Abstract
Protein hydrolysates are plant biostimulants containing amino acids, oligopeptides, and peptides in their composition. When supplied to plants, protein hydrolysates (HPs) have been identified to improve nitrogen metabolism, enhance the activity of antioxidant enzymes, boost plant defense response to stresses, and positively impact the quantity and quality of products. Soybean is a crucial global commodity, with nitrogen being the primary nutrient for crop development as it directly affects productivity. This study aimed to evaluate the effect of an HP-based biostimulant on the N metabolism in nodulated soybean plants and their productivity. A greenhouse experiment was conducted to test two modes of application of the 0.20% HP-based biostimulant. Soybean plants, growing in pots, were treated with 0.20% HP either via seed treatment or foliar application (at growth stages V3 and V5). Activities of enzymes and compounds related to N metabolism, gene expression, and productivity components were analyzed. It was observed that the mode of application did not significantly influence the results. The application of HPs increased the concentration of nitrate, amino acids, and ureides in soybean leaves. It also positively altered the expression of genes such as nitrate reductase, urease, and asparagine. Additionally, it enhanced productivity, resulting in plants with a greater number and weight of pods and grains. Therefore, it is possible to consider HPs as a stimulator for increasing soybean productivity, even under non-stressing conditions.
1. Introduction
The European Biostimulants Industry Council (EBIC) defines biostimulants as materials containing substances and/or microorganisms that, when applied to plants or the rhizosphere, stimulate natural processes to improve nutrient absorption, nutrient efficiency, abiotic stress tolerance, and crop quality (https://biostimulants.eu/ accessed on 18 July 2024). Protein hydrolysates (HPs) are a type of biostimulant composed of amino acids, oligopeptides, and peptides derived from the chemical or enzymatic hydrolysis of proteins from agro-industrial residues, which can be plant- or animal-based [1]. When used as biostimulants, HPs enhance nitrogen absorption and assimilation, regulate enzymes involved in nitrogen metabolism and their structural genes, improve response efficiency to biotic and abiotic stresses, and increase the production of secondary metabolites and the activity of antioxidant enzymes [2,3,4,5,6].
Several studies have highlighted the potential of biostimulant products for soybean (Glycine max L. Merril), with most research concentrating on improving tolerance to water stress. These studies have shown that biostimulant application helps plants adjust their turgor in relation to leaf temperature [7], increases nutrient concentration in grains, and enhances various growth parameters [8]. Additionally, biostimulants have been found to modify the expression of stress-responsive genes [9], boost antioxidant and photosynthetic activity, and enhance assimilate production [10].
Soybean cultivation serves a wide range of purposes, including feed and meal production, oil extraction, and processing into products such as paints, cosmetics, and pharmaceuticals [11,12]. Consequently, soybean is a major global agricultural commodity. In Brazil alone, soybean cultivation spans 45 million hectares, yielding 162 million tons of grains [13]. Given the significance of soybean production and the growing global demand for food, there is a pressing need to enhance soybean productivity and that of other crops within the same cultivated area. At the same time, it is crucial to mitigate the environmental impacts of agricultural practices by adopting more sustainable resources [14,15].
Nitrogen (N) is a crucial nutrient for all crops. Increasing the rate of nitrogen assimilation in soybeans can enhance productivity, as the number of grains is closely related to the number of pods retained, which depends on nitrogen availability during the flowering period [16]. In this context, biostimulants can be used to improve nitrogen metabolism efficiency and achieve higher yields.
In this study, we aimed to evaluate the effect of a protein hydrolysate-based biostimulant on nitrogen metabolism in nodulated soybean plants and their productivity. We hypothesize that HPs can have specific action in parts of the metabolism and in our case, we investigated the N metabolism.
2. Material and Methods
The experiment used soybean seeds of the cv. Intact RR2 Pro 57HO123 TP IPRO (HO-Genetics), inoculated with Bradyrhizobium elkanii and B. japonicum (Masterfix, Stoller Brazil, Campinas, SP, Brazil). These seeds were sown in 10 L pots filled with a soil mixture composed of soil, organic gardening substrate, and vermiculite (2:1:1, v/v/v). A concentration of 0.20% HP was tested for both seed and foliar applications, based on previous results with tomato and soybean seedlings where this concentration was found effective for promoting seedling growth. The HP was obtained by acid treatment of commercial hydrolyzed collagen and further alkalinization to pH 4.5 with KOH. The HP was not amended with any other nutrient. It had 0.5% protein, determined using Bradford reagent (BioRad, São Paulo, Brazil), and 21% free amino acids, determined using the protocol of Yemm et al. [17]. The final K concentration in the concentrate HP was 5.5%, measured with a flame photometer.
