MutL Significantly Regulates the Formation of Biofilms in Bacillus amyloliquefaciens YT1
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. The Establishment of WT B. amyloliquefaciens YT1 Mutant Libraries and the Isolation of the mutL Gene
2.3. Construction of the ΔmutL Mutant and Tagging Strains with GFP
2.4. Antifungal Activity of YT1 and ΔmutL Mutant against R. solani
2.5. Colonization Detection in Rice Plants Using Confocal Microscopy and Assessment of Biocontrol Efficacy against Rice Sheath Blight
2.6. Genomic Transcript Levels Analyzed between WT B. amyloliquefaciens YT1 and the ΔmutL Mutant Using the Illumina HiSeqTM2500 Platform
3. Results
3.1. The Acquisition of the mutL Gene
3.2. No Significant Difference in the Antibacterial Activity
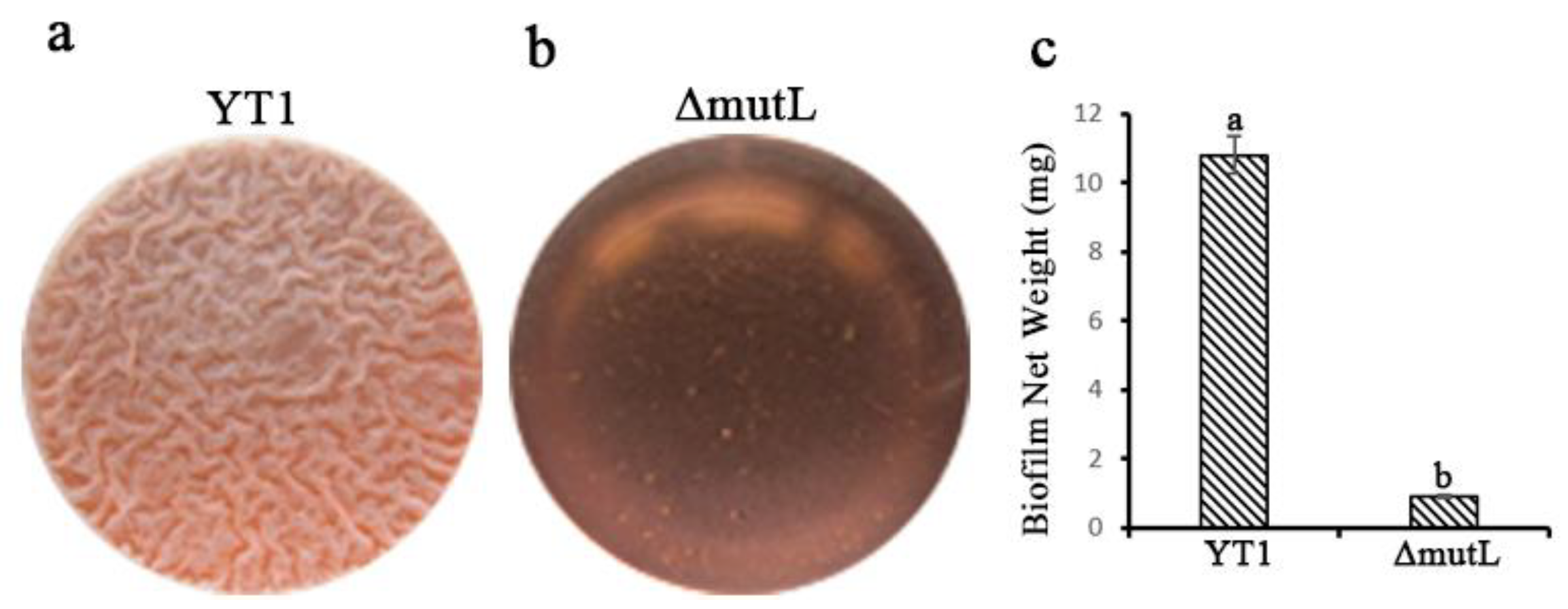
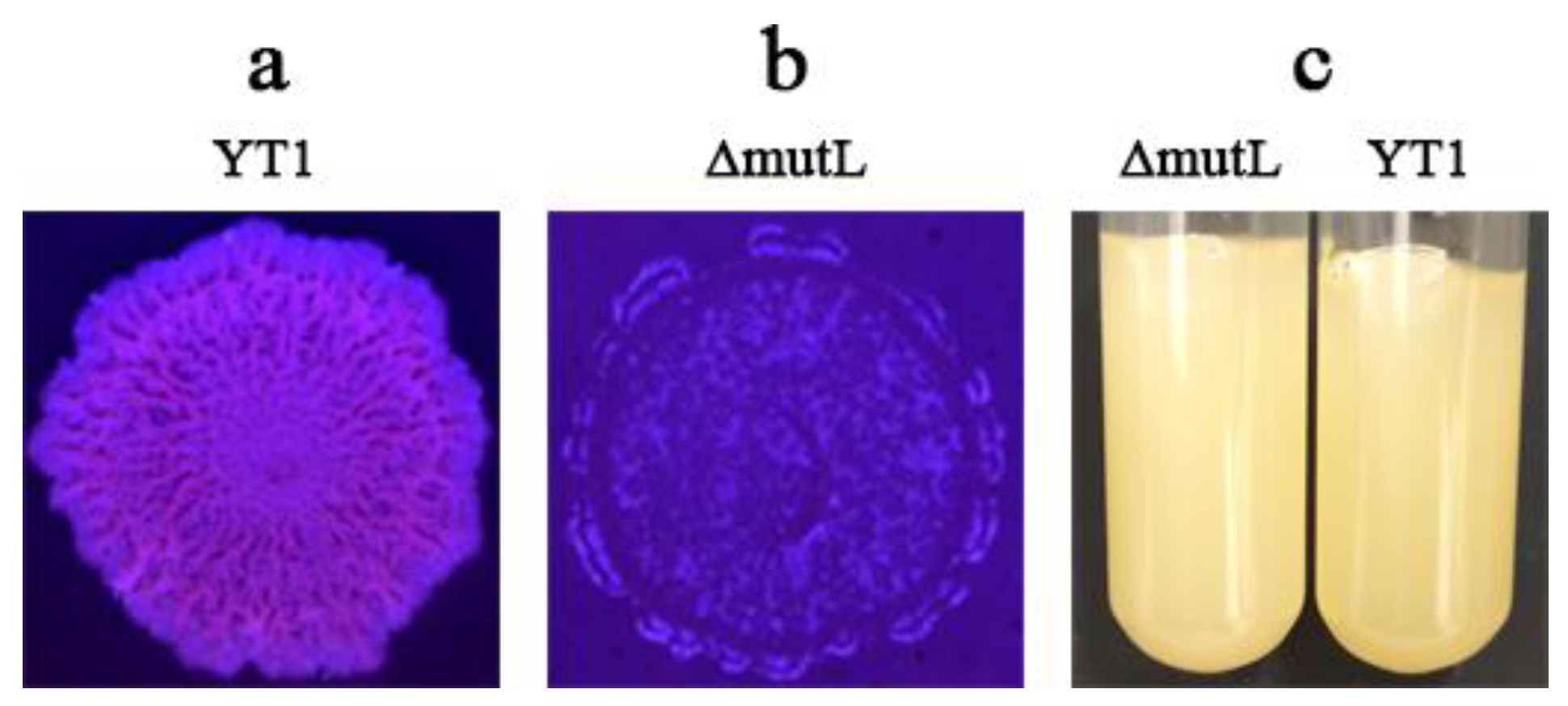
3.3. Significant Decrease in Colonization Ability of the Mutant ΔmutL
3.4. Significant Reduction in the Biocontrol Efficacy of the ΔmutL Mutant against Rice Sheath Blight
3.5. Comparative Analysis of Differential Gene Expression in B. amyloliquefaciens YT1 and the ΔmutL Mutant
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haggag, W.M.; Timmusk, S. Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. J. Appl. Microbiol. 2008, 104, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Wang, Y.; Li, J.; Shen, Q.; Zhang, R. Enhanced root colonization and biocontrol activity of Bacillus amyloliquefaciens SQR9 by abrB gene disruption. Appl. Microbiol. Biotechnol. 2013, 97, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Kantiwal, U.; Pandey, J. Efficient Inhibition of Bacterial Biofilm through Interference of Protein–Protein Interaction of Master Regulator Proteins: A Proof of Concept Study with SinR-SinI Complex of Bacillus subtilis. Appl. Biochem. Biotechnol. 2023, 195, 1947–1967. [Google Scholar] [CrossRef] [PubMed]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zeng, N.; Li, C.; Li, Z.; Zhang, N.; Li, B. Fungal biofilm formation and its regulatory mechanism. Heliyon 2024, 10, e32766. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, Y.; Mao, W.; Peng, Y.; Han, X.; Jin, H.; Xu, J.; Chang, L.; Hou, Y.; Shen, X. Functional versatility of Zur in metal homeostasis, motility, biofilm formation, and stress resistance in Yersinia pseudotuberculosis. Microbiol. Spectr. 2024, 12, e3723–e3756. [Google Scholar] [CrossRef]
