Identification of Fusarium spp. Associated with Chickpea Root Rot in Montana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling and Recovery of Isolates
2.2. Genomic DNA Isolation and Amplification
2.3. Amplicon Sequencing
2.4. Aggressiveness Tests in the Greenhouse
2.5. Data Analysis
2.6. Phylogenetic Analysis
3. Results
3.1. Identification and Prevalence of Fusarium Species
3.2. Aggressiveness Assessment Tests
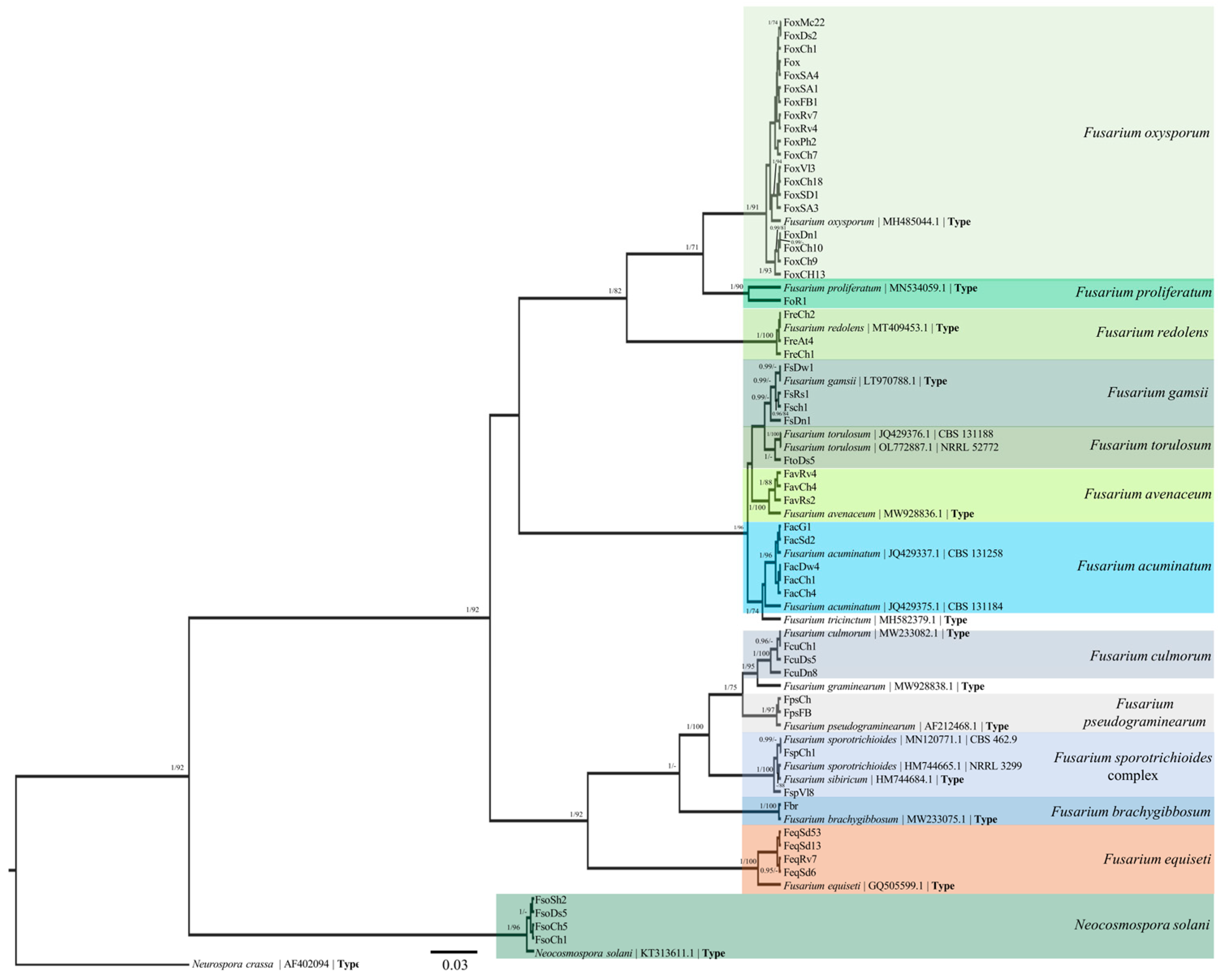
3.3. TEF-1α Sequences and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.W.; Ford, R. Chickpea. In Pulses, Sugar and Tuber Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 109–121. [Google Scholar]
- Van der Maessen, L.J.G. Cicer L., a Monograph of the Genus, with Special Reference to the Chickpea (Cicer arietinum L.), Its Ecology and Cultivation; Wageningen University and Research: Wageningen, The Netherlands, 1972. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 29 May 2004).
