The Use of Aerobic Urban Sewage Sludge in Agriculture: Potential Benefits and Contaminating Effects in Semi-Arid Zones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling of Vegetal Material and Soil
2.3. Analytical Methods
2.3.1. Physicochemical and Chemical Parameters
2.3.2. Soil Respiration and Biochemical Parameters
2.3.3. Pathogenic Microorganisms
2.3.4. Organic Contaminants
2.4. Statistical Analysis
3. Results
3.1. Effects on Soil Characteristics
3.1.1. Agronomic, Microbiological, and Biochemical Soil Parameters
3.1.2. Potential Hazardous Parameters
3.2. Effects on Plants
4. Discussion
4.1. Agronomic, Microbiological, and Biochemical Soil Parameters
4.2. Potential Hazardous Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagmur, M.; Arpah, D.; Gulser, F. The effects of sewage sludge treatment on triticale straw yield and its chemical contents in rainfed condition. J. Anim. Plant Sci. 2017, 27, 971–977. [Google Scholar]
- Melo, W.; Delarica, D.; Guedes, A.; Lavezzo, L.; Donha, R.; de Araújo, A.; de Melo, G.; Macedo, F. Ten years of application of sewage sludge on tropical soil: A balance sheet on agricultural crops and environmental quality. Sci. Total Environ. 2018, 643, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; Leal, T.T.; Araújo, A.S.; Araújo, R.M.; Gomes, R.L.; Melo, W.J.; Singh, R.P. Effect of different tannery sludge compost amendment rates on growth, biomass accumulation, and yield responses of Capsicum plants. Waste Manag. 2010, 30, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, M.G.M.; Ali, O.A.M.; Abdelaal, A.I.N.; Lin, X.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation, and wheat productivity. Sci. Rep. 2020, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.; Berlanga, J.G.; Tormos, I.; Garcia, C. Organic versus inorganic fertilizers: Response of soil properties and crop yield. AIMS Geosci. 2021, 7, 415–439. [Google Scholar] [CrossRef]
- Suhadolc, M.; Schroll, R.; Hagn, A.; Dörfler, U.; Schloter, M.; Lobnik, F. Single application of sewage sludge: Impact on the quality of an alluvial agricultural soil. Chemosphere 2010, 81, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Cherfouh, R.; Lucas, Y.; Derridj, A.; Merdy, P. Long-term, low technicality sewage sludge amendment and irrigation with treated wastewater under Mediterranean climate: Impact on agronomical soil quality. Environ. Sci. Pollut. Res. 2018, 25, 35571–35581. [Google Scholar] [CrossRef] [PubMed]
- Dhanker, R.; Chaudhary, S.; Goyal, S.; Garg, V.K. Influence of urban sewage sludge amendment on agricultural soil parameters. Environ. Technol. Innov. 2021, 23, 101642. [Google Scholar] [CrossRef]
- Hamdi, H.; Hechmi, S.; Khelil, M.N.; Zoghlami, I.R.; Benzarti, S.; Mokni-Tlili, S.; Hassen, A.; Jedidi, N. Repetitive land application of urban sewage sludge: Effect of amendment rates and soil texture on fertility and degradation parameters. Catena 2019, 172, 11–20. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Sulewska, H.; Niewiadomska, A.; Panasiewicz, K.; Borowiak, K.; Ratajczak, K. The influence of sewage sludge and a consortium of aerobic microorganisms added to the soil under a willow plantation on the biological indicators of transformation of organic nitrogen compounds. Pol. J. Environ. Stud. 2018, 27, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, K.; Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Borowiak, K.; Budka, A.; Ratajczak, K. The effect of sewage sludge and BAF inoculant on plant condition and yield as well as biochemical and microbial activity of soil in willow (Salix viminalis L.) culture as an energy crop. PeerJ 2019, 7, e6434. [Google Scholar] [CrossRef] [PubMed]
- Pourcher, A.M.; Françoise, P.B.; Virginie, F.; Agnieszka, G.; Vasilica, S.; Gérard, M. Survival of faecal indicators and enteroviruses in soil after land-spreading of municipal sewage sludge. Appl. Soil Ecol. 2007, 35, 473–479. [Google Scholar] [CrossRef]
- Clarke, B.O.; Smith, S.R. Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- Smith, S.R. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Philos. Trans. R. Soc. A 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.; Wang, M.J.; Padgett, E.; Beck, A.J. Analysis of 4-nonylphenols, phthalates, and polychlorinated biphenyls in soils and biosolids. Chemosphere 2005, 61, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P. Changes of polycyclic aromatic hydrocarbons during composting of sewage sludges with chosen physico-chemical properties and PAHs content. Chemosphere 2007, 67, 582–591. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Working Document on Sludge 3rd Draft; Document 27th April 2000; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- European Commission. Ex-Post Evaluation of Certain Waste Stream Directives. European Commission. 2014. Available online: https://ec.europa.eu/environment/waste/pdf/target_review/Final%20Report%20Ex-Post.pdf (accessed on 26 February 2022).