- Zegadło, K.; Gieroń, M.; Żarnowiec, P.; Durlik-Popińska, K.; Kręcisz, B.; Kaca, W.; Czerwonka, G. Bacterial motility and its role in skin and wound infections. Int. J. Mol. Sci. 2023, 24, 1707. [Google Scholar] [CrossRef] [PubMed]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; González-Pastor, J.E.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Monterrosa, R.; Kankel, S.; Götze, S.; Barnett, R.; Stallforth, P.; Kovács, Á.T. Lysinibacillus fusiformis M5 induces increased complexity in Bacillus subtilis 168 colony biofilms via hypoxanthine. J. Bacteriol. 2017, 199, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Almirón, J.; Navarro, Y.; Magno-Pérez-Bryan, M.C.; Molina-Santiago, C.; Pearson, J.R.; Díaz-Martínez, L.; de Vicente, A.; Pérez-García, A.; Romero, D. Dual functionality of the TasA amyloid protein in Bacillus physiology and fitness on the phylloplane. Nat. Commun. 2020, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, N.; Ren, Y.; Cui, T. Transcriptomic and biochemical analysis of metabolic remodeling in Bacillus subtilis MSC4 under Benzo [a] pyrene stress. Chemosphere 2024, 353, 141637. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, F.; Chen, Y.; Jin, C.; Guo, J.; Chai, Y. Poly-γ-glutamic acids contribute to biofilm formation and plant root colonization in selected environmental isolates of Bacillus subtilis. Front. Microbiol. 2016, 7, 1811. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer-Shaanan, Y.; Sibony-Nevo, O.; Bloom-Ackermann, Z.; Suissa, R.; Steinberg, N.; Kartvelishvily, E.; Brumfeld, V.; Kolodkin-Gal, I. Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. Npj Biofilms Microbiomes 2016, 2, 15031. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Kolter, R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol. Rev. 2010, 34, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Bakalakos, M.; Ampadiotaki, M.; Vlachos, C.; Sipsas, N.; Pneumaticos, S.; Vlamis, J. Molecular Mechanisms of Biofilm Formation on Orthopaedic Implants: Review of the Literature. Maedica 2024, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.T.; Gallegos-Monterrosa, R. Phenotypic plasticity: The role of a phosphatase family in the genetic regulation of Bacillus. Mol. Microbiol. 2023, 120, 20–31. [Google Scholar]
- Guttenplan, S.B.; Blair, K.M.; Kearns, D.B. The EpsE flagellar clutch is bifunctional and synergizes with EPS biosynthesis to promote Bacillus subtilis biofilm formation. PLoS Genet. 2010, 6, e1001243. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, M.R.; Mardanova, A.M.; Rudakova, N.L.; Pudova, D.S. Bistability and formation of the biofilm matrix as adaptive mechanisms during the stationary phase of Bacillus subtilis. Microbiology 2021, 90, 20–36. [Google Scholar] [CrossRef]
