Humic Acids Combined with Dairy Slurry as Fertilizer Can Increase Alfalfa Yield and Reduce Nitrogen Losses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Dairy Slurry Properties
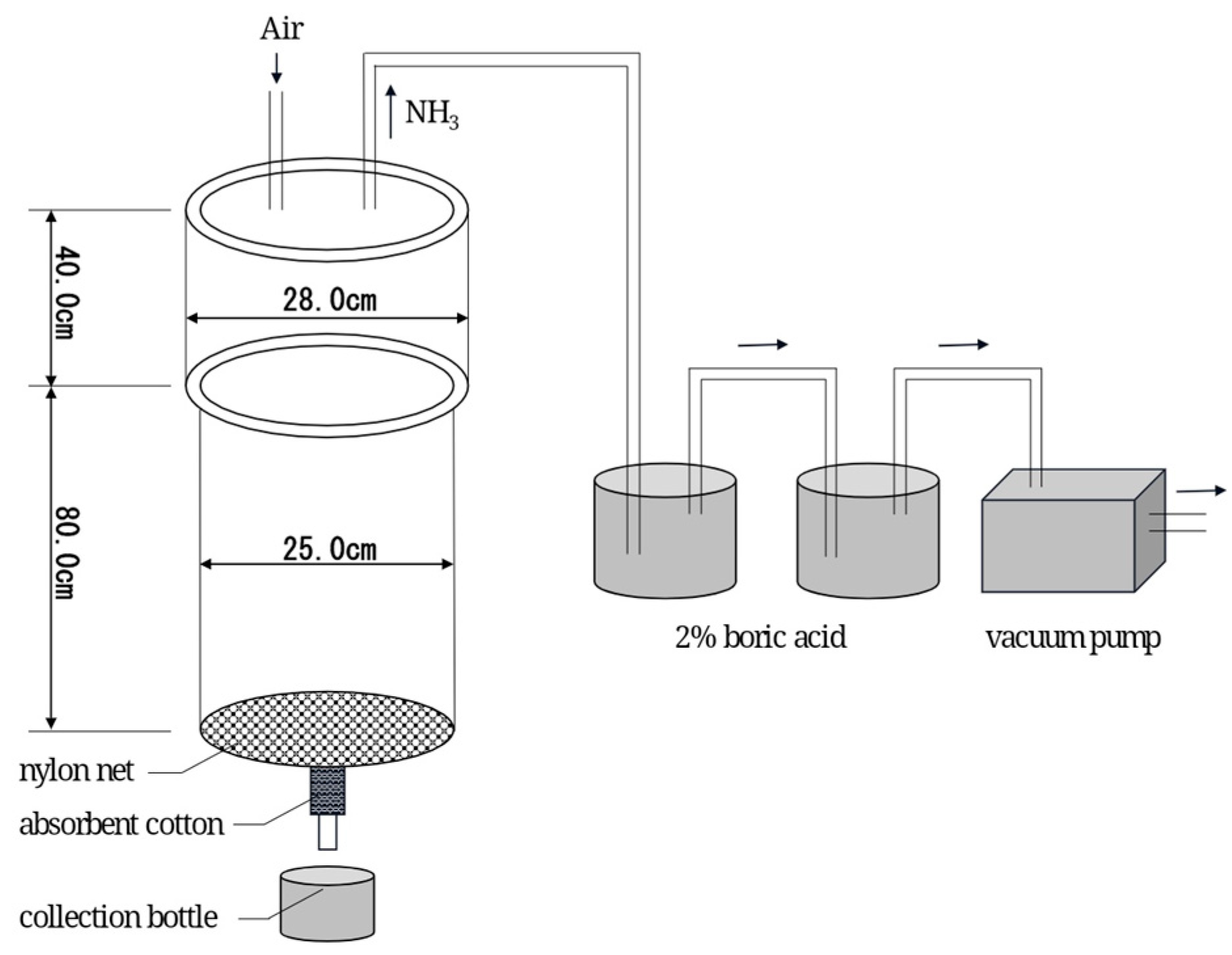
2.2. Lysimeter Installation
2.3. Experimental Design
2.4. Sample Collection and Measurement
2.5. Statistical Analysis
