Quality of Red Clover Forage in Different Organic Production Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Experimental Design
2.2. Analytical Methods
2.3. Calculations and Statistical Analyses
- ME—metabolic energy.
- kl—coefficient of ME utilization in lactation processes.
- kbp—coefficient of ME utilization in livestock processes and for growth.
- PDIF—proteins digested in the small intestine from feed.
- PDIMN—microbial protein synthesized in the rumen using available nitrogen.
- PDIME—microbial protein synthesized in the rumen using available energy.
- DOMF—digestible organic matter fermentable in the rumen.
- CP—crude protein.
- r—theoretical coefficient of CP distribution in the rumen (in sacco).
- sjp—actual intestinal digestibility of feed protein that is not broken down in the rumen.
3. Results
3.1. Chemical Composition and Mineral Content of Red Clover Herbage
3.2. Chemical Composition and Fermentation Pattern of Red Clover Silage
3.3. Changes in True Protein Content during the Ensiling of Red Clover
3.4. Feed Value of Red Clover Herbage and Silage
4. Discussion
4.1. Chemical Composition and Mineral Content of Red Clover Herbage
4.2. Chemical Composition and Fermentation Pattern of Red Clover Silage
4.3. Changes in True Protein Content during the Ensiling of Red Clover
4.4. Feed Value of Red Clover Herbage and Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J. The use of red clover (Trifolium pratense) in soil fertility-building: A Review. Field Crops Res. 2018, 221, 38–49. [Google Scholar] [CrossRef]
- Topp, C.F.E.; Doyle, C.J. Modeling the comparative productivity and profitability of grass and legume systems of silage production in northern Europe. Grass Forage Sci. 2004, 59, 274–292. [Google Scholar] [CrossRef]
- Boller, B.; Posselt, U.K.; Veronesi, F. Fodder Crops and Amenity Grasses; Springer: New York, NY, USA, 2010; pp. 395–437. [Google Scholar]
- Purwin, C.; Pysera, B.; Żuk-Gołaszewska, K.; Antoszkiewicz, Z.; Gołaszewski, J.; Fijałkowska, M.; Lipiński, K. Fermentation and proteolysis during the ensilage of wilted and unwilted diploid and tetraploid red clover. J. Cent. Europ. Agric. 2011, 12, 179–194. [Google Scholar] [CrossRef][Green Version]
- Lee, M.R.F.; Theobald, V.J.; Gordon, N.; Leyland, M.; Tweed, J.K.S.; Fychan, R.; Scollan, N.D. The effect of high polyphenol oxidase grass silage on metabolism of polyunsaturated fatty acids and nitrogen across the rumen of beef steers. J. Anim. Sci. 2014, 92, 5076–5087. [Google Scholar] [CrossRef]
- Grabber, J.H. Forage management effects on protein and fiber fractions, protein degradability, and dry matter yield of red clover conserved as silage. Anim. Feed Sci. Technol. 2009, 154, 284–291. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Fychan, R.; Tweed, J.K.S.; Gordon, N.; Theobald, V.; Yadav, R.; Marshall, A. Nitrogen and fatty acid rumen metabolism in cattle offered high or low polyphenol oxidase red clover silage. Animal 2019, 13, 1623–1634. [Google Scholar] [CrossRef]
- Niderkorn, V.; Copani, G.; Martin, C.; Maxin, G.; Torrent, A.; Anglard, F.; Rochette, Y.; Ginane, C. Effects of including bioactive legumes in grass silage on digestion parameters, nitrogen balance and methane emissions in sheep. Grass Forage Sci. 2019, 74, 626–635. [Google Scholar] [CrossRef]
- Doel, J.M. Accumulation and Recovery of Nitrogen in Mixed Farming Systems Using Legumes and Other fertility Building Crops. Doctoral Dissertation, Coventry University in association with the Royal Agricultural College, Cirencester, UK, 2012. [Google Scholar]