2.1. Treatments
The seed treatment occurred at the time of sowing when 1 mL of 0.20% HP was applied to each pot, over the seeds. Foliar application was performed at vegetative stages V3 and V5 [18], using a pressurized CO2 sprayer with a pressure of 2 bars. Six plants were used for each treatment. Control plants remained untreated with HP. The experiment was conducted in a greenhouse, irrigated with a drip system, and once a week, the plants received 200 mL of a nutrient solution (MgSO4—246.5 g L−1, CaSO4—1.72 g L−1, KH2PO4—136 g L−1—K2SO4—87.6 g L−1, CaCl2—111 g L−1), plus a micronutrient solution prepared according to Hoagland and Arnon [19] without N. The experiment was repeated twice; in the first experiment, biochemical and gene expression parameters were evaluated at stage R5.1 of the plants, and in the second experiment, productivity was assessed at full plant maturity [18].
2.2. Enzymatic and Biochemical Analyses
Leaves were collected for RN activity determination. The newest fully expanded leaf from each plant was collected, placed in plastic bags, and transported to the laboratory in a cooler with ice, where in vivo analysis was carried out [20]. Leaves collected and kept in a freezer at −20 °C were used for the determination of the enzyme glutamine synthetase (GS) [21] and glutamate synthetase (GOGAT) [22], with the protein concentration in the enzymatic extracts obtained by the Bradford reagent method (Bio-Rad). The concentration of chlorophyll and carotenoids in the leaves was determined after extraction in DMSO [23]. The foliar contents of nutrients N, phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) were determined in the leaves by plasma emission spectrometry (ICP-OES; JobinYvon, JY50P Longjumeau, France). Nitrogen compounds such as nitrate [24], free amino acids [17], and ureides [25] were analyzed in lyophilized and ground leaves, as well as in xylem sap collected and kept in a freezer at −20 °C. To collect the xylem sap, the plants in the greenhouse were watered abundantly and on the following day, between 8 and 10 a.m., the sap was collected [26]. With the help of pruning shears, a cut was made just below the cotyledonary node of the plants, separating the aerial part from the roots. Then, in the cut region, a more precise cut was made with a razor blade, and the region was washed with milliQ water and quickly dried with absorbent paper. The sap that accumulated at the cut site was collected using a pipette, avoiding touching the cut surface. Samples were collected until there was a volume of at least 0.5 mL of sap. During collection, the samples were deposited in Eppendorf tubes that remained on ice. After collection, the material was stored at −20 °C until use.
2.3. Gene Expression Analysis RT-qPCR
We analyzed the expression of NR, nitrite reductase (NiR), GS, GOGAT, nitrate transporter NTR1, asparagine synthetase, arginase, and urease (Table S1). The genes CYP2 and ACTII were used for gene expression normalization [27]. Total RNA was extracted from three replicates of leaves and roots using Trizol reagent (Sigma, Kawasaki, Japan), following the manufacturer’s instructions. The RNA was treated with “Turbo DNA-free” DNase (Ambion, Inc., Austin, TX, USA) and quantified using a spectrophotometer at 260 nm. RNA integrity was checked by 1.5% agarose gel electrophoresis with ethidium bromide and UV light observation. Three µg of total RNA was used to synthesize the first strand with the SuperScript III First-Strand kit (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions. The primers were designed using the Primer 3 program (https://bioinfo.ut.ee/primer3-0.4.0/, accessed on 1 April 2019) (Table S1) using gene sequences obtained from the Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html, accessed on 1 April 2019). Real-time reverse transcription quantitative PCR (RT-qPCR) reactions were prepared with a final volume of 10 µL, containing 3 µL of diluted cDNA, 1.6 µL of milliQ water, 0.2 µL of sense primer, 0.2 µL of antisense primer, and 5 µL of Sybr Green Master Mix (BioRad, Hercules, CA, USA). A StepOnePlus Real-Time PCR System (Thermo Fisher, Waltham, MA, USA) was used for gene amplification (95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s as melting curve). Seven biological replicates were performed for each treatment. Relative expression quantification was determined by comparing transcriptional expression between target genes and reference genes using the 2−ΔCt method [28].