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Hernandez, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Tontti, T.; Poutiainen, H.; Heinonen-Tanski, H. Efficiently treated sewage sludge supplemented with nitrogen and potassium is a good fertilizer for cereals. Land Degrad. Dev. 2017, 28, 742–751. [Google Scholar] [CrossRef]
- Tejada, M. Application of different organic wastes in a soil polluted by cadmium: Effects on soil biological properties. Geoderma 2009, 153, 254–268. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Laturnus, F.; von Arnold, K.; Grøn, C. Organic contaminants from sewage sludge applied to agricultural soils. False alarm regarding possible problems for food safety? Environ. Sci. Pollut. Res. 2007, 14, 53–60. [Google Scholar] [CrossRef]
- Sieciechowicz, A.; Sadecka, Z.; Myszograj, S.; Włodarczyk-Makula, M.; Wiśniowska, E.; Turek, A. Occurrence of heavy metals and PAHs in soil and plants after application of sewage sludge to soil. Desalin. Water Treat. 2014, 52, 4014–4026. [Google Scholar] [CrossRef]
- Koyuncu, S. Occurrence of organic micropollutants and heavy metals in the soil after the application of stabilized sewage sludge. J. Environ. Health Sci. Eng. 2022, 20, 385–394. [Google Scholar] [CrossRef]
- The Council of the European Communities. EU Council Directive (91/676/EEC) of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Union 1991, L 375, 1–8. [Google Scholar]
- The Council of the European Communities. European Directive 86/278/EEC, of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Union 1986, L 181, 6–12. [Google Scholar]
- Murcia, F.J. Lodos de Depuradora: Una Visión Integral para su Posible Aplicación a Suelos Desde una Perspectiva Agrícola (Sewage Sludge: A Comprehensive Vision for Its Possible Application to Soils from an Agricultural Perspective). Ph D. Thesis, University of Murcia, Murcia, Spain, 2013.
- Ondoño, S.; Martínez Sánchez, J.J.; Moreno, J.L. The inorganic components of Green roof substrates impacts the growth of Mediterranean plant species as well as the C and N sequestration potential. Ecol. Indic. 2016, 61, 739–752. [Google Scholar] [CrossRef]
- CEBAS-CSIC Ionomic Laboratory Service. Available online: http://www.cebas.csic.es/general_spain/ionomica.htlm (accessed on 26 February 2022).
- Hernandez, T.; García, C. Estimación de respiración microbiana del suelo (Stimation of soil respiration). In Técnicas de Análisis de Parámetros Bioquímicos en Suelos. Actividades Enzimáticas y Biomasa Microbiana (Techniques of Analysis of Biochemical Parameters in Soils. Enzymatic Activities and Microbial Biomass); Garcia, C., Gil-Soitres, F., Hernandez, T., Trasar-Cepeda, C., Eds.; Mundi-Prensa: Madrid, Spain, 2003; pp. 311–346. [Google Scholar]
- García, C.; Hernandez, M.T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.K.; Linkins, A.E.; Mcclaugherty, C.A.; Rayburn, L.; Repert, D.; Weiland, T. Wood decomposition: Nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 1993, 74, 1586–1593. [Google Scholar] [CrossRef]
