A Temporary Immersion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Culture Conditions
2.3. Variable Costs Comparison
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Girdziute, L.; Besuspariene, E.; Nausediene, A.; Novikova, A.; Leppala, J.; Jakob, M. Youth’s (Un)willingness to work in agriculture sector. Front. Public Health 2022, 10, 937657. [Google Scholar] [CrossRef] [PubMed]
- Poosappan, S.; Palanisamy, V.; Marimuthu, M.; Madheswaran, D. A study on farmers agricultural labour management problems during pre-pandemic time. In AIP Conference Proceedings, Proceedings of the 24TH Topical Conference on Radio-Frequency Power in Plasmas, Annapolis, MD, USA, 26–28 September 2023; American Institute of Physics: College Park, MD, USA, 2023. [Google Scholar]
- Kulus, D. Selected aspects of ornamental plants micropropagation in Poland and worldwide. Nauk. Przyr. 2015, 4, 10–25. [Google Scholar]
- Ahloowalia, B.S.; Savangikar, V.A. Low cost energy and labour. In International Atomic Energy Agency, Low-cost options for tissue culture technology in developing countries. In Proceedings of the Technical Meeting Organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vienna, Austria, 3–7 June 2002; pp. 41–46. [Google Scholar]
- Pożoga, M.; Olewnicki, D.; Wójcik-Gront, E.; Latocha, P. An Efficient Method of Pennisetum × advena ‘Rubrum’ Plantlets Production Using the Temporary Immersion Bioreactor Systems and Agar Cultures. Plants 2023, 12, 1534. [Google Scholar] [CrossRef] [PubMed]
- Pożoga, M.; Olewnicki, D. The Impact of the Organization of the Working Day on Production Efficiency in the Laboratory of Plant Tissue Cultures; Scientific Papers of Silesian University of Technology–Organization and Management Series; Silesian University of Technology Publishing House: Warsaw, Poland, 2023; p. 170. [Google Scholar]
- Martre, P.; Lacan, D.; Just, D.; Teisson, C. Physiological effects of temporary immersion on Hevea brasiliensis callus. Plant Cell Tissue Organ Cult. 2001, 67, 25–35. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Freire-Seijo, M.; Barbón, R.; Concepción-Hernández, M.; Mendoza-Rodríguez, M.F.; Torres-García, S. Effect of sucrose on physiological and biochemical changes of proliferated shoots of Bambusa vulgaris Schrad. Ex Wendl in temporary immersion. Plant Cell Tissue Organ Cult. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Costa, B.N.S.; Neto, A.R.; Chagas, P.C.; Chagas, E.A.; Pasqual, M.; Vendrame, W.A. Silicon in the Anatomy and Physiology of Banana Plant Leaves Under Temporary Immersion Bioreactors. Agric. Environ. Sci. 2021, 7, 10. [Google Scholar] [CrossRef]
- Piao, X.; Chakrabarty, D.; Hahn, E.; Peak, K. A simple method for mass production of potato microtubes using a bioreactor system. Curr. Sci. 2003, 84, 1129–1132. [Google Scholar]