2.4. Plant Growth and Yield Attributes
After collecting the material for laboratory analysis, the dry weights of leaves, stems, and roots were determined. The plants were at stage R5.1. Nodules were counted, and their fresh and dry weights (dried for 72 h at 70 °C) were measured. The productivity assessment was conducted during the second repetition of the experiment. Parameters analyzed included plant height, number of productive nodes, number of pods per plant, dry weight of pods per plant, number of seeds per pod and per plant, dry weight of seeds, and seed weight.
2.5. Statistical Analysis
The experiment was designed as a completely randomized design with factors including mode of application (seed and foliar), treatment (0.20% HP and control), and six replicates, except for gene expression (seven replicates) and nutrient analysis (three replicates). Data were first tested for normality using the Shapiro–Wilk test and for homogeneity of variances using the Bartlett test. Variables not meeting test assumptions underwent logarithmic transformation. Analysis of variance (ANOVA) was then performed, and means were compared using the Tukey test with a 5% significance level. All analyses were conducted using R software v.4.0.0 (http://www.r-project.org/ accessed on 18 July 2024).
3. Results
3.1. Enzymatic and Biochemical Analysis
The results of the analyses for photosynthetic pigments, nitrogenous compounds, and enzyme activity are presented in Table 1. The mode of application is specified only for parameters with significant differences. Treatment with HP increased nitrate content in soybean leaves compared to control plants, with no variation based on the mode of application. However, ureide content in the xylem sap differed by application mode, being higher when HP was applied to seeds. In contrast, control plants had higher ureide content in the xylem sap compared to those treated with HP via foliar application.

Table 1.
Photosynthetic pigments, nitrogen compound, and enzyme activities of soybean plants treated with HP.
The amino acid content in the leaves and xylem sap was influenced by the HP treatment. While the mode of application did not affect the amino acid levels, plants treated with HP had a higher content of total free amino acids. GOGAT (glutamate synthase) activity remained unchanged with foliar HP application but was reduced when HP was applied via seed. There were no differences in GOGAT activity based on the mode of application.
No significant changes were observed in most of the foliar nutrients analyzed (Table 2). Only phosphorus (P) and potassium (K) showed a reduction in content in plants treated with HP at 0.20%. The mode of application, whether seed or foliar, did not have a noticeable effect on these nutrient levels.

Table 2.
Nutrient concentrations in the leaves of soybean plants treated with HP.
3.2. Gene Expression Analyses
In the gene expression analyses of soybean leaves, significant differences were observed for urease and nitrate reductase (RN) (Table 3). The mode of application is shown only for those parameters with significant differences. Application of HP to soybean seeds increased urease expression compared to untreated plants or those treated via foliar application. RN expression also increased in the leaves, but there was no difference in expression based on the mode of application. In the root expression results, foliar application of HP increased asparagine synthetase expression, while seed application resulted in lower expression compared to control plants. Similar trends were observed for RN, with foliar treatment showing no significant difference.

Table 3.
Relative expression of nitrogen metabolism genes in leaf and root of soybean plants treated with HP.
3.3. Plant Growth and Yield Attributes
The growth parameters of the plant analyzed are presented in Table 4. A significant difference was observed in root dry weight between control and HP-treated plants, with variations depending on the mode of application. Foliar application of HP resulted in a greater root dry weight compared to the control, and it was also more effective than seed application. However, there were no significant effects of the treatments on the dry weight of leaves, stems, or nodules.

Table 4.
Growth features of soybean plants treated with 0.20% protein hydrolysate.
In the experiment conducted until plant maturity to evaluate productivity parameters, it was visually observed that the plants treated with the biostimulant maintained green leaves for a longer period (Figure 1). Additionally, the treatment with HP significantly altered almost all analyzed productivity parameters (Figure 2). Although plants treated with HP were not taller than the control plants, they exhibited a higher number of productive nodes, particularly when the biostimulant was applied via foliar spray. Furthermore, these plants showed an increase in the number and dry weight of pods, the number of seeds per plant and per pod, and seed dry weight.
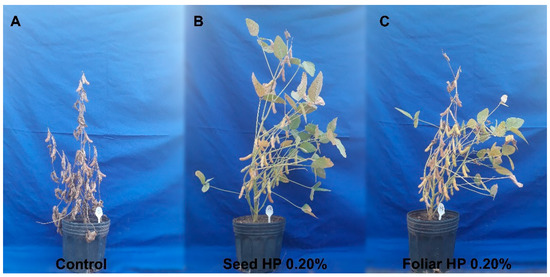
Figure 1.