- Aparicio, I.; Santos, J.L.; Alonso, E. Simultaneous sonication-assisted extraction, and determination by gas chromatography–Mass spectrometry, of di-(2-ethylhexyl)phthalate, nonylphenol, nonylphenol ethoxylates and polychlorinated biphenyls in sludge from wastewater treatment plants. Anal. Chim. Acta 2007, 584, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Aparicio, I.; Alonso, E. A new method for the routine analysis of LAS and PAH in sewage sludge by simultaneous sonication-assisted extraction prior to liquid chromatographic determination. Anal. Chim. Acta 2007, 605, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Beyer, L.; Wachendorf, C.; Elsner, D.C.; Knabe, R. Suitability of dehydrogenase activity assay as an index of soil biological activity. Biol. Fertil. Soils 1993, 16, 52–56. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Ronchi, V.; Bauer, C. Kinetic parameters of dehydrogenase in the assessment of the response of soil to vermicompost and inorganic fertilisers. Biol. Fertil. Soils 2000, 32, 479–483. [Google Scholar] [CrossRef]
- Ramulu, U.S. Reuse of Municipal Sewage Sludge in Agriculture; Scientific Publisher: Jodhpur, India, 2001. [Google Scholar]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Kim, H.G.; Jeong, S.; Lee, S.; Lee, K.B.; Lee, S.H.; Choi, J.W. Phosphorous recovery from sewage sludge using calcium silicate Hydrates. Chemosphere 2018, 193, 1087–1093. [Google Scholar] [CrossRef]
- Smith, J.; Johnson, L.; Davis, R. Effects of sewage sludge amendments on soil properties and crop performance. J. Environ. Agric. 2023, 12, 456–472. [Google Scholar]
- García, C.; Hernandez, T.; Coll, M.D.; Ondoño, S. Organic amendments for soil restoration in arid and semiarid areas: A review. AIMS Environ. Sci. 2017, 4, 640–676. [Google Scholar] [CrossRef]
- Parat, C.; Chaussod, R.; Lévêque, J.; Andreux, F. Long-term effects of metal-containing farmyard manure and sewage sludge on soil organic matter in a fluvisol. Soil Biol. Biochem. 2005, 37, 673–679. [Google Scholar] [CrossRef]
- Dhanker, R.; Chaudhary, S.; Goyal, S.; Kumar, R. Soil microbial properties and functional diversity in response to sewage sludge amendments. Arch. Agron. Soil Sci. 2020, 68, 809–822. [Google Scholar] [CrossRef]
- Roy, T.; Biswas, D.R.; Ghosh, A.; Patra, A.K.; Singh, R.D.; Sarkar, A.; Biswas, S.S. Dynamics of culturable microbial fraction in an inceptisol under short-term amendment with municipal sludge from different sources. Appl. Soil Ecol. 2019, 136, 116–121. [Google Scholar] [CrossRef]
- Ekmekyapar, F.; Çeltikli, D.O. Effects of linear alkylbenzene sulfonate on agricultural soil and its degradation. Fresenius Environ. Bull. 2014, 23, 3188–3192. [Google Scholar]
- Jensen, J.; Hans, L.; Holmstrup, M.; Krog, P.H.; Elsgaard, L. Effects and risk assessment of linear alkylbenzene sulfonates in agricultural soil. 5. Probabilistic risk assessment of linear alkylbenzene sulfonates in sludge-amended soils. Environ. Toxicol. Chem. 2009, 20, 1690–1697. [Google Scholar]
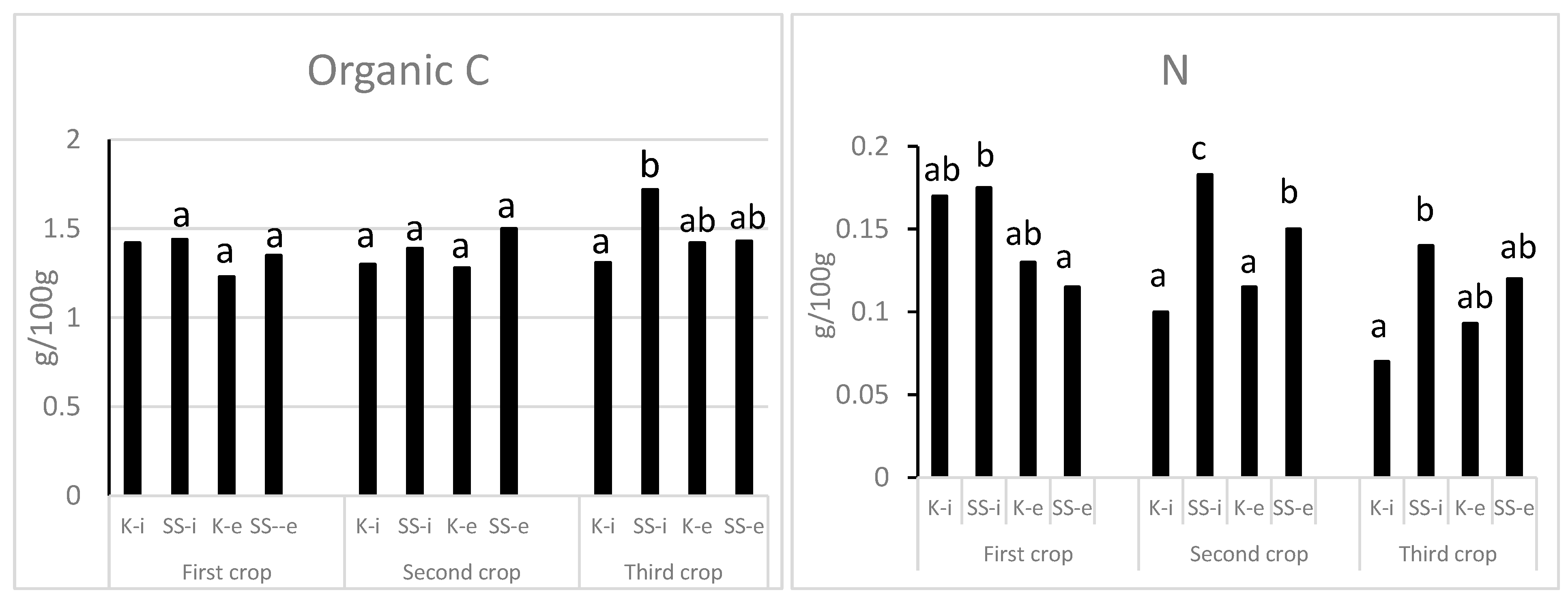

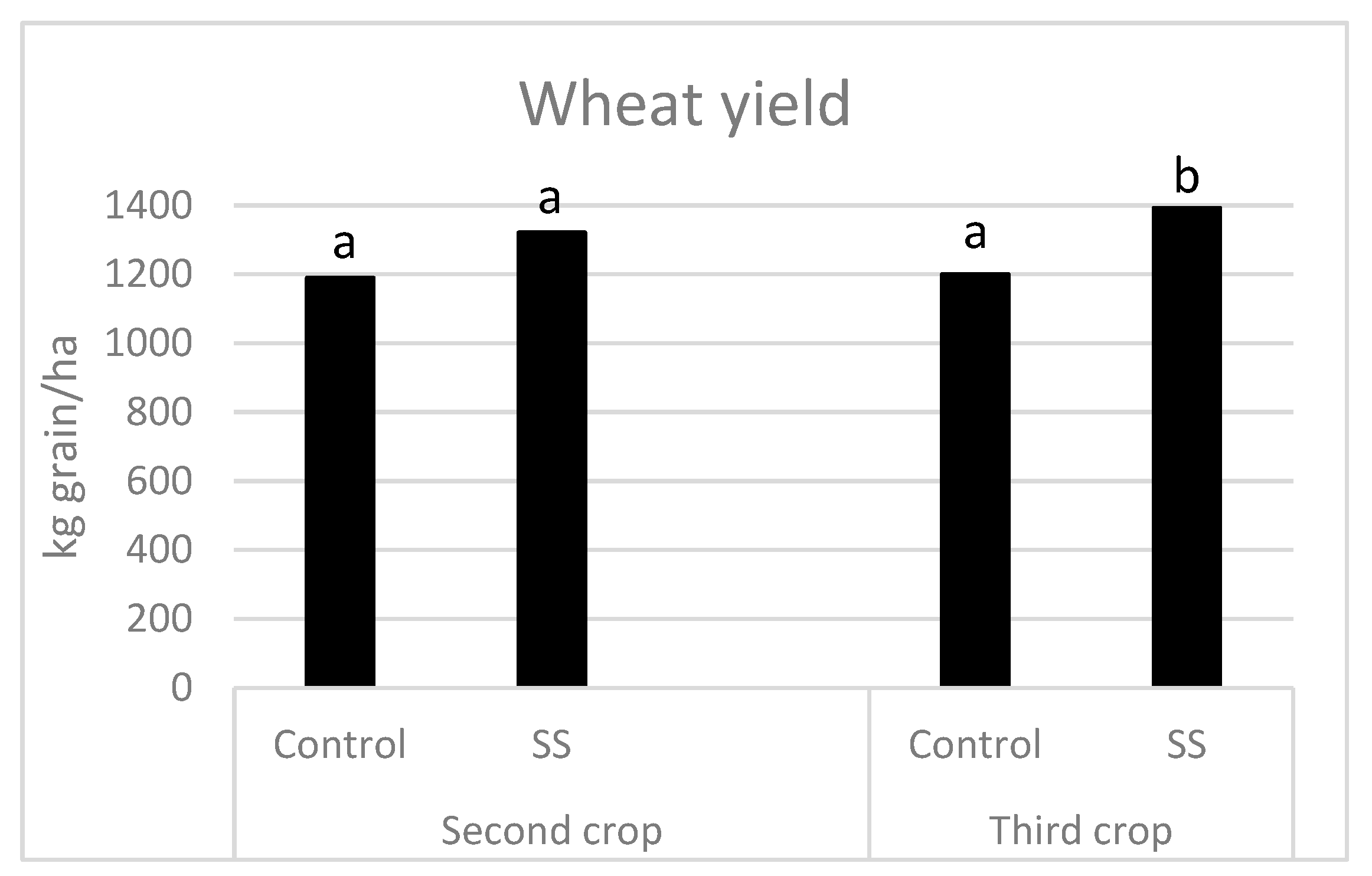
| Parameters | SS First Crop | SS Second Crop | EU Limit for Heavy Metals in SS [30] |
|---|---|---|---|
| Moisture (%) | 81.00 (1.24) | 84.90 (0.99) | |
| pH | 6.75 (0.04) | 6.49 (0.03) | |
| Electrical conductivity (dS/m) | 2.63 (0.06) | 2.18 (0.08) | |