3. Results
3.1. Yield of Alfalfa
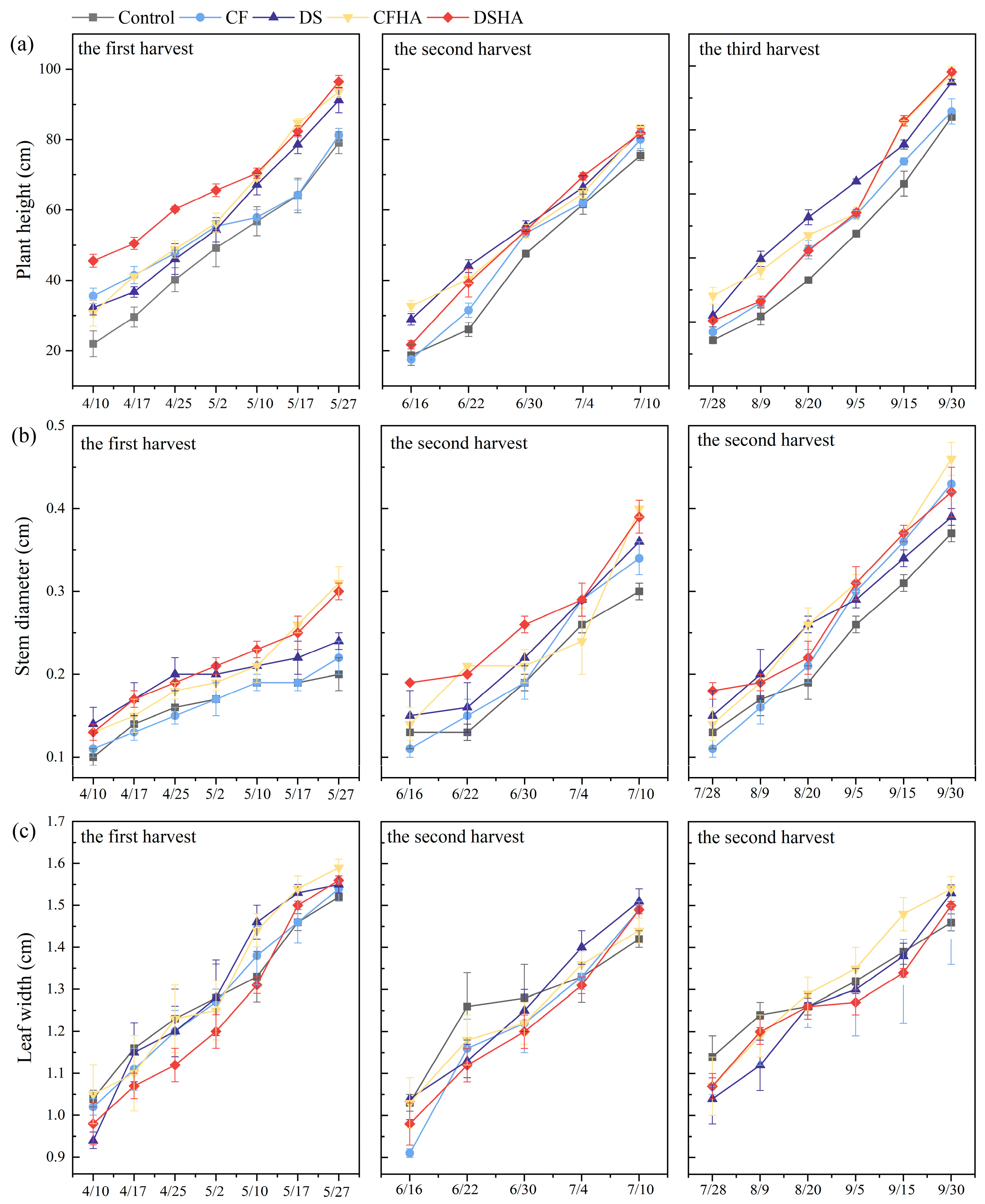
3.2. Agronomic Traits and Growth Rate of Alfalfa
3.3. Photosynthetic Characteristics