- Dugan, F.M.; Lupien, S.L.; Hu, J. Fungal plant pathogens associated with emerging crops in North America: A challenge for plant health professionals. Plant Health Prog. 2017, 18, 221–229. [Google Scholar] [CrossRef]
- USA Dry Pea, Lentil, and Chickpea Production. Available online: https://agresearch.montana.edu/wtarc/producerinfo/agronomy-nutrient-management/Pulses/USADryPeaCouncil%20FactSheet.pdf (accessed on 28 May 2024).
- Attaway, D. Clemson Scientist Discover Plant Genes to Help Grow S.C. Economy. Available online: https://news.clemson.edu/clemson-scientists-discover-plant-genes-to-help-boost-s-c-economy/ (accessed on 23 May 2024).
- Miller, P.R.; Holmes, J.A. Cropping sequences effects of four broadleaf crops on four cereal crops in the northern Great Plains. Agron. J. 2005, 97, 189–200. [Google Scholar] [CrossRef]
- Muehlbauer, F.J.; Sarker, A. Economic importance of chickpea: Production, value, and world trade. In The Chickpea Genome; Springer: Cham, Switzerland, 2017; pp. 5–12. [Google Scholar] [CrossRef]
- Boukid, F. Chickpea (Cicer arietinum L.) protein as a prospective plant- based ingredient: A review. Inter. J. Food Sci. Technol. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Karalija, E.; Vergata, C.; Basso, M.F.; Negussu, M.; Zaccai, M.; Grossi-de-Sa, M.F.; Martinelli, F. Chickpeas’ tolerance of drought and heat: Current knowledge and next steps. Agronomy 2022, 12, 2248. [Google Scholar] [CrossRef]
- Singh, R.; Pratap, T.; Singh, D.; Singh, G.; Singh, A.K. Effect of phosphorus, sulphur and biofertilizers on growth attributes and yield of chickpea (Cicer arietinum L.). J. Pharmacogn. Phytochem. 2018, 7, 3871–3875. [Google Scholar]
- United States Department of Agriculture National Agricultural Statistic Service [USDA NASS]. Statistics by Subject Results. 2020. Available online: https://www.nass.usda.gov/Statistics_by_State/Montana/Publications/Special_Interest_Reports/agfacts.pdf (accessed on 23 April 2020).
- Zhou, Y. Chickpea Cultivar Evaluation and Intercropping for Disease Management and Yield. Master’s Dissertation, Montana State University, Bozeman, MT, USA, 2022. [Google Scholar]
- Bahr, L.; Castelli, M.V.; Barolo, M.I.; Mostacero, N.R.; Tosello, M.E.; López, S.N. Ascochyta blight: Isolation, characterization, and development of a rapid method to detect inhibitors of the chickpea fungal pathogen Ascochyta rabiei. Fungal Biol. 2016, 120, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.M.; Jukanti, A.K.; Varshney, R.K. Impact of genomic technologies on chickpea breeding strategies. Agronomy 2012, 2, 199–221. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Kumar, S.; Patil, B.S.; Sharma, M.; Kemal, S.; Ontagodi, T.P.; Datta, S.; Patil, P.; Chaturvedi, S.K.; Sultana, R.; et al. Narrowing yield gaps through genetic improvement for Fusarium wilt resistance in three pulse crops of the semi-arid tropics. SABRAO J. Breed. Genet. 2013, 45, 341–370. Available online: http://oar.icrisat.org/id/eprint/1079 (accessed on 27 May 2024).