| Volatile organic matter (%) | 94.47 (0.47) | 98.30 (0.63) | |
| Organic C (g/100 g) | 41.09 (0.12) | 40.24 (0.20) | |
| N (g/100 g) | 6.36 (0.03) | 7.06 (0.02) | |
| P (g/100 g) | 1.11 (0.01) | 0.82 (0.01) | |
| K (g/100 g) | 0.68 (0.02) | 0.31 (0.01) | |
| Fe (g/100 g) | 0.21 (0.04) | 0.22 (0.06) | |
| Mg (g/100 g) | 0.49 (0.01) | 0.43 (0.03) | |
| Mn (mg/kg) | 92.83 (4.82) | 63.60 (3.97) | |
| Ca (g/100 g) | 2.38 (0.14) | 1.36 (0.06) | |
| Na (g/100 g) | 0.16 (0.02) | 0.18 (0.02) | |
| Mo (mg/kg) | 5.00 (0.48) | 3.17 (0.38) | |
| Chlorides (mg/L) | 546.80 (13.2) | 244.20 (9.47) | |
| Sulphates (mg/L) | 590.00 (12.48) | 127.40 (11.36) | |
| Phosphates (mg/L) | 639.50 (11.05) | 804.40 (9.32) | |
| Bromides (mg/L) | <0.1 | <0.1 | |
| Ni (mg/kg) | 10.60 (0.44) | 18.53 (0.69) | 400 |
| Cu (mg/kg) | 105.81 (1.69) | 62.79 (0.93) | 1750 |
| Cd (mg/kg) | 0.50 (0.10) | 0.14 (0.07) | 40 |
| Zn (mg/kg) | 326.80 (2.24) | 292.10 (7.83) | 4000 |
| Hg (mg/kg) | 0.33 (0.01) | <0.10 | 10 |
| As (mg/kg) | 1.35 (0.01) | 1.60 (0.03) | 40 |
| Cr (mg/kg) | 22.05 (2.05) | 40.95 (2.33) | 1500 |
| Pb (mg/kg) | 21.99 (1.05) | 11.07 (0.99) | 1200 |
| Salmonella (in 25 g) | Absence | Absence | |
| Escherichia coli (CFU/g) | 169.000 | 17.000 |
| Parameters | SS First Year | SS Second Year | Proposed Limits [18] | |
|---|---|---|---|---|
| PAH (µg/kg) | Naftalene | 1.20 (0.01) | <LC | |
| Acenaphtylene | 142.50 (2.27) | <LC | ||
| Acenaphthene + Fluorene | 170.00 (1.79) | 57.09 (2.01) | ||
| Fenantrene | 1.10 (0.05) | 105.00 (1.03) | ||
| Anthracene | 20.25 (1.18) | 2.66 (0.89) | ||
| Fluorantene | 34.95 (2.26) | <LC | ||
| Pyrenean | 86.45 (3.50) | 128.00 (6.47) | ||
| Benzo[a]anthracene | 3.17 (0.06) | 16.50 (0.93) | ||
| Crisene | 2.48 (0.48) | 3.92 (0.19) | ||
| Benzo[b]fluorantheon | 0.81 (0.02) | 1.39 (0.03) | ||
| Benzo[k]fluorantheon | 1.25 (0.04) | 0.42 (0.01) | ||
| Benzo[a]pyrenean | 3.71 (0.02) | 1.00 (0.02) | ||
| Dibenzo[ah]anthracene | 2.05 (0.06) | <LC | ||
| Benzo[ghi]perilene | <LC | 4.08 (0.24) | ||
| Indeno[123cd]pyrenean | 3.17 (0.29) | <LC | ||
| ΣPAH (µg/kg) | 469.50 (5.17) | 320.00 (3.20) | 6000 µg/kg | |
| LAS (mg/kg) | C10 | 21.65 (1.03) | 0.46 (0.03) | |
| C11 | 133.00 (1.22) | 2.24 (0.19) | ||
| C12 | 214.00 (2.56) | 1.80 (0.08) | ||
| C13 | 259.00 (2.83) | 1.40 (0.08) | ||
| ΣLAS (mg/kg) | 627.00 (3.27) | 5.91 (0.72) | 2600 mg/kg | |
| NPE (µg/kg) | NP2E0 | 0.04 (0.01) | <LC | |
| NP1E0 | 0.24 (0.01) | 0.01 (0.00) | ||
| NP | <LC | 0.18 (0.01) | ||
| ΣNPE (µg/kg) | 1.66 (0.03) | 0.19 (0.01) | 50.000 µg/kg | |
| DEHP (mg/kg) | 0.81 (0.01) | 0.02 (0.00) | 100 mg/kg | |
| PCB (µg/kg) | PCB 101 | 15.65 (1.06) | <LC | |
| PCB 118 | <LC | <LC | ||
| PCB 138 | <LC | <LC | ||
| PCB 153 | <LC | 2.36 (0.61) | ||
| PCB 18 | <LC | <LC | ||
| PCB 180 | 17.60 (1.02) | <LC | ||
| PCB 28 | <LC | <LC | ||
| PCB 52 | 11.90 (0.93) | <LC | ||
| ΣPCB (µg/kg) | <LC | 2.36 (0.06) | 800 µg/kg | |
| FIRST CROP | Control-Initio | SS-Initio | Control-End | SS-End |
|---|---|---|---|---|
| pH | 8.99 (0.04) | 9.08 (0.05) | 9.18 (0.05) | 9.12 (0.03) |
| Electrical conductivity (µS/cm) | 118.03 (5.32) | 119.33 (6.12) | 114.3 (6.27) | 122.17 (5.28) |