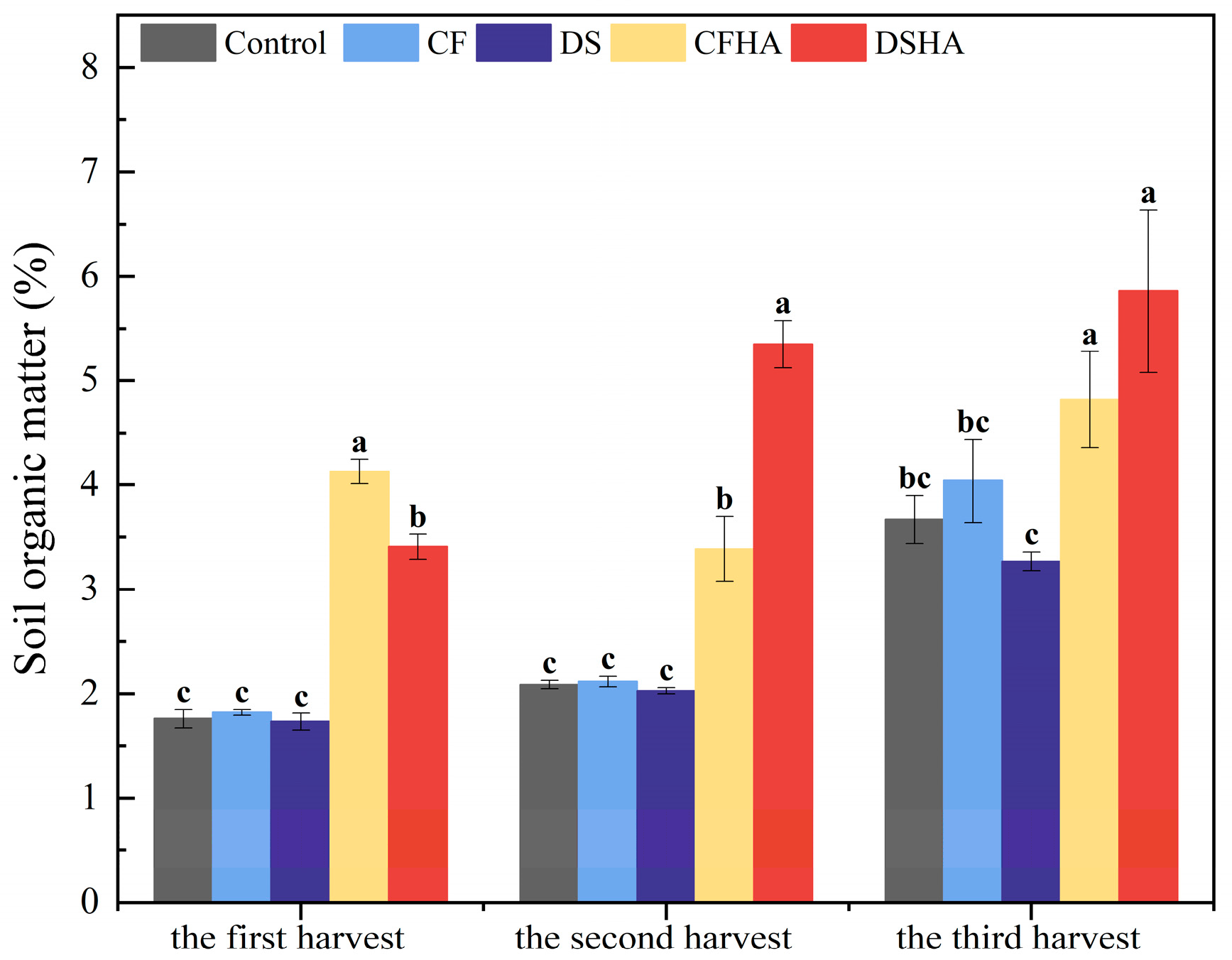
3.4. Soil Organic Matter, Soil pH, and Urease Activity
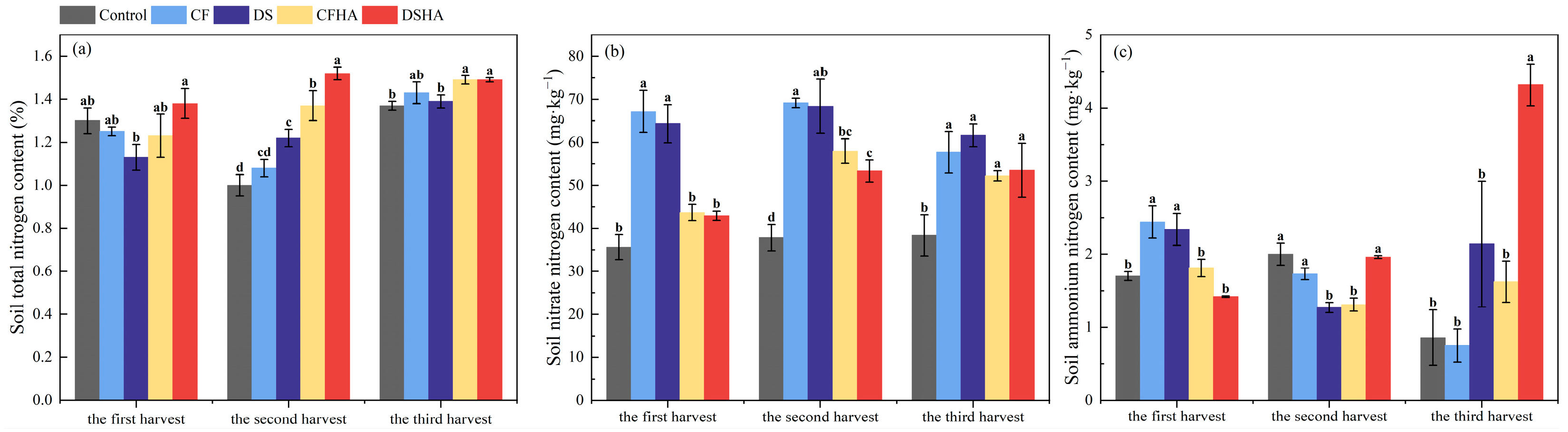
3.5. Soil Total Nitrogen, Nitrate Nitrogen, and Ammonium Nitrogen