- Halila, I.; Rubio, J.; Millán, T.; Gil, J.; Kharrat, M.; Marrakchi, M. Resistance in chickpea (Cicer arietinum) to Fusarium wilt race ‘0’. Plant Breed. 2010, 129, 563–566. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, Y.; Wang, Y.; Jones, C.; Feindel, D.; Harding, M.; Feng, J. Phylogenetic, phenotypic and host range characterization of five Fusarium species isolated from chickpea in Alberta, Canada. Can. J. Plant Pathol. 2021, 43, 651–657. [Google Scholar] [CrossRef]
- Moparthi, S.; Burrows, M.; Mgbechi-Ezeri, J.; Agindotan, B. Fusarium spp. associated with root rot of pulse crops and their cross pathogenicity to cereal crops in Montana. Plant Dis. 2020, 105, 548–557. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplificaiton and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Robideau, G.P.; De Cock, A.W.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Citty, D.; Desaulniers, N.; Eggerston, Q.; Gachon, C.M. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spcer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cruz, T.J.; Whitaker, B.K.; Proctor, R.H.; Broders, K.; Laraba, I.; Kim, H.S.; Brown, D.W.; O’Donnell, K.; Estrada-Rodríguez, T.L.; Lee, Y.H.; et al. FUSARIUM-ID v. 3.0: An updated, downloadable resource for Fusarium species identification. Plant Dis. 2022, 106, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, N.J.; Coffman, V.A.; Kraft, J.M. Sources of partial resistance to Fusarium root rot in the Pisum core collection. Plant Dis. 2003, 87, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.A.; Madden, L.V. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology 2004, 94, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Konietschke, F.; Hothorn, L.A.; Brunner, E. Rank-based multiple test procedures and simultaneous confidence intervals. Electron. J. Stat. 2012, 6, 738–759. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Abadi, S.; Azouri, D.; Mayrose, I.; Pupko, T. Model selection may not be a mandatory step for phylogeny reconstruction. Nat. Commun. 2019, 10, 934. [Google Scholar] [CrossRef]
- Gernhard, T. The conditioned reconstructed process. J. Theoretical. Biol. 2008, 253, 769–778. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, Vey016. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. Fig Tree Ver. 1.3.1. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 27 May 2024).
- Silvestro, D.; Michalak, I. RaxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2011, 12, 335–337. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Whitaker, B.K.; Laraba, I.; Proctor, R.H.; Brown, D.W.; Broders, K.; Kim, H.S.; McCormick, S.P.; Busman, M.; Aoki, T.; et al. DNA sequence-based identification of Fusarium: A work in progress. Plant Dis. 2022, 106, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; del Mar Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 11, 473–479. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Chittem, K.; Mathew, F.M.; Gregoire, M.; Lamppa, R.S.; Chang, Y.W.; Markell, S.G.; Bradley, C.A.; Barasubiye, T.; Goswami, R.S. Identification and characterization of Fusarium spp. associated with root rots of field pea in North Dakota. Eur. J. Plant Pathol. 2015, 143, 641–649. [Google Scholar] [CrossRef]
- Zitnick-Anderson, K.; Gargouri Jbir, T.; Carlson, A.; Postovit, S.; Pasche, J.; Kalil, A. Fusarium species associated with root rot of lentil (Lens culinaris) in North Dakota. Plant Health Prog. 2021, 22, 524–528. [Google Scholar] [CrossRef]