| Ammonium (mg N-NH4/kg) | <2.5 | <2.5 | <2.5 | <2.5 |
| Total P (g/100 g) | 0.03 (0.01) | 0.03 (0.00) | 0.02 (0.00) | 0.03 (0.01) |
| Total K (meq/100 g) | 0.69 (0.03) | 0.70 (0.02) | 0.55 (0.05) | 0.55 (0.06) |
| Fe (mg/kg) | 16,677 (450) | 16,709 (448) | 14,028 (515) | 17,384 (715) |
| Mg (g/100 g) | 1.29 (0.27) | 1.34 (0.27) | 1.65 (0.18) | 1.57(0.17) |
| Mn (mg/kg) | 286 (12.42) | 288 (13.29) | 182 (10.15) | 199 (11.59) |
| Ca (g/100 g) | 10.80 (0.71) | 11.65 (0.81) | 14.0 (0.80) | 16.63 (0.67) |
| Chlorides (mg/kg) | 71.43 (9.32) | 57.78 (7.43) | 91.57 (11.35) | 76.00 (10.51) |
| Nitrates (mg/kg) | 529.36 (120) | 561.41 (134) | 82.31 (23) | 250.11 (69) |
| Phosphates (mg/kg) | <1.0 | <1.0 | <1.0 | <1.0 |
| Sulphates (mg/kg) | 121.0 (49) | 89.93 (44) | 50.25 (15) | 74.66 (18) |
| SECOND CROP | ||||
| pH | 8.85 (0.06) | 8.61 (0.07) | 8.63 (0.07) | 8.58 (0.05) |
| Electrical conductivity (µS/cm) | 134.20 (20.02) | 174.48 (16.89) | 182.6 (19.36) | 293.42 (11.03) |
| Ammonium (mg N-NH4/kg) | <2.5 | 16.10 (0.05) | <2.5 | <2.5 |
| Total P (g/100 g) | 0.02 (0.01) | 0.03 (0.01) | 0.02 (0.01) | 0.03 (0.01) |
| Total K (meq/100 g) | 0.66 (0.07) | 0.63 (0.08) | 0.65 (0.06) | 0.56 (0.07) |
| Fe (mg/kg) | 11,200 (716) | 10,625 (1332) | 17,000 (1308) | 12,674 (2308) |
| Mg (g/100 g) | 1.38 (0.06) | 1.26 (0.09) | 1.39 (0.22) | 1.39 (0.23) |
| Mn (mg/kg) | 287.5 (27.15) | 286.88 (31.48) | 266.6 (30.27) | 242.63 (28.96) |
| Ca (g/100 g) | 8.6 (0.56) | 9.13 (0.75) | 10.7 (1.02) | 13.53 (2.77) |
| Chlorides (mg/kg) | 17.1 (1.05) | 17.2 (2.93) | 7.99 (0.96) | 7.49 (0.65) |
| Nitrates (mg/kg) | 23.3 (3.27) | 97.9 (22.55) | 6.21 (2.12) | 9.69 (2.87) |
| Phosphates (mg/kg) | <0.1 | <0.1 | <0.5 | <0.5 |
| Sulphates (mg/kg) | 15.28 (2.17) | 25.63 (5.32) | 4.65 (1.10) | 5.88 (1.21) |
| THIRD CROP | ||||
| pH | 8.61 (0.04) | 8.65 (0.05) | 8.50 (0.04) | 8.54 (0.03) |
| Electrical conductivity (µS/cm) | 116.3 (9.22) | 117.11 (10.73) | 127.5 (8.54) | 134.53 (5.91) |
| Ammonium (mg N-NH4/kg) | 8.79 (0.05) | 8.53 (0.05) | <2.5 | <2.5 |
| Total P (g/100 g) | 0.02 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.01) |
| Total K (meq/100 g) | 0.61 (0.04) | 0.55 (0.06) | 0.65 (0.05) | 0.69 (0.06) |
| Fe (mg/kg) | 14,375 (1.302) | 13,626 (1405) | 16,377 (1505) | 17,273 (2700) |
| Mg (g/100 g) | 1.00 (0.02) | 0.88 (0.03) | 1.47 (0.10) | 1.25 (0.11) |
| Mn (mg/kg) | 254.7(24.03) | 241.53(20.28) | 279.43 (23.17) | 276.73 (25.10) |
| Ca (g/100 g) | 10.1 (0.98) | 8.9 (1.05) | 13.2 (1.02) | 13.45 (0.97) |
| Chlorides (mg/kg) | 15.96 (1.32) | 15.40 (1.52) | 7.71 (1.29) | 9.54 (2.6) |
| Nitrates (mg/kg) | 19.25 (6.54) | 28.69 (9.17) | 13.01 (2.01) | 23.31 (1.92) |
| Phosphates (mg/kg) | <0.6 | 6.42 (0.43) | <0.1 | <0.1 |
| Sulphates (mg/kg) | 17.77 (2.53) | 22.71 (2.16) | 9.8 (0.06) | 16.65 (2.79) |
| FIRST CROP | Control-Initio | SS-Initio | Control-End | SS-End |
|---|---|---|---|---|
| Ni (mg/kg) | 11.46 (2.95) | 13.255 (3.00) | 8.90 (0. 63) | 9.54 (0.56) |
| Cu (mg/kg) | 39.08 (1.02) | 39.24 (1.19) | 4.99 (0.53) | 5.93 (0.77) |
| Zn (mg/kg) | 28.16 (1.23) | 27.84 (1.39) | 19.27 (1.45) | 20.98 (1.82) |
| As (mg/kg) | 0.74 (0.11) | 0.67 (0.12) | 4.32 (0.22) | 3.31 (0.44) |