- Żuk-Gołaszewska, K.; Purwin, C.; Pysera, B.; Wierzbowska, J.; Golaszewski, J. Yields and quality of green forage from red clover di-and tetraploid forms. J. Elem. 2010, 15, 757–770. [Google Scholar] [CrossRef]
- Franco, M.; Tapio, I.; Huuskonen, A.; Rinne, M. Fermentation quality and bacterial ecology of red clover dominated silage modulated by different management factors. Front. Anim. Sci. 2022, 3, 1080535. [Google Scholar] [CrossRef]
- Nykanen, A.; Granstedt, A.; Laine, A.; Kunttu, S. Yields and clover contents of leys of different ages in organic farming in Finland. Biol. Agric. Hortic. 2000, 18, 55–66. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Thomas, T.A. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Kostulak–Zielińska, M.; Potkański, A. Quality of baled grass-clover silages ensiled with chemical additives. Chemical composition. Ann. Anim. Sci. 2001, 1, 153–165. [Google Scholar]
- Gąsior, R. Oznaczanie lotnych kwasów tłuszczowych i kwasu mlekowego w kiszonkach i w treści żwacza. In Biuletyn Informacyjny Instytutu Zootechniki; Instytut Zootechniki: Balice, Poland, 2002. [Google Scholar]
- Purwin, C.; Fijalkowska, M.; Pysera, B.; Lipinski, K.; Sienkiewicz, S.; Piwczynski, D.; Puzio, N. Nitrogen fractions and amino acid content in alfalfa and red clover immediately after cutting and after wilting in the field. J. Elem. 2014, 19, 723–733. [Google Scholar] [CrossRef]
- Drobna, J.V.U.R.V.P.; Jancovic, J.S.P.U.N. Estimation of red clover (Trifolium pratense L.) forage quality parameters depending on the variety, cut and growing year. Plant Soil Environ. 2006, 52, 468. [Google Scholar] [CrossRef]
- Buxton, D.R.; Muck, R.E.; Harrison, J.H. Silage Science and Technology; American Society of Agronomy Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Tamm, S.; Bender, A. Variation of agronomic and quality characteristics of red clover. Czech J. Genet. Plant Breed. 2003, 39, 319. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Sun, X.; Chen, J.; Liu, L.; Rosanoff, A.; Xiong, X.; Zhang, Y.; Pei, T. Effects of magnesium fertilizer on the forage crude protein content depend upon available soil nitrogen. J. Agric. Food Chem. 2018, 66, 1743–1750. [Google Scholar] [CrossRef]
- Purwin, C. Quality of the grass and grass-legume silages making by baler technology. In Dissertations and Monographs; Wyd. UWM w Olsztynie: Olsztyn, Poland, 2007; Volume 127. (In Polish) [Google Scholar]
- Weissbach, F.; Auerbach, H. Hay-crop silages and the problem of fermentation quality. Int. Dairy Top. 2013, 12, 11–15. [Google Scholar]
- Elgersma, A.; Søegaard, K. Changes in nutritive value and herbage yield during extended growth intervals in grasslegume mixtures: Effects of species, maturity at harvest, and relationships between productivity and components of feed quality. Grass Forage Sci. 2018, 73, 78–93. [Google Scholar] [CrossRef]
- Wróbel, B.; Zielewicz, W. Nutritional value of red clover (Trifolium pratense L.) and birdsfoot trefoil (Lotus corniculatus L.) harvested in different maturity stages. J. Res. Appl. Agric. Eng. 2019, 64, 14–19. [Google Scholar]
- White, P.J.; Karley, A.J. Potassium. In Plant Cell Monographs 17, Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Tiwari, P.S.; Joshi, P.O.; Vyas, K.A.; Billore, D.S. Potassium nutrition in yield and quality improvement of soybean. In Proceedings of the International Symposium on “Importance of Potassium in Nutrient Management for Sustainable Crop Production in India”, New Delhi, India, 3–5 December 2001; Available online: https://www.ipipotash.org/uploads/udocs/Potassium%20Nutrition%20in%20Yield%20and%20Quality.pdf (accessed on 13 April 2020).
