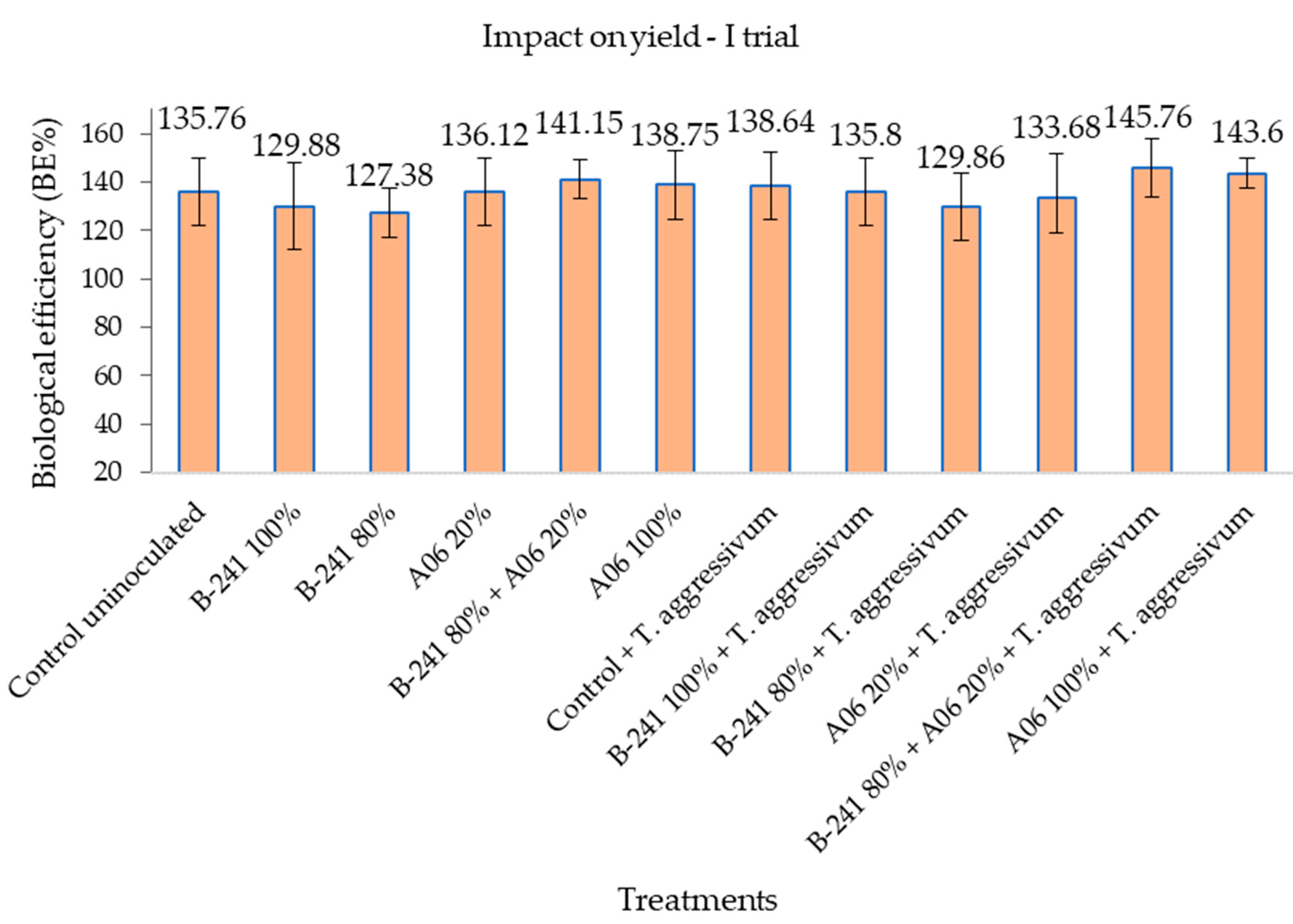
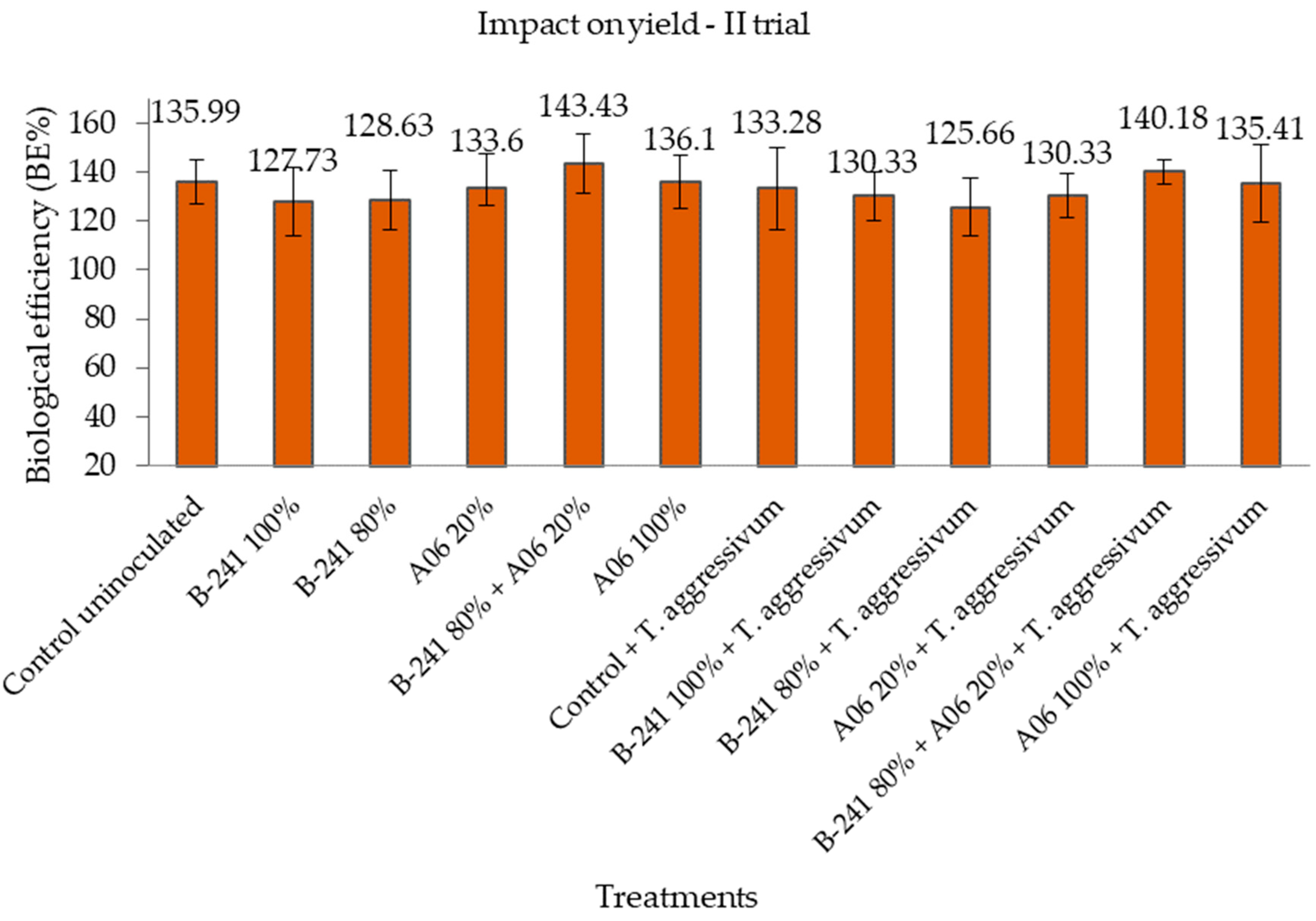
| Treatment | Trial | Biological Efficiency (BE%) in Mushroom Productivity | Efficacy (E%) in Suppressing Green Mould Disease Symptoms | ||||
|---|---|---|---|---|---|---|---|
| Observed BE% | Expected BE% | Synergy Factor | Observed E% | Expected E% | Synergy Factor | ||
| Mean ± SE b | Mean ± SE | ||||||
| Bacillus amyloliqufaciens B-241+ Streptomyces flavovirens A06 | I | 145.76 ± 2.44 | 89.94 | 1.62 | 61.93 ± 1.89 | 59.09 | 1.05 |
| II | 140.18 ± 5.87 | 92.22 | 1.52 | 69.37 ± 4.54 | 65.94 | 1.05 | |
| ANOVA Analysis | |||||||
| F | 12.32 | 0.48 | |||||
| p-value | 0.04 | 0.66 | |||||
| SEDs c | 1.58 | 1.9 | |||||
| df | 2 | 2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milijašević-Marčić, S.; Šantrić, L.; Luković, J.; Potočnik, I.; Grujić, N.; Drobnjaković, T.; Marčić, D. Altering Microbial Communities in Substrate to Stimulate the Growth of Healthy Button Mushrooms. Agriculture 2024, 14, 1152. https://doi.org/10.3390/agriculture14071152
Milijašević-Marčić S, Šantrić L, Luković J, Potočnik I, Grujić N, Drobnjaković T, Marčić D. Altering Microbial Communities in Substrate to Stimulate the Growth of Healthy Button Mushrooms. Agriculture. 2024; 14(7):1152. https://doi.org/10.3390/agriculture14071152
Chicago/Turabian StyleMilijašević-Marčić, Svetlana, Ljiljana Šantrić, Jelena Luković, Ivana Potočnik, Nikola Grujić, Tanja Drobnjaković, and Dejan Marčić. 2024. "Altering Microbial Communities in Substrate to Stimulate the Growth of Healthy Button Mushrooms" Agriculture 14, no. 7: 1152. https://doi.org/10.3390/agriculture14071152
APA StyleMilijašević-Marčić, S., Šantrić, L., Luković, J., Potočnik, I., Grujić, N., Drobnjaković, T., & Marčić, D. (2024). Altering Microbial Communities in Substrate to Stimulate the Growth of Healthy Button Mushrooms. Agriculture, 14(7), 1152. https://doi.org/10.3390/agriculture14071152