- Septi, A.; Luthfi, A.M.S.; Irda, S. Microbubers production by using Temporary Immersion System (TIS) bioreactor to potato varieties. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd Biennial Conference of Tropical Biodiversity, Makassar, Indonesia, 4–5 August 2021; IOP Publishing: Bristol, UK, 2021; Volume 886, p. 886. [Google Scholar]
- Escalona, M.; Lorenzo, F.J.; González, B.; Daquinta, M.; Gonzalez, O.J.; Desjardins, Y.; Borroto, C. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Scherer, R.F.; Garcia, A.C.; de Freitas Fraga, H.P.; Dal Vesco, L.L.; Steinmacher, D.A.; Guerra, M.P. Nodule cluster cultures and temporary immersion bioreactors as a high performance micropropagation strategy in pineapple (Ananas comosus var. comosus). Sci. Hortic. 2013, 151, 38–45. [Google Scholar] [CrossRef]
- Lawan, A.; Usman, I.S.; Nasir, A.U.; Abdulmalik, M. Micropropagation of pineapple (Ananas comosus L. var. Smooth cayenne) in temporary immersion bioreactor system (TIPS). Bayero J. Pure Appl. Sci. 2021, 12, 207–209. [Google Scholar]
- Abdulmalik, M.; Usman, I.S.; Nasir, A.U.; Lawan, A. Micropropagation of banana (Musa spp.) using temporary immersion bioreactor system. Bayero J. Pure Appl. Sci. 2021, 12, 197–200. [Google Scholar] [CrossRef]
- Uma, S.; Raju, K.; Kalpana, S.; Suthanthiram, B.; Saraswathi, M. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef] [PubMed]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S. Evaluation of temporary immersion bioreactors for in vitro micropropagation of banana (Musa spp.) and genetic fidelity assessment using flow cytometry and simple-sequence repeat markers. S. Afr. J. Bot. 2023, 157, 553–565. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary immersion systems (TISs): A comprehensive review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Berkov, S.; Claus, D.; Gerth, A.; Bastida, J.; Codina, C. Production of galanthamine by Leucojum aestivum shoots grown in different bioreactor systems. Appl. Biochem. Biotechnol. 2012, 167, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.S.; Pan, X.; Jin, L.; Xu, D.; Zhang, B.; Duns, G.J.; Shi, J.; Chen, J. Optimization of nutritional conditions using a temporary immersion bioreactor system for the growth of Bletilla striata pseudobulbs and accumulation of polysaccharides. Sci. Hortic. 2018, 240, 155–161. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Lyam, P.T.; Musa, M.L.; Jamaleddine, Z.O.; Okere, U.A.; Odofin, W.T. The potential of temporary immersion bioreactors (TISs) in meeting crop production demand in Nigeria. J. Biol. Life Sci. 2012, 3, 66–86. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees Struct. Funct. 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Sottile, F. Temporary Immersion System as an Innovative Approach for In Vitro Propagation of Sorbus domestica L. Horticulturae 2024, 10, 164. [Google Scholar] [CrossRef]