- Sawan, M.Z.; Fahmy, H.A.; Yousef, E.S. Direct and residual effects of nitrogen fertilization, foliar application of potassium and plant growth retardant on Egyptian cotton growth, seed yield, seed viability and seedling vigor. Acta Ecol. Sin. 2009, 29, 116–123. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Tomić, D.; Dalibor, T.; Vladeta, S.; Aleksandar, S.; Dragan, S.; Mirjana, R.; Knežević, J. Foliar fertilization with phosphorus and potassium in red clover seed production on an acidic soil. Acta Agric. Serbica 2020, 25, 51–57. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Bahaeldeen, B.M.; Fadlalla, A.H.; Elhadi, A.E. Effects of seedbed preparation and potassium application on alfalfa yield. J. Sci. Technol. 2009, 10, 1–10. [Google Scholar]
- Abusuwar, A.O.; Abdella, A.A. Effects of seedbed types and phosphorus fertilizer (TSP) on growth and yield of clitoria (Clitoria ternata L.). University of Khartoum. J. Agric. Investig. 2004, 2, 63–65. [Google Scholar]
- Parmar, P.; Sindhu, S.S. Potassium Solubilization by Rhizosphere Bacteria: Influence of Nutritional and Environmental Conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar]
- Kochian, L.V. Mechanisms of micronutrient uptake and translocation in plants. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 229–296. [Google Scholar]
- Lindsay, W.L. Inorganic equilibria affecting micronutrients in soils. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 89–112. [Google Scholar]
- Grusak, M.A. Plant macro-and micronutrient minerals. Encycl. Life Sci. 2001, 1–5. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Lipiński, K.; Pysera, B.; Wierzbowska, J.; Antoszkiewicz, Z.; Sienkiewicz, S.; Stasiewicz, M. The effect of ensiling in round bales on the content of nitrogen fractions in lucerne and red clover protein. J. Elem. 2015, 20, 285–291. [Google Scholar] [CrossRef]