- Blair, K.M.; Turner, L.; Winkelman, J.T.; Berg, H.C.; Kearns, D.B. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 2008, 320, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Qiao, J.; Li, P.; Zhang, L.; Qiao, Z.; Lin, L.; Yu, C.; Yang, Y.; Zubair, M.; Gu, Q. A novel Rap-Phr system in Bacillus velezensis NAU-B3 regulates surfactin production and sporulation via interaction with ComA. Appl. Microbiol. Biotechnol. 2020, 104, 10059–10074. [Google Scholar] [CrossRef] [PubMed]
- Marlow, V.L.; Porter, M.; Hobley, L.; Kiley, T.B.; Swedlow, J.R.; Davidson, F.A.; Stanley-Wall, N.R. Phosphorylated DegU manipulates cell fate differentiation in the Bacillus subtilis biofilm. J. Bacteriol. 2014, 196, 16–27. [Google Scholar] [CrossRef]
- Hobley, L.; Ostrowski, A.; Rao, F.V.; Bromley, K.M.; Porter, M.; Prescott, A.R.; MacPhee, C.E.; Van Aalten, D.M.; Stanley-Wall, N.R. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2013, 110, 13600–13605. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Filho, R.G.; Campos, A.C.; Souza, I.D.S.; Saramago, C.S.D.M.; de Lima E Silva, A.A. Production of Poly-γ-Glutamic Acid (γ-PGA) by Clinical Isolates of. Open Microbiol. J. 2020, 14, 30–37. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Shu, C.; Zhu, M.; Zhou, E. Isolation and characterization of a melanin from Rhizoctonia solani, the causal agent of rice sheath blight. Eur. J. Plant Pathol. 2015, 142, 281–290. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Zhang, C.; Wang, L.; Fan, C.; Zhong, C. Probing the growth and mechanical properties of Bacillus subtilis biofilms through genetic mutation strategies. Synth. Syst. Biotechnol. 2022, 7, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lander, S.M.; Prindle, A. Active pH regulation facilitates Bacillus subtilis biofilm development in a minimally buffered environment. Mbio 2024, 15, e3323–e3387. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, H.; Zou, J.; Wang, X.; Zhang, R.; Xiang, Y.; Chen, Z. Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2015, 99, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Luo, C.P.; Wang, X.Y. Construction of Bacillus subtilis Bs916 mutant libraries by transposon tagging and cloning the genes to the organism’s anti-bacterial activities. Sci. Agric. Sin. 2013, 46, 2232–2239. [Google Scholar]