Soybean plants treated with HP at the maturation stage. (A) Control plant, (B) plant with seed application of HP, and (C) plant with foliar application of HP.
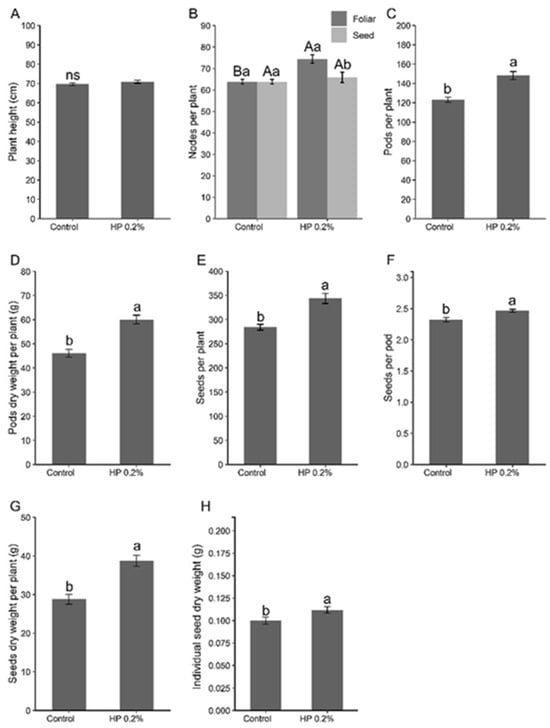
Figure 2.
Effect of the application of HP on (A) height, (B) number of productive nodes per plant, (C) number of pods per plant, (D) pod weight per plant, (E) seeds per plant, (F) seeds per pod, (G) seed weight per plant, and (H) dry weight per seed. Different uppercase letters indicate the statistical difference between application methods, while different lowercase letters indicate the statistical difference between treatments (Tukey test, p < 0.05). Bars represent standard deviation.
4. Discussion
The use of biostimulants in agriculture aims to stimulate the physiological processes of crops, primarily under conditions of abiotic stress, thereby improving nutrient use efficiency and product quality [9,29]. Due to the complexity of formulating HP, it is challenging to elucidate their mode of action and how they interfere with plant metabolism [30]. In the results presented in this study, an increase in productivity parameters was observed with the application of the biostimulant HP, such as the number and weight of pods and seeds per plant. Despite that, few biochemical and genetic analyzed parameters related to N metabolism changed significantly. The mode of HP application was not a determining factor in the examined results.
We decided to evaluate the nitrogen-related biochemical parameters and gene expression at the R5.1 phenological stage when seeds are beginning to accumulate biomass [18]. This stage was chosen because the activities of nitrate reductase (NR) and nitrite reductase (NiR), as well as the nitrate (NO3) concentration in the xylem sap, remain high during pod filling [16]. Additionally, the nitrogen content from biological fixation is near its maximum at this stage [31]. Therefore, further evaluations at more frequent intervals during plant development are necessary to determine the specific points at which the application of protein hydrolysates (HPs) influences metabolism, thereby justifying the observed increase in productivity.
There were changes in the activity of the enzymes NR, urease, and asparagine synthetase. NR catalyzes the initial step of reducing absorbed nitrate to ammonia, while urease hydrolyzes urea into ammonia and carbon dioxide, and asparagine synthetase synthesizes asparagine, an amino acid involved in N transport, mainly in seeds and roots [32]. The increase in the expression of genes such as nitrate reductase (NR) and urease in leaves was not accompanied by a corresponding increase in enzyme activity. Additionally, there was an accumulation of nitrogenous compounds influenced by the application of HP. Considering that ureides are compounds originating from biological nitrogen fixation (symbiosis with Bradyrhizobium), an increase in ureides, amino acids, and nitrate in the leaves was observed. In contrast, in a study involving 14-day-old maize seedlings grown hydroponically, the application of alfalfa-based HP increased the dry root mass of the plants but resulted in a lower concentration of nitrate in the leaves [3]. These results contrast with our findings, which show an increase in leaf nitrate. The authors of the maize study explained the reduction in nitrate by the higher activity of NR and glutamine synthetase (GS) enzymes [3].