- Bretag, T.W.; Mebalds, M.I. Pathogenicity of fungi isolated from Cicer arietinum (chickpea) grown in northwestern Victoria. Aust. J. Exp. Agric. 1987, 27, 141–148. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Strelkov, S.E.; Hwang, S.F. Genetic diversity and aggressiveness of Fusarium spp. isolated from canola in Alberta, Canada. Plant Dis. 2014, 98, 727–738. [Google Scholar] [CrossRef]
- Okello, P.N.; Petrovic, K.; Singh, A.K.; Kontz, B.; Mathew, F.M. Characterization of species of Fusarium causing root rot of soybean (Glycine max L.) in South Dakota, USA. Canada. Can. J. Plant Pathol. 2020, 42, 560–571. [Google Scholar] [CrossRef]
- Cother, E.J. Identification and control of root-rot fungi in Cicer arietinum (chickpea). Plant Dis. Rep. 1977, 61, 736–740. [Google Scholar]
- Cruz, A.F.; Hamel, C.; Yang, C.; Matsubara, T.; Gan, Y.; Singh, A.K.; Kuwada, K.; Ishii, T. Phytochemicals to suppress Fusarium head blight in wheat–chickpea rotation. Phytochemistry 2012, 78, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.; Nawrocki, J.; Kucmierz, J. Fungal diseases of chickpea (Cicer arietinum L.) cultivated in the south region of Poland. Plant Prot. Sci. 2002, 38, 332–335. [Google Scholar] [CrossRef]
- Jiménez-Fernández, D.; Navas-Cortés, J.A.; Montes-Borrego, M.; Jiménez-Díaz, R.M.; Landa, B.B. Molecular and pathogenic characterization of Fusarium redolens, a new causal agent of Fusarium yellows in chickpea. Plant Dis. 2011, 95, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Bouhadida, M.; Jendoubi, W.; Gargouri, S.; Beji, M.; Kharrat, M.; Chen, W. First report of Fusarium redolens causing Fusarium yellowing and wilt of chickpea in Tunisia. Plant Dis. 2017, 101, 1038. [Google Scholar] [CrossRef]
- Saeedi, S.; Jamali, S. Molecular characterization and distribution of Fusarium isolates from uncultivated soils and chickpea plants in Iran with special reference to Fusarium redolens. J. Plant Pathol. 2021, 103, 167–183. [Google Scholar] [CrossRef]
- Westerlund, F.V.; Campbell, R.N.; Kimble, K.A. Fungal root rots and wilt of chickpea in California. Phytopathology 1974, 64, 432–436. [Google Scholar]
- Dugan, F.M.; Lupien, S.L.; Hernandez-Bello, M.; Peever, T.L.; Chen, W. Fungi resident in chickpea debris and their suppression of growth and reproduction of Didymella rabiei under laboratory conditions. J. Phytopathol. 2005, 153, 431–439. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Simpfendorfer, S.; Holland, J.; Bambach, R.; Moore, K.J.; Rebetzke, G.J. Effect of previous crops on crown rot and yield of durum and bread wheat in northern NSW. Aust. J. Agric Res. 2004, 55, 321–334. [Google Scholar] [CrossRef]
- Ali, H.Z.; Hameed, M.S.; Abdulrahman, A.A.; Saood, H.M. First report on Fusarium brachygibbosum isolate FIR 16_ITS isolated from Iraqi wheat plant. J. Ecol. Eng. 2020, 21, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Namsi, A.; Rabaaoui, A.; Masiello, M.; Moretti, A.; Othmani, A.; Gargouri, S.; Gdoura, R.; Werbrouck, S.P. First report of leaf wilt caused by Fusarium proliferatum and F. brachygibbosum on Date Palm (Phoenix dactylifera) in Tunisia. Plant Dis. 2021, 105, 1217. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Li, J.; Zheng, W.M.; Su, X.H.; Xing, G.Z.; Li, S.J.; Zhang, Z.Y.; Li, C.J.; Wang, J.; Chen, Y.G.; et al. First report of root rot of tobacco caused by Fusarium brachygibbosum in China. Plant Dis. 2021, 105, 4170. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names-restyling the Fusarium incarnatum-equiseti species complex. Persoonia. Mol. Phylo. Evol. Fung. 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Adnani, M.; El Hazzat, N.; Msairi, S.; El Alaoui, M.A.; Mouden, N.; Selmaoui, K.; Benkirane, R.; Ouazzani Touhami, A.; Douira, A. Exploring the efficacy of a Trichoderma asperellum-based seed treatment for controlling Fusarium equiseti in chickpea. Egypt. J. Biol. Pest Co. 2024, 34, 7. [Google Scholar] [CrossRef]
- Younesi, H.; Darvishnia, M.; Bazgir, E.; Chehri, K. Morphological, molecular and pathogenic characterization of Fusarium spp. associated with chickpea wilt in western Iran. J. Plant Prot. Res. 2021, 61, 402–413. [Google Scholar]
- Duarte-Leal, Y.; Martínez-Coca, B.; Echevarria-Hernández, A.; do Carmo de Souza, E.S.; Miller, R.N.G.; Café-Filho, A.C. First report of vascular wilt caused by Fusarium proliferatum on chickpea in Cuba. N. Dis. Rep. 2020, 41, 32. [Google Scholar] [CrossRef]
- Yu, H.; Hwang, S.F.; Strelkov, S.E. The Host Range of Fusarium proliferatum in Western Canada. Pathogens 2024, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.M.D.; Leandro, L.F.; Munkvold, G.P. Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology 2013, 103, 822–832. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Pandey, S.; Parida, S.K. Breeding, genetics, and genomics approaches for improving Fusarium wilt resistance in major grain legumes. Front. Gen. 2020, 11, 1001. [Google Scholar] [CrossRef]
- Mannur, D.M.; Babbar, A.; Thudi, M.; Sabbavarapu, M.M.; Roorkiwal, M.; Yeri, S.B.; Bansal, V.P.; Jayalakshmi, S.K.; Singh Yadav, S.; Rathore, A.; et al. Super Annigeri 1 and improved JG 74: Two Fusarium wilt-resistant introgression lines developed using marker-assisted backcrossing approach in chickpea (Cicer arietinum L.). Molec. Breed. 2019, 39, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Ghosh, R.; Tarafdar, A.; Rathore, A.; Chobe, D.R.; Kumar, A.V.; Gaur, P.M.; Samineni, S.; Gupta, O.; Singh, N.P.; et al. Exploring the genetic cipher of chickpea (Cicer arietinum L.) through identification and multi-environment validation of resistant sources against Fusarium wilt (Fusarium oxysporum f. sp. ciceris). Front. Sustain. Food Syst. 2019, 3, 78. [Google Scholar] [CrossRef]
- Navas-Cortés, J.A.; Hau, B.; Jiménez-Díaz, R.M. Yield loss in chickpeas in relation to development of Fusarium wilt epidemics. Phytopathology 2000, 90, 1269–1278. [Google Scholar] [CrossRef] [PubMed]

| 95% CI for | ||||||
|---|---|---|---|---|---|---|
| Fusarium spp. | Isolate | MDR y | z | Lower | Upper | |
| F. avenaceum | Fav 1 | 5 | 1001.7 | 0.96 | 0.96 | 0.97 |
| F. avenaceum | Fav 2 | 3 | 793.5 | 0.76 | 0.67 | 0.86 |
| F. avenaceum | Fav 3 | 5 | 976.2 | 0.94 | 0.91 | 0.97 |
| F. avenaceum | Fav 4 | 4 | 976.7 | 0.94 | 0.92 | 0.95 |
| F. avenaceum | Fav 5 | 5 | 1006.1 | 0.97 | 0.96 | 0.97 |
| F. accuminatum | Fac 6 | 1 | 362.0 | 0.35 | 0.25 | 0.44 |
| F. accuminatum | Fac 7 | 1 | 330.7 | 0.32 | 0.24 | 0.40 |
| F. accuminatum | Fac 8 | 1 | 417.4 | 0.40 | 0.30 | 0.50 |
| F. accuminatum | Fac 9 | 2 | 544.2 | 0.52 | 0.42 | 0.62 |
| F. accuminatum | Fac 10 | 2 | 488.3 | 0.47 | 0.36 | 0.58 |