- Mamun, N.H.; Egertsdotter, U.; Aidun, C.K. Bioreactor technology for clonal propagation of plants and metabolite production. Front. Biol. 2015, 10, 177–193. [Google Scholar] [CrossRef]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a New Temporary Immersion Bioreactor System for Micropropagation of Cultivars of Eucalyptus, Birch and Fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pożoga, M.; Olewnicki, D.; Jabłońska, L. In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Appl. Sci 2019, 9, 2272. [Google Scholar] [CrossRef]
- Elum, Z.A.; Etowa, E.B.; Ogonda, A.U. Economics of cucumber production in rivers state, Nigeria. Agro-Sci. 2016, 15, 48. [Google Scholar] [CrossRef]
- Takayama, S.; Akita, M. The types of bioreactors used for shoots and embryos. Plant Cell Tissue Organ Cult. 1994, 39, 147–156. [Google Scholar] [CrossRef]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Werner, S.; Maschke, R.; Eibl, D.; Eibl, R. Bioreactor Technology for Sustainable Production of Plant Cell-Derived Products. In Bioprocessing of Plant In Vitro Systems; Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Cham, Switzerland, 2017; pp. 413–432. [Google Scholar]
- Escalona, M.; Samson, G.; Borroto, C.; Desjardins, Y. Physiology of Effects of Temporary Immersion Bioreactors on Micropropagated Pineapple Plantlets. In Vitro Cell. Dev. Biol. Plant 2003, 39, 651–656. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Murch, S.; Liu, C.; Romero, R.; Saxena, P. In vitro Culture and Temporary Immersion Bioreactor Production of Crescentia cujete. Plant Cell Tissues Organ Cult. 2004, 73, 63–68. [Google Scholar] [CrossRef]
- Tomar, U.K.; Negi, U.; Sinha, A.K.; Kumar, P. An overview of the economic factors influencing micropropagation. My For. 2007, 43, 523–534. [Google Scholar]
- Bhoite, H.A.; Palshikar, G.S. Plant tissue culture: A review. World J. Pharm. Sci. 2014, 2, 565–572. [Google Scholar]
- Harris, R.E.; Mason, E.B. Two machines for in vitro propagation of plants in liquid media. Can. J. Plant Sci. 1983, 63, 311–316. [Google Scholar] [CrossRef]
- Tisserat, B.; Vandercook, C.E. Development of an automated plant culture system. Plant Cell Tissue Organ Cult. 1985, 5, 107–117. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Serrano-Fuentes, M.K.; Sorcia-Morales, M.; Bello-Bello, J.J. Temporary Immersion Bioreactors for Sugarcane Multiplication and Rooting. In Micropropagation Methods in Temporary Immersion Systems; Methods in Molecular Biology; Ramírez-Mosqueda, M.A., Cruz-Cruz, C.A., Eds.; Springer: Cham, Switzerland, 2024; Volume 2759, pp. 53–61. [Google Scholar]
- López, C.Q.; Corral, P.; Lorrain-Lorrette, B. Use of a temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot cultures of Thapsia garganica. Plant Methods 2018, 14, 79. [Google Scholar] [CrossRef]
- Erst, G.; Karakulov, A. Rooting and acclimatization of in vitro propagated microshoots of the Ericaceae. J. Appl. Hortic. 2019, 20, 176–180. [Google Scholar] [CrossRef]