- Purwin, C.; Fijałkowska, M.; Kowalik, B.; Skórko-Sajko, H.; Nogalski, Z.; Pysera, B. The effect of bale density and addition of formic acid on the in situ dry matter and crude protein degradation of lucerne, red clover and red fescue silages. J. Anim. Feed Sci. 2014, 23, 177–184. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow Bucks, UK, 1991. [Google Scholar]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silage. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of Ensiling. In W: Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; Volume 42, pp. 31–93. [Google Scholar]
- Huhtanen, P.; Khalili, H.; Nousiainen, J.I.; Rinne, M.; Jaakkola, S.; Heikkilä, T.; Nousuaunen, J. Prediction of the relative intake potential of grass silage by dairy cows. Livest. Prod. Sci. 2002, 73, 111–130. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Pysera, B.; Lipiński, K.; Strusińska, D. Changes of nitrogen compounds during ensiling of high protein herbages—A review. Ann. Anim. Sci. 2015, 15, 289–305. [Google Scholar] [CrossRef]
- Owens, V.N.; Albrecht, K.A.; Muck, R.E.; Duke, S.H. Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total non-structural carbohydrates. Crop Sci. 1999, 39, 1873–1880. [Google Scholar] [CrossRef]
- Krawutschke, M.; Kleen, J.; Weiher, N.; Loges, R.; Taube, F.; Gierus, M. Changes in crude protein fractions of forage legumes during the spring growth and summer regrowth period. J. Agric. Sci. 2013, 151, 72–90. [Google Scholar] [CrossRef]