- Steinberg, N.; Rosenberg, G.; Keren-Paz, A.; Kolodkin-Gal, I. Collective vortex-like movement of Bacillus subtilis facilitates the generation of floating biofilms. Front. Microbiol. 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiao, H.; Huang, L.; Buchenauer, H.; Han, Q.; Kang, Z.; Gong, Y. Biological control of take-all in wheat by endophytic Bacillus subtilis E1R-j and potential mode of action. Biol. Control 2009, 49, 277–285. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Feng, H.; Huang, L.; Kang, Z. Biological control of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in greenhouse and field trials. Crop Prot. 2013, 43, 201–206. [Google Scholar] [CrossRef]
- Gao, X.; Gong, Y.; Huo, Y.; Han, Q.; Kang, Z.; Huang, L. Endophytic Bacillus subtilis strain E1R-J is a promising biocontrol agent for wheat powdery mildew. Biomed. Res. Int. 2015, 2015, 462645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.K.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Zhou, X.G.; Sudini, H.; Groth, D.E.; Raju, S.K.; Miller, M.E. Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef]
- Krishna Kumar, K.V.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Miller, M.E.; Sudini, H.; Surendranatha Reddy, E.S.; Zhou, X.G.; Groth, D.E. Ultrastructural studies on the interaction between Bacillus subtilis MBI 600 (Integral®) and the rice sheath blight pathogen, Rhizoctonia solani. Afr. J. Microbiol. Res. 2013, 7, 2078–2086. [Google Scholar]
- Branda, S.S.; Chu, F.; Kearns, D.B.; Losick, R.; Kolter, R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006, 59, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Csh Perspect. Biol. 2010, 2, a398. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; Vlamakis, H.; Losick, R.; Kolter, R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011, 80, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Guarné, A. The functions of MutL in mismatch repair: The power of multitasking. Prog. Mol. Biol. Transl. 2012, 110, 41–70. [Google Scholar]
- Liu, J.; He, D.; Li, X.; Gao, S.; Wu, H.; Liu, W.; Gao, X.; Zhou, T. γ-Polyglutamic acid (γ-PGA) produced by Bacillus amyloliquefaciens C06 promoting its colonization on fruit surface. Int. J. Food Microbiol. 2010, 142, 190–197. [Google Scholar] [CrossRef]
- Liu, J.; Hanne, J.; Britton, B.M.; Bennett, J.; Kim, D.; Lee, J.; Fishel, R. Cascading MutS and MutL sliding clamps control DNA diffusion to activate mismatch repair. Nature 2016, 539, 583–587. [Google Scholar] [CrossRef] [PubMed]


| Strain/Plasmid | Description | Source or Reference |
|---|---|---|
| B. amyloliquefaciens YT1 | WT strain | CGMCC * NO. 29889 |
| ΔmutL | ΔmutL::Specr *, YT1 derivative | This study |
| ΔYT1-gfp | ΔYT1-gfp::Cmr *, YT1 tagged with green fluorescent protein | This study |
| ΔmutL-gfp | ΔmutL:: Specr *, ΔmutL tagged with green fluorescent protein | This study |