- Wojtania, A.; Markiewicz, M.; Góraj-Koniarska, J. Ex vitro rooting, acclimatization and genetic stability of Lonicera caerulea var. kamtschatica. J. Hortic. Res. 2020, 28, 61–70. [Google Scholar] [CrossRef]

| Ingredients for Medium Preparation | Wholesale Prices of Ingredients (in USD) | Ingredients for 1 L of Medium Preparation | Costs of Ingredients for 1 L of Medium Preparation (in USD) |
|---|---|---|---|
| MS medium Duchefa 100 L | 55.60 | MS medium Duchefa (1 L) | 0.56 |
| Sucrose (food sugar) 1 kg (1000 g) | 1.23 | Sucrose (20 g) | 0.02 |
| Agar Ducheffa 25 kg (25,000 g) | 2132.04 | agar Ducheffa (7 g) | 0.60 |
| BAP Ducheffa 25 g (25,000 mg) | 122.67 | BAP Ducheffa (1 mg) | 0.005 * |
| IBA Ducheffa 25 g (25,000 mg) | 75.64 | IBA Ducheffa (0.5 mg) | 0.002 * |
| NAA Ducheffa 100 g (100,000 mg) | 27.75 | NAA Ducheffa (0.5 mg) | 0.001 * |
| Total cost per 1 litre of agar-based multiplication medium | 1.19 | ||
| Total cost per 1 litre of TIS medium | 0.59 | ||
| Total cost per 1 litre of rooting medium | 1.18 | ||
| Work Hour | Agar Cultures | TIS | Agar Rooting |
|---|---|---|---|
| 1 | 14.4 ± 0.7 | 27.1 ± 2.1 | 18.9 ± 0.8 |
| 2 | 15.9 ± 1.5 | 28.8 ± 2.7 | 20.4 ± 1.4 |
| 3 | 17.1 ± 1.0 | 30.1 ± 2.2 | 22.0 ± 1.4 |
| 4 | 20.8 ± 0.7 | 36.6 ± 1.8 | 26.3 ± 1.1 |
| 5 | 19.3 ± 1.4 | 34.2 ± 1.0 | 25.5 ± 1.1 |
| 6 | 18.1 ± 1.0 | 32.3 ± 2.2 | 23.7 ± 1.5 |
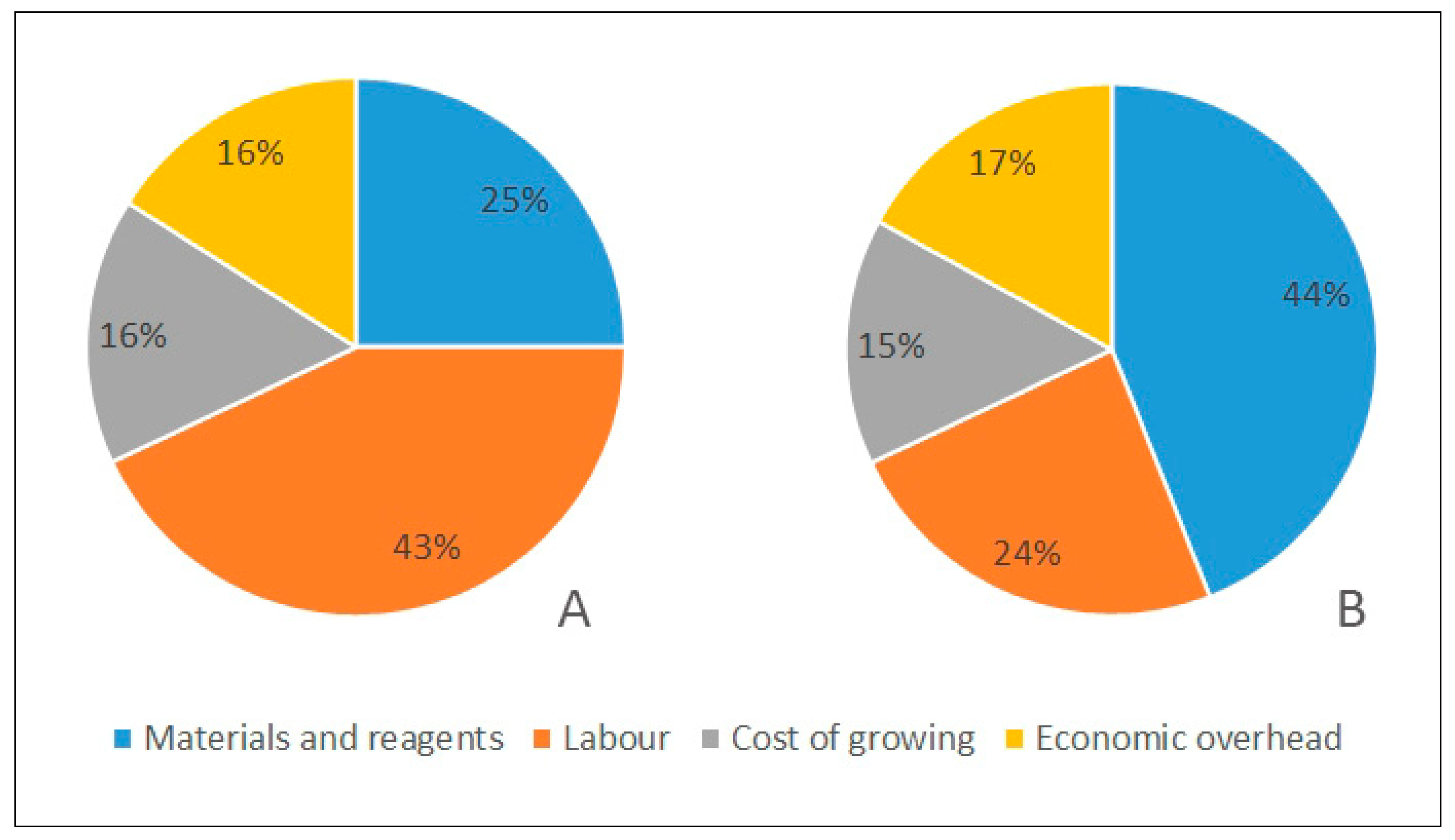
| Tissue Culture Type | Materials and Reagents | Labour | Cost of Growing | Economic Overhead | The Total Cost of a Single Container with 10 Explants | The Total Cost of a Single Plant |
|---|---|---|---|---|---|---|
| Agar cultures | 0.179 | 0.309 | 0.114 | 0.120 | 0.723 | 0.004 |
| TIS | 0.326 | 0.172 | 0.113 | 0.122 | 0.734 | 0.002 |
| Agar rooting | 0.178 | 0.238 | 0.043 | 0.092 | 0.551 | 0.055 |
| Single-rooted agar plant | 0.068 | |||||
| Single rooted TIS plant | 0.067 |
| Specification | Months | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Number of plants in agar cultures | 10 | 10 | 195 | 195 | 3802 |
| Number of plants in TIS | 10 | 362 | 13,104 | 474,379 | 17,172,530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pożoga, M.; Olewnicki, D.; Latocha, P. A Temporary Immersion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’. Agriculture 2024, 14, 1177. https://doi.org/10.3390/agriculture14071177
Pożoga M, Olewnicki D, Latocha P. A Temporary Immersion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’. Agriculture. 2024; 14(7):1177. https://doi.org/10.3390/agriculture14071177
Chicago/Turabian StylePożoga, Mariusz, Dawid Olewnicki, and Piotr Latocha. 2024. "A Temporary Immersion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’" Agriculture 14, no. 7: 1177. https://doi.org/10.3390/agriculture14071177
APA StylePożoga, M., Olewnicki, D., & Latocha, P. (2024). A Temporary Immersion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’. Agriculture, 14(7), 1177. https://doi.org/10.3390/agriculture14071177