- Marković, J.; Dinić, B.; Blagojević, M.; Anđelković, B.; Babić, S.; Petrović, M.; Terzić, D. Effects of alfalfa and red clover cultivars on protein fractions by CNCPS system of analyzis. In Proceedings of the 4th International Congress, New Perspectives and Challenges of Sustainable Livestock Production, Belgrade, Serbia, 7–9 October 2015; pp. 796–802. [Google Scholar]
- Hart, E.H.; Onime, L.A.; Davies, T.E.; Morphew, R.M.; Kingston-Smith, A.H. The effects of PPO activity on the proteome of ingested red clover and implications for improving the nutrition of grazing cattle. J. Proteom. 2016, 141, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.L.; Hatfield, R.D. Polyphenol oxidase and o-diphenols inhibit postharvest proteolysis in red clover and alfalfa. Crop Sci. 2006, 46, 662–670. [Google Scholar] [CrossRef]
- IZPIB-INRA. Normy Żywienia Przeżuwaczy. In Wartość Pokarmowa Francuskich i Krajowych Pasz dla Przeżuwaczy; IZ PIB: Cracow, Poland, 2009. (In Polish) [Google Scholar]
- Sheaffer, C.C.; Cash, D.; Ehlke, N.J.; Henning, J.C.; Jewett, J.G.; Johnson, K.D.; Peterson, M.A.; Smith, M.; Hansen, J.L.; Viands, D.R. Entry × environment interactions for alfalfa forage quality. Agron. J. 1998, 90, 774–780. [Google Scholar] [CrossRef]
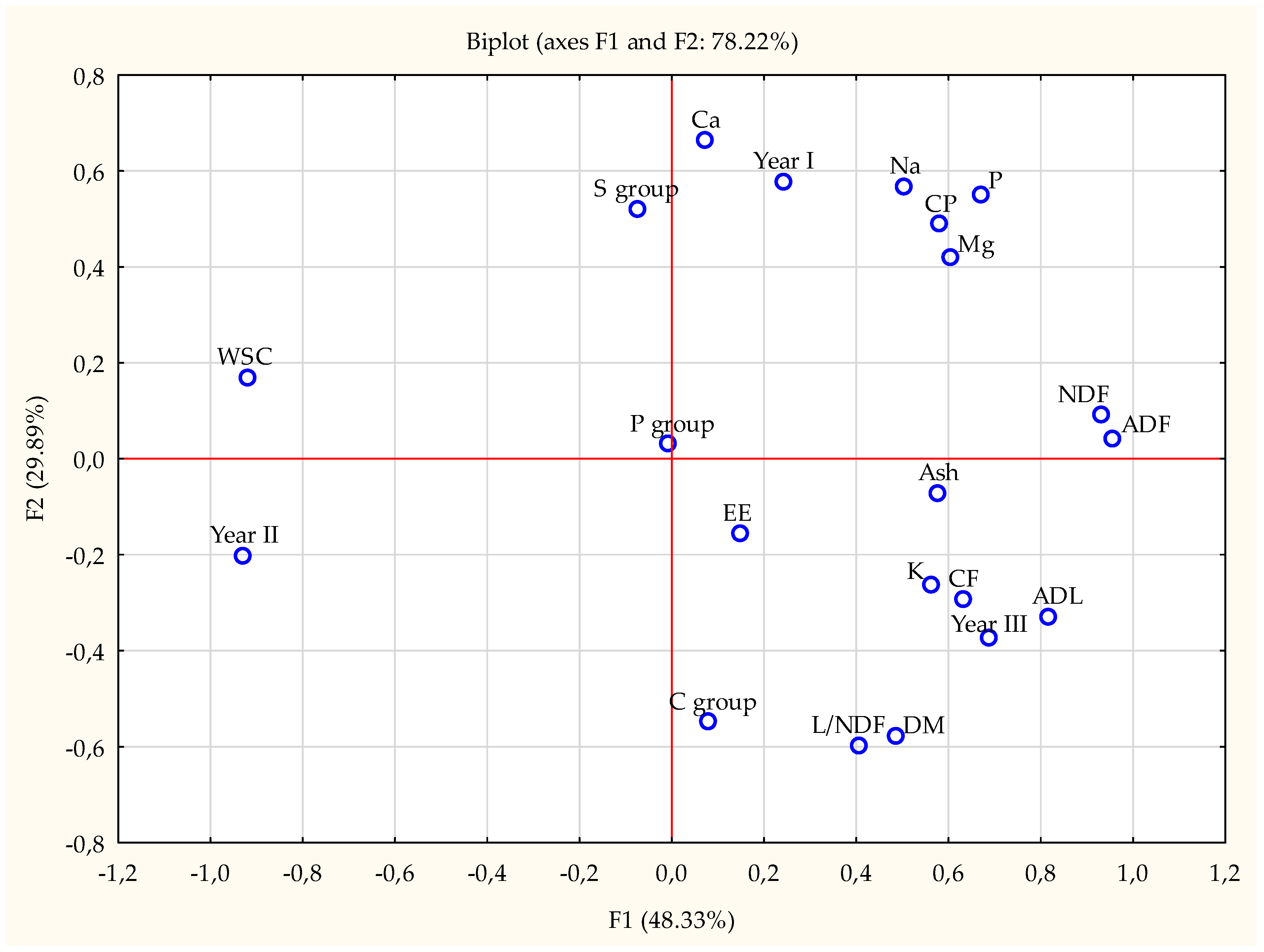
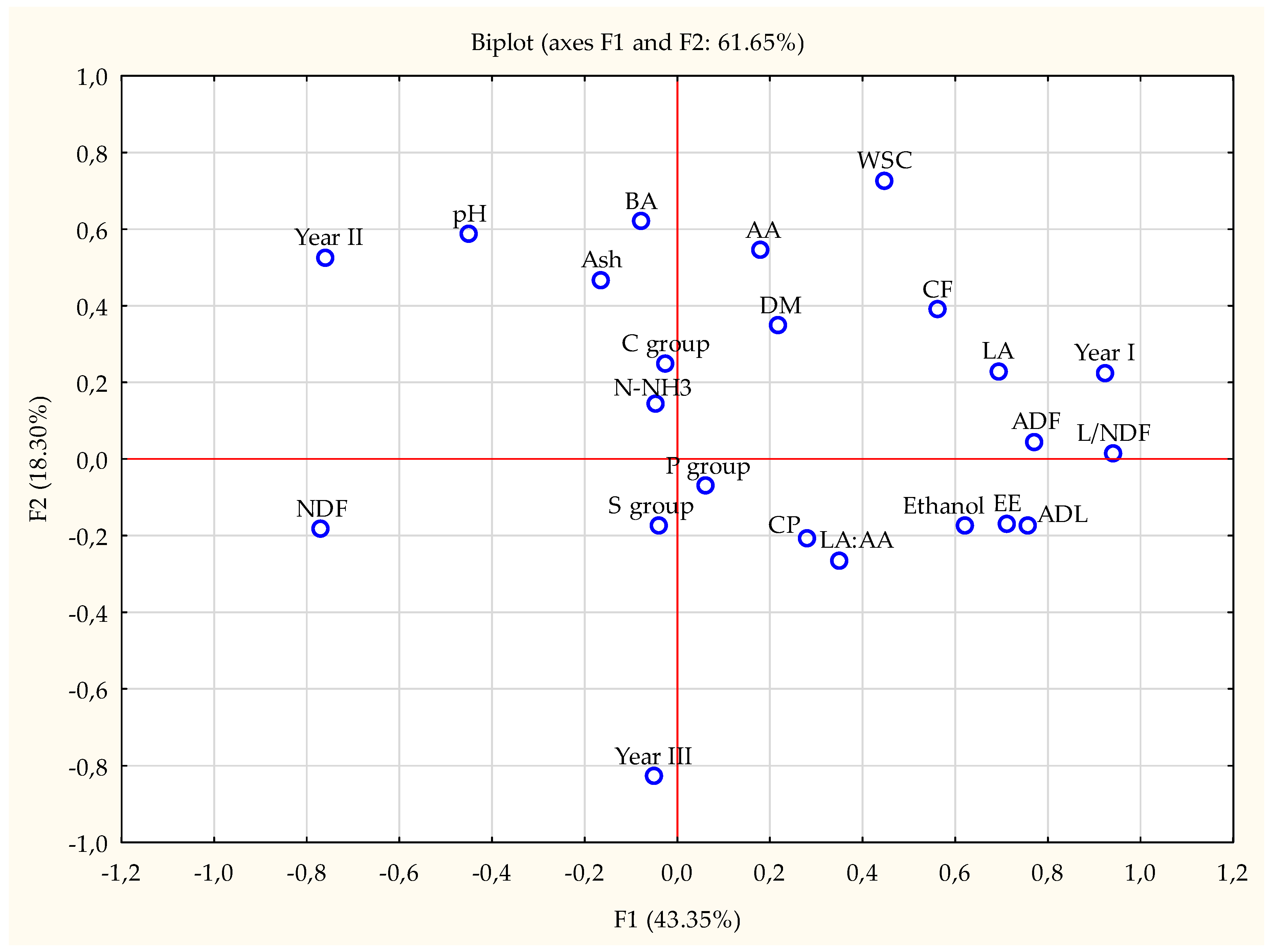
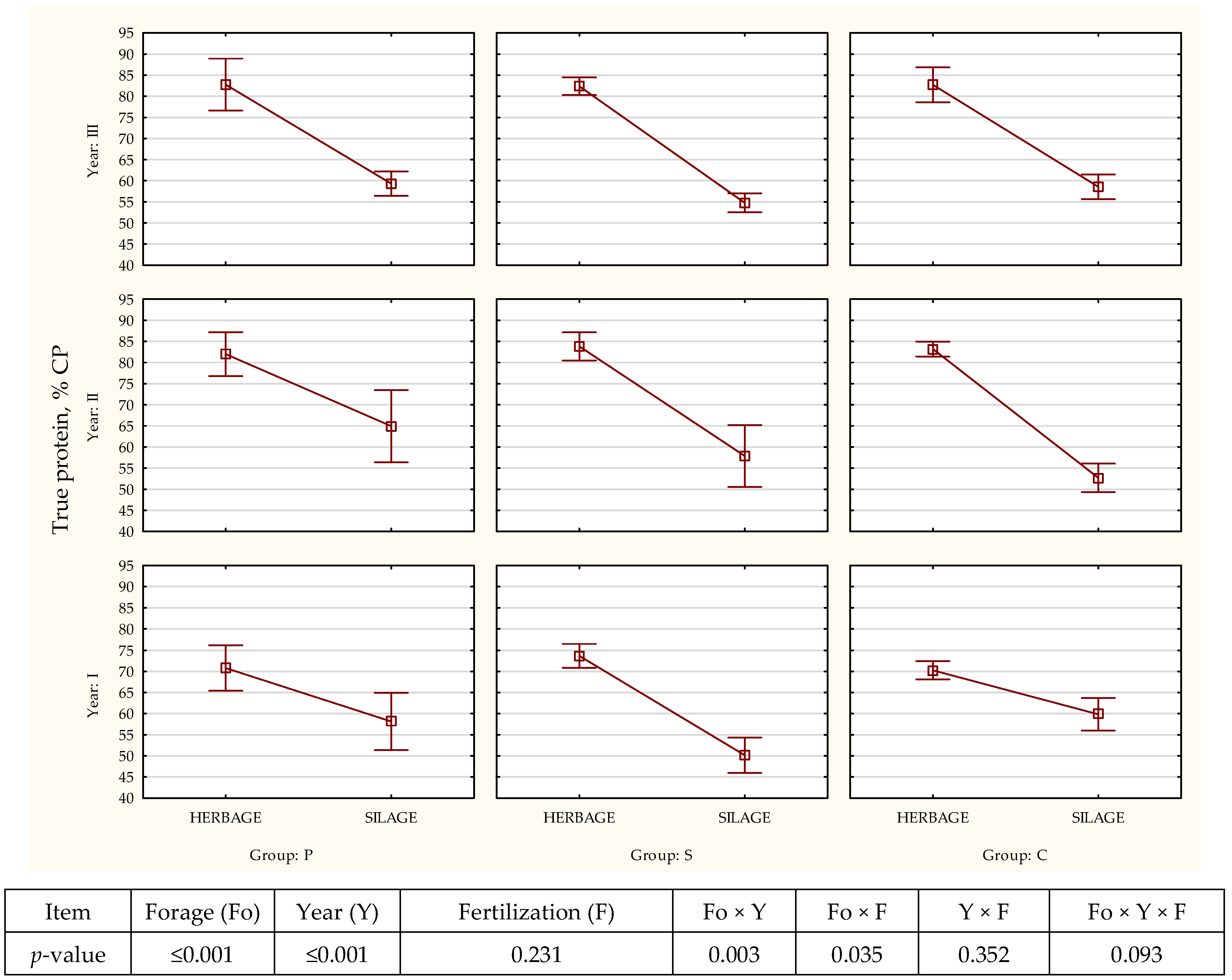
| Item | Year | Fertilization | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | P | S | Year (Y) | Fertilization (F) | Interaction (Y × F) | ||
| DM 1, g∙kg−1 FM | 147 bd | 164 bc | 217 a | 184 a | 177 ab | 167 b | 4.823 | ≤0.001 | 0.031 | 0.036 |
| Chemical composition, g∙kg−1 DM | ||||||||||
| Ash | 87.6 a | 73.3 b | 89.8 a | 87.9 | 82.2 | 80.6 | 1.929 | ≤0.001 | 0.280 | 0.002 |