| Cr (mg/kg) | 28.04 (0.99) | 28.23 (1.07) | 13,19 (1.82) | 15.86 (1.02) |
| Pb (mg/kg) | 22.13 (1.13) | 20.37 (1.36) | 11,00 (0.43) | 9.58 (0.41) |
| E. coli (UFC/g) | <10 | <40 | <10 | <10 |
| Salmonella in 25 g | Absence | Absence | Absence | Absence |
| SECOND CROP | ||||
| Ni (mg/kg) | 10.95 (1.03) | 10.60 (1.29) | 11.49 (0.83) | 11.01 (0.99) |
| Cu (mg/kg) | 5.96 (0.65) | 6.16 (0.68) | 7.72 (0.53) | 7.64 (0.86) |
| Zn (mg/kg) | 19.23 (1.27) | 19.92 (2.15) | 28.98 (4.32) | 26.41 (5.86) |
| As (mg/kg) | 1.30 (0.15) | 1.07 (0.18) | 0.42 (0.33) | 0.81 (0.56) |
| Cr (mg/kg) | 25.40 (2.53) | 24.47 (3.11) | 32.89 (2.63) | 29.03 (4.09) |
| Pb (mg/kg) | 14.90 (0.99) | 12.90 (1.10) | 20.84 (1.80) | 16.50 (2.30) |
| E. coli (UFC/g) | <10 | <10 | <10 | <10 |
| Salmonella in 25 g | Absence | Absence | Absence | Absence |
| THIRD CROP | ||||
| Ni (mg/kg) | 10.45 (0.99) | 9.75 (1.07) | 12.82 (1.03) | 12.59 (1.12) |
| Cu (mg/kg) | 7.89 (0.76) | 7.93 (0.82) | 8.78 (0.63) | 9.32 (0.76) |
| Zn (mg/kg) | 19.20 (1.53) | 20.00 (1.78) | 22.51 (1.42) | 24.29 (1.99) |
| As (mg/kg) | 0.76 (0.19) | 0.50 (0.20) | 0.73 (0.15) | 0.55 (0.19) |
| Cr (mg/kg) | 28.17 (1.86) | 26.36 (2.28) | 29.39 (1.36) | 29.77 (2.59) |
| Pb (mg/kg) | 17.22 (0.47) | 15.06 (0.57) | 19.25 (0.72) | 18.03 (0.84) |
| Escherechia coli (CFU/g) | <10 | <10 | <10 | <10 |
| Salmonella in 25 g | Absence | Absence | Absence | Absence |
| Organic Contaminant | Initio First Crop | Initio Second Crop | End Third Crop | ||||
|---|---|---|---|---|---|---|---|
| Control | SS | Control | SS | Control | SS | ||
| PAH (ng/kg) | Naftalene | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ |
| Acenaphtylene | 395 (7.89) | 122 (5.93) | <LQ | <LQ | <LQ | <LQ | |
| Acenaphthene + Fluorene | 429 (8.32) | 637 (10.24) | <LQ | <LQ | <LQ | <LQ | |
| Fenantrene | 34 (2.25) | 36.8 (3.26) | 879 (22.32) | 1150 (91.24) | 19.38 (1.67) | 6.60 (0.86) | |
| Anthracene | 368 (7.23) | 436.8 (4.82) | <LQ | <LQ | 2.68 (0.27) | 0.69 (0.06) | |
| Fluorantene | 378 (9.47) | 440 (10.50) | 769 (9.24) | 1035 (0.76) | <LQ | <LQ | |
| Pyrenean | 233 (9.43) | 443 (10.22) | 5063 (115.24) | 11,239 (187.89) | 5.98 (0.89) | 4.48 (1.22) | |
| Benzo[a]anthracene | 357 (7.28) | 467 (9.53) | 736 (11.04) | 238 (7.31) | <LQ | <LQ | |
| Crisene | 181 (2.25) | 178 (3.32) | 234 (5.24) | 511 (7.32) | 10.83 (0.89) | 0.37 (0.03) | |
| Benzo[b]fluorantheon | 112 (5.09) | 101 (2.17) | 493 (5.76) | 331 (4.28) | 6.70 (0.99) | <LQ | |
| Benzo[k]fluorantheon | 162 (3.27) | <LQ | 56 (1.43) | 246 (3.89) | 9.73 (1.14) | <LQ | |
| Benzo[a]pyrenean | <LQ | 244 (2.12) | <LQ | <LQ | 8.70 (0.57) | <LQ | |
| Dibenzo[ah]anthracene | 390 (5.23) | 321 (6.09) | <LQ | <LQ | <LQ | <LQ | |
| Benzo[ghi]perilene | <LQ | <LQ | 335 | 744 | 1.76 | <LQ | |
| Indeno[123cd]pyrenean | <LQ | <LQ | <LQ | <LQ | 4.78 | <LQ | |
| ΣPAH (ng/kg) | 3039 (18.22) | 3427 (15.77) | 8565 (96.84) | 15,494 (117.32) | 70.54 (8.93) | 12.14 (1.12) | |
| LAS (µg/kg) | Q10 | 0.83 (0.03) | 1191 (4.86) | 25 (1.16) | 22 (1.93) | 4.34 (0.78) | 0.63 (0.76) |
| Q11 | 5.18 (0.57) | 6195 (5.92) | 128 (2.18) | 61 (2.45) | 6.32 (0.89) | <LQ | |
| Q12 | 7.32 (086) | 8888 (3.97) | 187 (3.91) | 126 (3.27) | 11.86 (1.65) | 0.82 (0.06) | |