Mamatha et al. [33] investigated the application of different doses of plant-based HP on soybean, pepper, and chickpea grown under thermal and water stress conditions. They found that foliar application of the biostimulant at a dose of 4 mL L−1 increased the photosynthetic efficiency of the treated plants. This treatment also boosted the productivity of soybean, chickpea, and pepper crops by 17%, 30%, and 25%, respectively, mirroring the results observed in our study with soybean plants. The increased productivity in plants treated with biostimulants is attributed to the action of phytohormones, enhanced defense responses to biotic and abiotic stresses, maintenance of water balance in plants and soil, and improved nutrient uptake [1,34,35]. Here, we did not observe significant changes in nutrient levels because of HP application.
Maintaining leaf area during grain filling can be crucial for achieving higher plant productivity [36]. Francesca et al. [37] attributed the greater biomass production in tomato plants treated with HP and subjected to thermal and water stress to the presence of amino acids in the biostimulant, which promoted the action of phytohormones that stimulated plant development. The retention of leaves at the end of maturation observed in this study can be attributed to the action of phytohormones such as cytokinin. Cytokinin influences cell division, leaf senescence, nutritional signaling, and stress tolerance, all of which are triggered by the biostimulant [38]. Similarly, Aremu et al. [39] applied an algae extract-based biostimulant to Eucomis autumnalis plants grown hydroponically for five weeks. They observed a higher concentration of cytokinin in the leaves of the treated plants, as well as increased root mass and bulb size.
The nutrient absorption in soybeans increases significantly from the V3 vegetative stage, reaching a maximum proportion close to the R5.3 stage [16,31]. At reproductive stage R5, soybean plants have fully developed pods and seeds at the beginning of development [18]. Sampling plants at this developmental stage did not reveal significant differences in many of the evaluated nitrogen metabolism parameters, such as chlorophyll and carotenoid content, and the activity of NR, GS, and GOGAT enzymes, as well as gene expression. However, there was a notable impact on productivity parameters, indicating a positive effect of the application on soybean plants.
Biostimulants, such as HP, hold great potential for increasing the productivity of crops and enhancing sustainability, even in the absence of stress conditions [4,40]. However, further research is needed to elucidate the mechanisms behind the increased productivity in soybeans. Variations in biostimulants—such as differences in manufacturing processes, components, dosage, timing, and application methods—as well as the plant genotype, can lead to diverse results [41,42].
5. Conclusions
In recent years, agriculture has seen a shift towards more sustainable strategies due to concerns about the intensive use of pesticides and fertilizers. Incorporating biostimulant products into plant management offers a more sustainable approach to mitigate productivity losses caused by stress conditions, enhance crop quality and yield, and improve nutrient and resource use efficiency. Biostimulants, such as HPs, show the potential to increase soybean crop productivity by boosting pod and grain production. While it is not definitive that HP effects are solely due to the stimulation of nitrogen metabolism, our data suggest that increased production in soybean plants may be due to alterations in nitrogen metabolism. Additionally, HP application appears to influence pod maturation, likely by affecting hormonal control.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture14081205/s1: Table S1: Primers used in the RT-PCR reactions.
Author Contributions
D.C.H.E., D.F., M.R. and J.L.C.B. executed the experiment and conducted data analyses; D.C.H.E. wrote the first manuscript draft; P.M. planned the experiment, helped with data analyses, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
D.C.H.E. thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES and Fundação de Amparo à Pesquisa do Estado de São Paulo—Fapesp for MSc (2019/20211-2) fellowships. D.F. thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES for a postdoctoral fellowship. M.R. and J.L.C.B. thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for doctoral fellowships. P.M. thanks the National Council for Scientific and Technological Development (CNPq-Brazil) for a research fellowship.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
For additional information contact the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant Activity of Two Protein Hydrolyzates in the Growth and Nitrogen Metabolism of Maize Seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum Lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz-Walec, E.; Olszewska, M. Biostimulants in the Production of Forage Grasses and Turfgrasses. Agriculture 2023, 13, 1796. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal Imaging of Soybean Response to Drought Stress: The Effect of Ascophyllum Nodosum Seaweed Extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of Seaweed Extract on the Growth, Yield and Nutrient Uptake of Soybean (Glycine Max) under Rainfed Conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed Extract Improve Drought Tolerance of Soybean by Regulating Stress-Response Genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef] [PubMed]
- 1do Rosário Rosa, V.; Farias dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased Soybean Tolerance to Water Deficiency through Biostimulant Based on Fulvic Acids and Ascophyllum Nodosum (L.) Seaweed Extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Chen, K.-I.; Erh, M.-H.; Su, N.-W.; Liu, W.-H.; Chou, C.-C.; Cheng, K.-C. Soyfoods and Soybean Products: From Traditional Use to Modern Applications. Appl. Microbiol. Biotechnol. 2012, 96, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef] [PubMed]
- CONAB Acompanhamento Da Safra Brasileira: Grãos—Safra 2023/2022. 2o Levantamento. 2023. Available online: https://www.Conab.Gov.Br/Info-Agro/Safras/Graos/Boletim-Da-Safra-de-Graos (accessed on 23 February 2024).