| F. redolens | Fre 11 | 2 | 724.6 | 0.70 | 0.62 | 0.77 |
| F. redolens | Fre 12 | 2 | 591.0 | 0.57 | 0.46 | 0.67 |
| F. redolens | Fre 13 | 2 | 527.2 | 0.51 | 0.38 | 0.63 |
| F. redolens | Fre 14 | 2 | 551.7 | 0.53 | 0.42 | 0.64 |
| F. redolens | Fre 15 | 2 | 630.6 | 0.61 | 0.51 | 0.70 |
| N. solani | Fso 16 | 1 | 370.3 | 0.36 | 0.27 | 0.45 |
| N. solani | Fso 17 | 2 | 736.1 | 0.71 | 0.63 | 0.79 |
| N. solani | Fso 18 | 3 | 802.0 | 0.77 | 0.72 | 0.82 |
| N. solani | Fso 19 | 1 | 465.7 | 0.45 | 0.36 | 0.54 |
| N. solani | Fso 20 | 1 | 386.5 | 0.37 | 0.29 | 0.46 |
| F. brachygibbosum | Fbr 21 | 2 | 695.1 | 0.67 | 0.64 | 0.70 |
| F. oxysporum | Fox 22 | 2 | 716.3 | 0.69 | 0.61 | 0.77 |
| F. oxysporum | Fox 23 | 2 | 553.2 | 0.53 | 0.45 | 0.61 |
| F. oxysporum | Fox 24 | 2 | 513.6 | 0.49 | 0.41 | 0.58 |
| F. oxysporum | Fox 25 | 1 | 426.1 | 0.41 | 0.32 | 0.50 |
| F. oxysporum | Fox 26 | 1 | 283.5 | 0.27 | 0.23 | 0.32 |
| F. equiseti | Feq 27 | 1 | 276.0 | 0.26 | 0.25 | 0.28 |
| F. equiseti | Feq 28 | 1 | 315.6 | 0.30 | 0.25 | 0.35 |
| F. equiseti | Feq 29 | 1 | 335.4 | 0.32 | 0.26 | 0.38 |
| F. equiseti | Feq 30 | 1 | 370.3 | 0.36 | 0.27 | 0.45 |
| F. torulosum | Fto 31 | 1 | 276.0 | 0.26 | 0.25 | 0.28 |
| F. torulosum | Fto 32 | 1 | 283.5 | 0.27 | 0.23 | 0.32 |
| F. torulosum | Fto 33 | 2 | 551.4 | 0.53 | 0.42 | 0.64 |
| F. torulosum | Fto 34 | 2 | 556.5 | 0.53 | 0.44 | 0.63 |
| F. gamsii | Ftr 35 | 0 | 141.0 | 0.14 | 0.08 | 0.19 |
| F. gamsii | Ftr 36 | 1 | 465.7 | 0.45 | 0.36 | 0.54 |
| F. gamsii | Ftr 37 | 1 | 276.0 | 0.26 | 0.25 | 0.28 |
| F. gamsii | Ftr 38 | 1 | 401.3 | 0.39 | 0.28 | 0.49 |
| F. culmorum | Fcu 39 | 2 | 647.1 | 0.62 | 0.56 | 0.68 |
| F. culmorum | Fcu 40 | 1.5 | 497.1 | 0.48 | 0.38 | 0.58 |
| F. culmorum | Fcu 41 | 1 | 488.8 | 0.47 | 0.36 | 0.57 |
| F. pseudograminearum | Fps 42 | 2 | 622.3 | 0.60 | 0.50 | 0.69 |
| F. pseudograminearum | Fps 43 | 1.5 | 473.3 | 0.45 | 0.36 | 0.55 |
| F. pseudograminearum | Fps 44 | 1.5 | 502.7 | 0.48 | 0.37 | 0.60 |
| F. sporotrichioides | Fsp 45 | 2 | 638.9 | 0.61 | 0.53 | 0.70 |
| F. sporotrichioides | Fsp 46 | 2 | 607.6 | 0.58 | 0.50 | 0.67 |
| F. sporotrichioides | Fsp 47 | 1 | 417.9 | 0.40 | 0.31 | 0.50 |
| F. sporotrichioides | Fsp 48 | 2 | 604.7 | 0.58 | 0.48 | 0.68 |
| F. sporotrichioides | Fsp 49 | 2 | 584.5 | 0.56 | 0.48 | 0.64 |
| F. proliferatum | Fpr 50 | 1 | 310.9 | 0.30 | 0.23 | 0.37 |
| F. proliferatum | Fpr 51 | 2 | 521.1 | 0.50 | 0.41 | 0.59 |
| control | 0 | 30.5 | 0.03 | 0.02 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moparthi, S.; Perez-Hernandez, O.; Burrows, M.E.; Bradshaw, M.J.; Bugingo, C.; Brelsford, M.; McPhee, K. Identification of Fusarium spp. Associated with Chickpea Root Rot in Montana. Agriculture 2024, 14, 974. https://doi.org/10.3390/agriculture14070974
Moparthi S, Perez-Hernandez O, Burrows ME, Bradshaw MJ, Bugingo C, Brelsford M, McPhee K. Identification of Fusarium spp. Associated with Chickpea Root Rot in Montana. Agriculture. 2024; 14(7):974. https://doi.org/10.3390/agriculture14070974
Chicago/Turabian StyleMoparthi, Swarnalatha, Oscar Perez-Hernandez, Mary Eileen Burrows, Michael J. Bradshaw, Collins Bugingo, Monica Brelsford, and Kevin McPhee. 2024. "Identification of Fusarium spp. Associated with Chickpea Root Rot in Montana" Agriculture 14, no. 7: 974. https://doi.org/10.3390/agriculture14070974