| Q13 | 4.85 (0.06) | 6213 (5.16) | 148 (7.42) | 41 (4.18) | 5.49 (1.18) | <LQ | |
| ΣLAS (µg/kg) | 18.18 (1.27) | 25,914 (12.04) | 488 (3.23) | 250 (3.82) | 28.01 (2.26) | 1.45 (0.09) | |
| NPE (µg/kg) | NP2EO | 0.38 (0.01) | 0.37 (0.06) | <LQ | <LQ | 0.14 (0.01) | <LQ |
| NP1E0 | 1.25 (0.19) | 1.12 (0.06) | <LQ | <LQ | <LQ | <LQ | |
| NP | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| ΣNPE (µg/kg) | 1.63 (0.09) | 1.49 (0.19) | <LQ | <LQ | 0.14 (0.01) | <LQ | |
| DEHP (µg/kg) | DEHP | 11.3 (1.11) | 12.63 (1.02) | 12.9 (1.05) | 2.38 (0.10) | 0.17 (0.03) | <LQ |
| PQB (µg/kg) | PQB 101 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ |
| PQB 118 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 138 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 153 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 18 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 180 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 28 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| PQB 52 | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| ΣPQB (µg/kg) | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ | |
| Second Crop | Third Crop | |||||||
|---|---|---|---|---|---|---|---|---|
| Straw | Grain | Straw | Grain | |||||
| Control | Amended Soil | Control | Amended Soil | Control | Amended Soil | Control | Amended Soil | |
| C, g/100 g | 43.71 (0.24) | 43.54 (0.30) | 43.80 (0.24) | 43.71 (0.77) | 43.31 (0.64) | 43.58 (0.54) | 43.89 (0.06) | 44.21 (0.12) |
| N, g/100 g | 2.06 (0.18) | 2.52 (0.24) | 0.27 (0.08) | 0.45 (0.11) | 1.72 (0.08) | 2.16 (0.35) | 0.67 (0.07) | 0.86 (0.16) |
| P, g/100 g | 0.34 (0.01) | 0.37 (0.05) | 0.01 (0.01) | 0.02 (0.01) | 0.07 (0.02) | 0.07 (0.05) | 0.32 (0.04) | 0.31 (0.02) |
| K, g/100 g | 0.72 (0.05) | 0.76 (0.08) | 0.98 (0.12) | 1.25 (0.63) | 1.06 (0.25) | 1.31 (0.20) | 0.40 (0.05) | 0.41 (0.02) |
| Ca, g/100 g | 0.07 (0.01) | 0.09 (0.00) | 0.18 (0.06) | 0.38 (0.10) | 0.27 (0.06) | 0.33 (0.14) | 0.05 (0.00) | 0.04 (0.00) |
| Mg, g/100 g | 0.13 (0.00) | 0.15 (0.01) | 0.05 (0.02) | 0.08 (0.02) | 0.07 (0.01) | 0.09 (0.03) | 0.13 (0.01) | 0.13 (0.01) |
| Na, g/100 g | 0.005 (0.00) | 0.003 (0.00) | 0.01 (0.00) | 0.01 (0.00) | 0.04 (0.02) | 0.04 (0.02) | 0.03 (0.01) | 0.04 (0.02) |
| Fe, mg/kg | 49.95 (8.99) | 50.17 (5.76) | 70.25 (0.80) | 112.01 (12.08) | 129.30 (20.27) | 76.83 (25.89) | 54.84 (12.88) | 39.68 (4.19) |
| Mn, mg/kg | 73.38 (5.09) | 70.75 (7.63) | 23.55 (7.46) | 27.58 (6.28) | 49.10 (4.82) | 50.91 (7.19) | 46.86 (4.02) | 42.40 (1.71) |
| Mo, mg/kg | 0.54 (0.01) | 0.46 (0.09) | 0.67 (0.35) | 0.71 (0.28) | 1.32 (0.48) | 0.58 (0.38) | 1.20 (1.14) | 0.27 (0.09) |
| As, mg/kg | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cd, mg/kg | <0.01 | <0.01 | 0.01 (0.00) | <0.01 | <0.01 | <0.01 | 0.01 (0.00) | <0.01 |
| Cu, mg/kg | 5.81 (0.44) | 6.18 (0.23) | 1.22 (0.24) | 1.72 (0.27) | 2.28 (0.18) | 2.28 (0.69) | 4.78 (0.02) | 4.07 (0.16) |
| Cr, mg/kg | 0.64 (0.43) | 0.57(0.31) | 13.98 (8.30) | 16.08 (6.36) | 12.79 (2.20) | 3.93 (3.53) | 5.65 (1.85) | 2.70 (1.28) |
| Ni, mg/kg | 0.58 (0.18) | 0.51 (0.22) | 0.45 (0.09) | 0.58 (0.28) | 1.59 (0.44) | 1.18 (0.22) | 1.17 (0.37) | 1.00 (0.48) |