| CP | 192 a | 150 bd | 172 bc | 166 b | 175 a | 174 a | 2.705 | ≤0.001 | 0.038 | 0.064 |
| EE | 25.4 b | 26.4 b | 28.8 a | 25.6 | 27.3 | 27.7 | 0.443 | 0.004 | 0.102 | 0.012 |
| CF | 246 b | 241 b | 282 a | 258 | 257 | 255 | 3.200 | ≤0.001 | 0.944 | 0.417 |
| NDF | 526 a | 400 b | 534 a | 492 | 487 | 481 | 8.943 | ≤0.001 | 0.894 | 0.249 |
| ADF | 364 bc | 298 bd | 388 a | 351 | 351 | 349 | 5.551 | ≤0.001 | 0.977 | 0.035 |
| ADL | 67.5 a | 49.4 b | 73.3 a | 67.7 a | 64.4 ab | 58.1 b | 1.901 | ≤0.001 | 0.006 | 0.005 |
| WSC | 160 bc | 228 a | 105 bd | 154 b | 167 ab | 172 a | 7.374 | ≤0.001 | 0.020 | 0.003 |
| L/NDF | 0.13 | 0.12 | 0.14 | 0.14 a | 0.14 a | 0.12 b | 0.002 | 0.057 | 0.014 | 0.097 |
| Item | Year | Fertilization | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | P | S | Year (Y) | Fertilization (F) | Interaction (Y × F) | ||
| P | 0.176 bd | 0.227 bc | 0.277 a | 0.146 b | 0.260 a | 0.273 a | 0.005 | ≤0.001 | 0.038 | 0.021 |
| K | 1.140 bd | 1.560 bc | 1.980 a | 1.147 bd | 1.391 bc | 1.683 a | 0.568 | 0.001 | ≤0.001 | ≤0.001 |
| Mg | 0.035 bd | 0.243 bc | 0.273 a | 0.164 | 0.257 | 0.270 | 0.004 | ≤0.001 | 0.313 | 0.038 |
| Ca | 0.905 | 1.050 | 1.073 | 0.967 b | 1.060 ab | 1.170 a | 0.028 | 0.927 | 0.009 | ≤0.001 |
| Na | 0.010 b | 0.009 b | 0.022 a | 0.011 b | 0.021 a | 0.020 ab | 0.002 | ≤0.001 | 0.045 | 0.040 |
| Item | Year | Fertilization | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | P | S | Year (Y) | Fertilization (F) | Interaction (Y × F) | ||
| DM 1, g∙kg−1 FM | 321 a | 313 a | 297 b | 311 | 310 | 313 | 2.235 | ≤0.001 | 0.123 | 0.019 |
| Chemical composition, g∙kg−1 DM | ||||||||||
| Ash | 102 ab | 109 a | 92.0 b | 102 | 108 | 99.9 | 2.100 | 0.002 | 0.716 | 0.001 |