| Plasmids | ||
| pUC19 | Cloning vector, Ampr * | TakaRa U07650 |
| pDG1728 | Cloning vector, Ampr Specr * | BGSC No. ECE114 |
| pUCSpec | pUC19 carrying spectinomycin cassette from pDG1728 | This study |
| pUCSpec-MutL | pUCSpec carrying 741 bp fragment MutL | This study |
| pRp22-gfp | This study |
| Treatment | 4 d | 8 d | 12 d | |||
|---|---|---|---|---|---|---|
| Lesion Size (cm2) | Control Efficiency (%) | Lesion Size (cm2) | Control Efficiency (%) | Lesion Size (cm2) | Control Efficiency (%) | |
| YT1 | 1.4 (0.1) b | 63.2 a | 7.8 (0.3) c | 50.6 a | 16.5 (0.7) c | 42.9 a |
| ΔmutL | 3.2 (0.2) a | 15.8 b | 13.7 (0.4) ab | 13.3 b | 25.8 (0.9) ab | 10.7 b |
| CK | 3.8 (0.2) a | 15.8 (0.4) a | 28.9 (0.9) a | |||
| Gene | Relative Expression Level | Function | |
|---|---|---|---|
| B. amyloliquefaciens YT1 | ΔmutL | ||
| senN | 1 | 0.41 | SenN transcriptional regulator |
| capB | 1 | 0.44 | γ-PGA synthesis |
| appA-D | 1 | 0.44 | Periplasmic oligopeptide-binding protein |
| nprE | 1 | 0.52 | Extracellular neutral metalloprotease |
| epsA-B | 1 | 0.6 | Substrate for biofilm formation |
| tasA | 1 | 0.87 | Substrate for biofilm formation |
| sipW-yqxM | 1 | 1.37 | Type I signal peptidase protein for biofilm |
| kinA-D | 1 | 1.58 | Two-component sensor histidine kinase |
| spo0A | 1 | 1.8 | Response regulator |
| degQ | 1 | 1.96 | Pleiotropic regulator |
| sinR | 1 | 2.09 | Master regulator of biofilm formation |
| abrB | 1 | 2.5 | Transcriptional regulator for transition state |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Chen, M.; Li, B.; Chen, H.; Wang, H.; Wang, S.; Luan, B.; Liu, B. MutL Significantly Regulates the Formation of Biofilms in Bacillus amyloliquefaciens YT1. Agriculture 2024, 14, 1193. https://doi.org/10.3390/agriculture14071193
Zhou H, Chen M, Li B, Chen H, Wang H, Wang S, Luan B, Liu B. MutL Significantly Regulates the Formation of Biofilms in Bacillus amyloliquefaciens YT1. Agriculture. 2024; 14(7):1193. https://doi.org/10.3390/agriculture14071193
Chicago/Turabian StyleZhou, Huafei, Min Chen, Baoyan Li, Haining Chen, Hongtao Wang, Shaoli Wang, Binghui Luan, and Baoyou Liu. 2024. "MutL Significantly Regulates the Formation of Biofilms in Bacillus amyloliquefaciens YT1" Agriculture 14, no. 7: 1193. https://doi.org/10.3390/agriculture14071193
APA StyleZhou, H., Chen, M., Li, B., Chen, H., Wang, H., Wang, S., Luan, B., & Liu, B. (2024). MutL Significantly Regulates the Formation of Biofilms in Bacillus amyloliquefaciens YT1. Agriculture, 14(7), 1193. https://doi.org/10.3390/agriculture14071193