| Pb, mg/kg | 0.18 (0.04) | 0.17 (0.02) | 0.19 (0.09) | 0.19 (0.07) | 0.18 (0.03) | 0.20 (0.09) | 0.20 (0.00) | 0.17 (0.03) |
| Zn, mg/kg | 39.39 (2.04) | 46.93 (10.56) | 3.04 (0.83) | 3.48 (1.24) | 9.28 (1.15) | 8.06 (1.16) | 30.46 (1.29) | 29.64 (0.97) |
| E. coli CFU/g) | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Salmonella in 25 g | Absence | Absence | Absence | Absence | Absence | Absence | Absence | Absence |
| Grain | Straw | ||||
|---|---|---|---|---|---|
| Organic Contaminants | Control | Amended Soil | Control | Amended Soil | |
| ΣNPE (µg/kg) | <LQ | <LQ | <LQ | 14.17 (1.76) | |
| PAH (µg/kg) | Naftalene | <LQ | <LQ | <LQ | <LQ |
| Acenaphtylene | <LQ | <LQ | <LQ | <LQ | |
| Acenaphthene + Fluorene | <LQ | 18.9 | <LQ | <LQ | |
| Fenantrene | <LQ | LQ | <LQ | <LQ | |
| Anthracene | 107 (3.5) | <LQ | 61.0 (2.95) | <LQ | |
| Fluorantene | <LQ | <LQ | <LQ | 38.5 (1.24) | |
| Pyrenean | <LQ | <LQ | <LQ | <LQ | |
| Benzo[a]anthracene | <LQ | <LQ | <LQ | <LQ | |
| Crisene | <LQ | 25.3 | <LQ | <LQ | |
| Benzo[b]fluorantheon | 48 (2.80) | 90.0 (3.47) | 4.8 (0.28) | 24.8 (0.99) | |
| Benzo[k]fluorantheon | 8.4 (0.19) | <LQ | <LQ | <LQ | |
| Benzo[a]pyrenean | 1.8 (0.09) | <LQ | <LQ | <LQ | |
| Dibenzo[ah]anthracene | <LQ | <LQ | <LQ | 3.7 (0.22) | |
| Benzo[ghi]perilene | 2.1 (0.03) | <LQ | <LQ | <LQ | |
| Indeno[123cd]pyrenean | <LQ | <LQ | <LQ | <LQ | |
| Indeno[123cd]pireno | <LQ | <LQ | <LQ | <LQ | |
| ΣPAH (µg/kg) | 167.3 (10.7) | 134.2 (8.47) | 66.8 (5.32) | 67.0 (4.16) | |
| LAS (µg/kg) | C10 | 4.2 (0.59) | 2.6 (0.43) | 22.3 (2.57) | 17.0 (3.83) |
| C11 | 10.5 (1.05) | 4.0 (0.23) | 75.7 (8.79) | 61.8 (9.52) | |
| C12 | 23.5 (2.19) | 11.8 (1.43) | 139.5 (5.43) | 133.0 (4.86) | |
| C13 | 4.8 (0.25) | 1.2 (0.06) | 30.7 (5.12) | 40.3 (4.32) | |
| ΣLAS (µg/kg) | 43.0 (3.24) | 19.6 (2.32) | 268.2 (9.84) | 252.1 (11.02) | |
| NPE (µg/kg) | NP | <LQ | <LQ | <LQ | 9.02 |
| NP1EO | <LQ | <LQ | <LQ | 2.32 | |
| NP2EO | <LQ | <LQ | <LQ | 2.83 | |
| DEHP (µg/kg) | <LQ | <LQ | <LQ | <LQ | |
| PCB (µg/kg) | PCB 101 | <LQ | <LQ | <LQ | <LQ |
| PCB 118 | <LQ | <LQ | <LQ | <LQ | |
| PCB 138 | <LQ | <LQ | <LQ | <LQ | |
| PCB 153 | <LQ | <LQ | <LQ | <LQ | |
| PCB 18 | <LQ | <LQ | <LQ | <LQ | |
| PCB 180 | <LQ | <LQ | <LQ | <LQ | |
| PCB 28 | <LQ | <LQ | <LQ | <LQ | |
| PCB 52 | <LQ | <LQ | <LQ | <LQ | |
| ΣPCB (µg/kg) | <LQ | <LQ | <LQ | <LQ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, T.; López Aragón, R.F.; Garcia, C. The Use of Aerobic Urban Sewage Sludge in Agriculture: Potential Benefits and Contaminating Effects in Semi-Arid Zones. Agriculture 2024, 14, 983. https://doi.org/10.3390/agriculture14070983
Hernández T, López Aragón RF, Garcia C. The Use of Aerobic Urban Sewage Sludge in Agriculture: Potential Benefits and Contaminating Effects in Semi-Arid Zones. Agriculture. 2024; 14(7):983. https://doi.org/10.3390/agriculture14070983
Chicago/Turabian StyleHernández, Teresa, Román Francisco López Aragón, and Carlos Garcia. 2024. "The Use of Aerobic Urban Sewage Sludge in Agriculture: Potential Benefits and Contaminating Effects in Semi-Arid Zones" Agriculture 14, no. 7: 983. https://doi.org/10.3390/agriculture14070983