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a Better Understanding of the Genetic and Physiological Basis for Nitrogen Use Efficiency in Maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, P.S.; Jaworski, E.G. Patterns of Nitrogen Utilization in the Soybean. Planta 1975, 127, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Special Report 80; Iowa Agricultural Experiment Station, Iowa Cooperative External Series; Iowa State University: Ames, IA, USA, 1977. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soils; California Agricultural Experimental Station: Berkeley, CA, USA, 1950; 347p. [Google Scholar]
- Foy, C.D.; Fleming, A.L. Aluminum Tolerances of Two Wheat Genotypes Related to Nitrate Reductase Activities. J. Plant Nutr. 1982, 5, 1313–1333. [Google Scholar] [CrossRef]
- Rhodes, D.; Rendon, G.A.; Stewart, G.R. The Control of Glutamine Synthetase Level in Lemna Minor L. Planta 1975, 125, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Dougall, D.K. Evidence for the Presence of Glutamate Synthase in Extracts of Carrot Cells Cultures. Biochem. Biophys. Res. Commun. 1974, 58, 639–646. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil. Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential Analyses of Glyoxylate Derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.C.R.; Mazzafera, P.; Sodek, L. Flooding of the Root System in Soybean: Biochemical and Molecular Aspects of N Metabolism in the Nodule during Stress and Recovery. Amino Acids 2016, 48, 1285–1295. [Google Scholar] [CrossRef]
- de Souza, S.C.R.; Sodek, L.; Polacco, J.C.; Mazzafera, P. Urease Deficiency Alters Nitrogen Metabolism and Gene Expression in Urease-Null Soybean without Affecting Growth or Productivity under Nitrate Supply. Acta Physiol. Plant 2020, 42, 34. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A New Protein Hydrolysate-Based Biostimulant Applied by Fertigation Promotes Relief from Drought Stress in Capsicum Annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Balboa, G.R.; Ciampitti, I.A. Estimating Biological Nitrogen Fixation in Field-Grown Soybeans: Impact of B Value. Plant Soil. 2020, 446, 195–210. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.A.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Mamatha, B.C.; Rudresh, K.; Karthikeyan, N.; Kumar, M.; Das, R.; Taware, P.B.; Khapte, P.S.; Soren, K.R.; Rane, J.; Gurumurthy, S. Vegetal Protein Hydrolysates Reduce the Yield Losses in Off-Season Crops under Combined Heat and Drought Stress. Physiol. Mol. Biol. Plants 2023, 29, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum Chinensis L. Growth and Nutraceutical Properties Are Enhanced by Biostimulants in a Long-Term. Period: Chemical and Metabolomic Approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Tagliapietra, E.L.; Streck, N.A.; da Rocha, T.S.M.; Richter, G.L.; da Silva, M.R.; Cera, J.C.; Guedes, J.V.C.; Zanon, A.J. Optimum Leaf Area Index to Reach Soybean Yield Potential in Subtropical Environment. Agron. J. 2018, 110, 932–938. [Google Scholar] [CrossRef]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.-O.; Barone, A.; Rigano, M.M. Phenotyping to Dissect the Biostimulant Action of a Protein Hydrolysate in Tomato Plants under Combined Abiotic Stress. Plant Physiol. Biochem. 2022, 179, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and Regulation of Cytokinins in Tomato Fruit Development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Plačková, L.; Gruz, J.; Bíba, O.; Novák, O.; Stirk, W.A.; Doležal, K.; Van Staden, J. Seaweed-Derived Biostimulant (Kelpak®) Influences Endogenous Cytokinins and Bioactive Compounds in Hydroponically Grown Eucomis Autumnalis. J. Plant Growth Regul. 2016, 35, 151–162. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant Biostimulants: Physiological Responses Induced by Protein Hydrolyzed-Based Products and Humic Substances in Plant Metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of Natural Biostimulants on Yield and Nutritional Quality: An Example of Sweet Yellow Pepper (Capsicum Annuum L.) Plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of Growth, Yield, and the Nutraceutical and Antioxidative Potential of Soybean through the Use of Synthetic Biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).