| CP | 192 a | 171 b | 181 ab | 170 b | 181 a | 182 a | 3.116 | 0.019 | 0.014 | 0.042 |
| EE | 50.7 a | 38.8 bd | 44.2 bc | 42.0 | 43.2 | 44.1 | 0.835 | ≤0.001 | 0.352 | 0.160 |
| CF | 344 a | 314 b | 303 b | 316 | 319 | 321 | 3.362 | ≤0.001 | 0.381 | 0.078 |
| NDF | 457 b | 547 a | 551 a | 522 | 528 | 527 | 5.173 | ≤0.001 | 0.727 | 0.033 |
| ADF | 363 a | 320 bd | 339 bc | 340 | 334 | 332 | 3.133 | ≤0.001 | 0.150 | ≤0.001 |
| ADL | 80.9 a | 68.6 bd | 77.3 bc | 73.1 | 74.7 | 73.7 | 0.907 | ≤0.001 | 0.143 | 0.085 |
| WSC | 33.8 a | 22.5 bc | 9.73 bd | 26.1 a | 22.1 ab | 18.2 b | 1.408 | ≤0.001 | 0.031 | ≤0.001 |
| L/NDF | 0.18 a | 0.13 b | 0.14 b | 0.14 | 0.14 | 0.14 | 0.003 | ≤0.001 | 1.000 | 0.237 |
| Item | Year | Fertilization | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | P | S | Year (Y) | Fertilization (F) | Interaction (Y × F) | ||
| pH | 4.52 b | 5.06 a | 4.44 b | 4.88 | 4.68 | 4.75 | 0.059 | 0.045 | 0.055 | 0.022 |
| Fermentation pattern, g∙kg−1 DM 1 | ||||||||||
| LA | 47.2 a | 27.3 b | 26.6 b | 33.2 ab | 35.9 a | 27.13 b | 1.528 | ≤0.001 | 0.023 | 0.031 |
| AA | 17.8 | 15.8 | 12.8 | 15.9 | 15.8 | 14.8 | 0.789 | 0.075 | 0.330 | 0.079 |
| BA | 3.29 ab | 3.84 a | 1.93 b | 3.81 | 2.69 | 3.19 | 0.253 | 0.007 | 0.612 | 0.013 |
| Ethanol | 5.47 a | 1.53 bd | 3.77 bc | 2.21 b | 3.27 a | 3.75 b | 0.297 | ≤0.001 | 0.006 | ≤0.001 |
| LA:AA | 3.02 a | 2.05 b | 2.26 ab | 2.36 ab | 2.71 a | 1.97 b | 0.145 | 0.019 | 0.016 | 0.028 |
| N-NH3, g∙kg−1 TN | 90.0 | 99.3 | 77.1 | 80.9 b | 112 a | 80.9 b | 0.297 | 0.752 | 0.036 | 0.031 |
| Item | Year | Fertilization | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | P | S | Year (Y) | Fertilization (F) | Interaction (Y × F) | ||
| Herbage, g∙kg−1 DM 1 | ||||||||||
| UFL | 0.83 a | 0.81 a | 0.75 b | 0.79 | 0.80 | 0.80 | 0.009 | 0.024 | 0.998 | 0.014 |
| UFV | 0.77 a | 0.75 a | 0.68 b | 0.73 | 0.73 | 0.74 | 0.011 | 0.038 | 0.998 | 0.012 |
| PDIN | 121 a | 94.0 bd | 108 bc | 104 | 110 | 109 | 3.272 | 0.018 | 0.980 | 0.043 |
| PDIE | 93 a | 84 b | 85 b | 86 | 88 | 88 | 1.194 | 0.048 | 0.998 | 0.055 |
| Silage, g∙kg−1 DM | ||||||||||
| UFL | 0.70 | 0.70 | 0.71 | 0.70 | 0.70 | 0.70 | 0.002 | 0.989 | 1.000 | 0.999 |
| UFV | 0.60 | 0.60 | 0.61 | 0.60 | 0.61 | 0.61 | 0.002 | 0.998 | 0.998 | 0.999 |
| PDIN | 110 | 103 | 105 | 104 | 106 | 106 | 0.902 | 0.998 | 0.998 | 0.998 |
| PDIE | 56 | 55 | 55 | 54 | 55 | 55 | 0.236 | 0.998 | 0.988 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwin, C.; Żuk-Gołaszewska, K.; Tyburski, J.; Borsuk-Stanulewicz, M.; Stefańska, B. Quality of Red Clover Forage in Different Organic Production Systems. Agriculture 2024, 14, 1159. https://doi.org/10.3390/agriculture14071159
Purwin C, Żuk-Gołaszewska K, Tyburski J, Borsuk-Stanulewicz M, Stefańska B. Quality of Red Clover Forage in Different Organic Production Systems. Agriculture. 2024; 14(7):1159. https://doi.org/10.3390/agriculture14071159
Chicago/Turabian StylePurwin, Cezary, Krystyna Żuk-Gołaszewska, Józef Tyburski, Marta Borsuk-Stanulewicz, and Barbara Stefańska. 2024. "Quality of Red Clover Forage in Different Organic Production Systems" Agriculture 14, no. 7: 1159. https://doi.org/10.3390/agriculture14071159
APA StylePurwin, C., Żuk-Gołaszewska, K., Tyburski, J., Borsuk-Stanulewicz, M., & Stefańska, B. (2024). Quality of Red Clover Forage in Different Organic Production Systems. Agriculture, 14(7), 1159. https://doi.org/10.3390/agriculture14071159