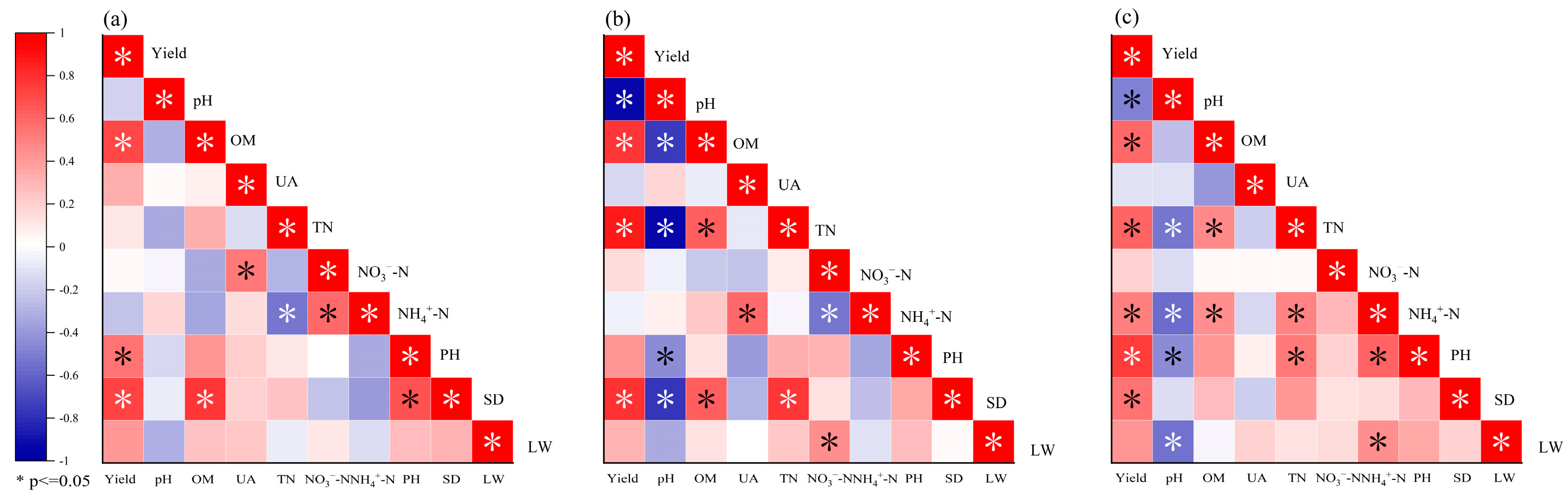
3.6. The Relationships between Alfalfa Yield and Soil Physicochemical Properties
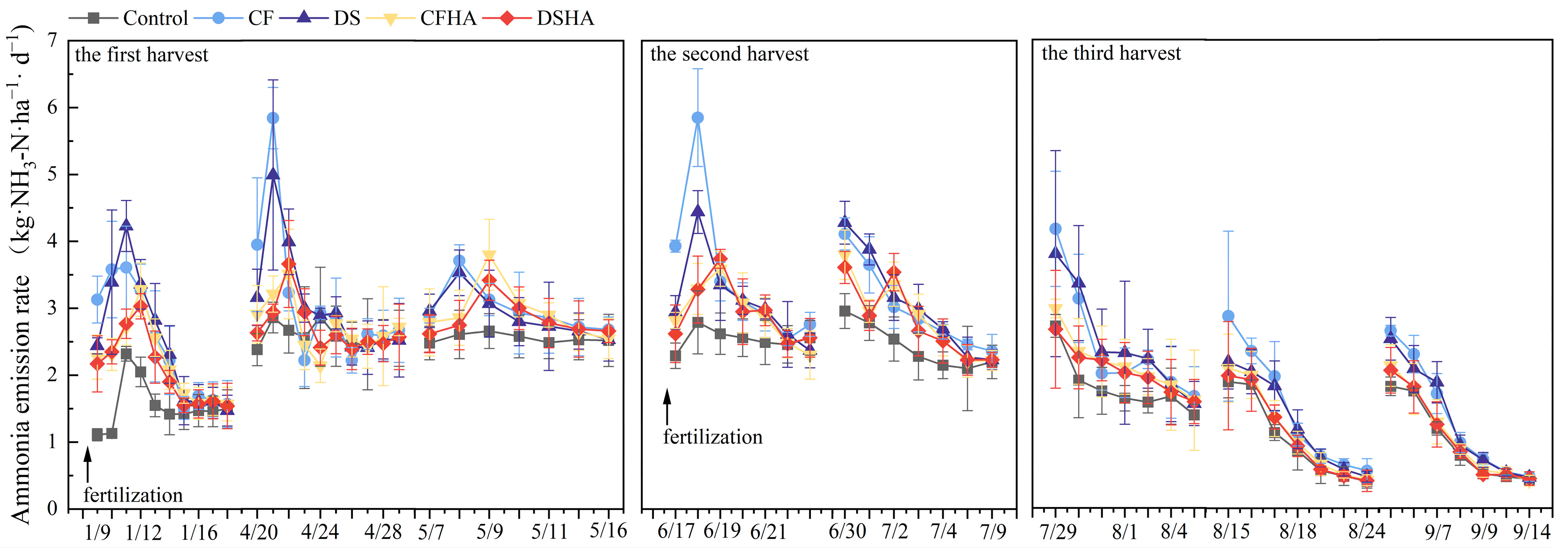
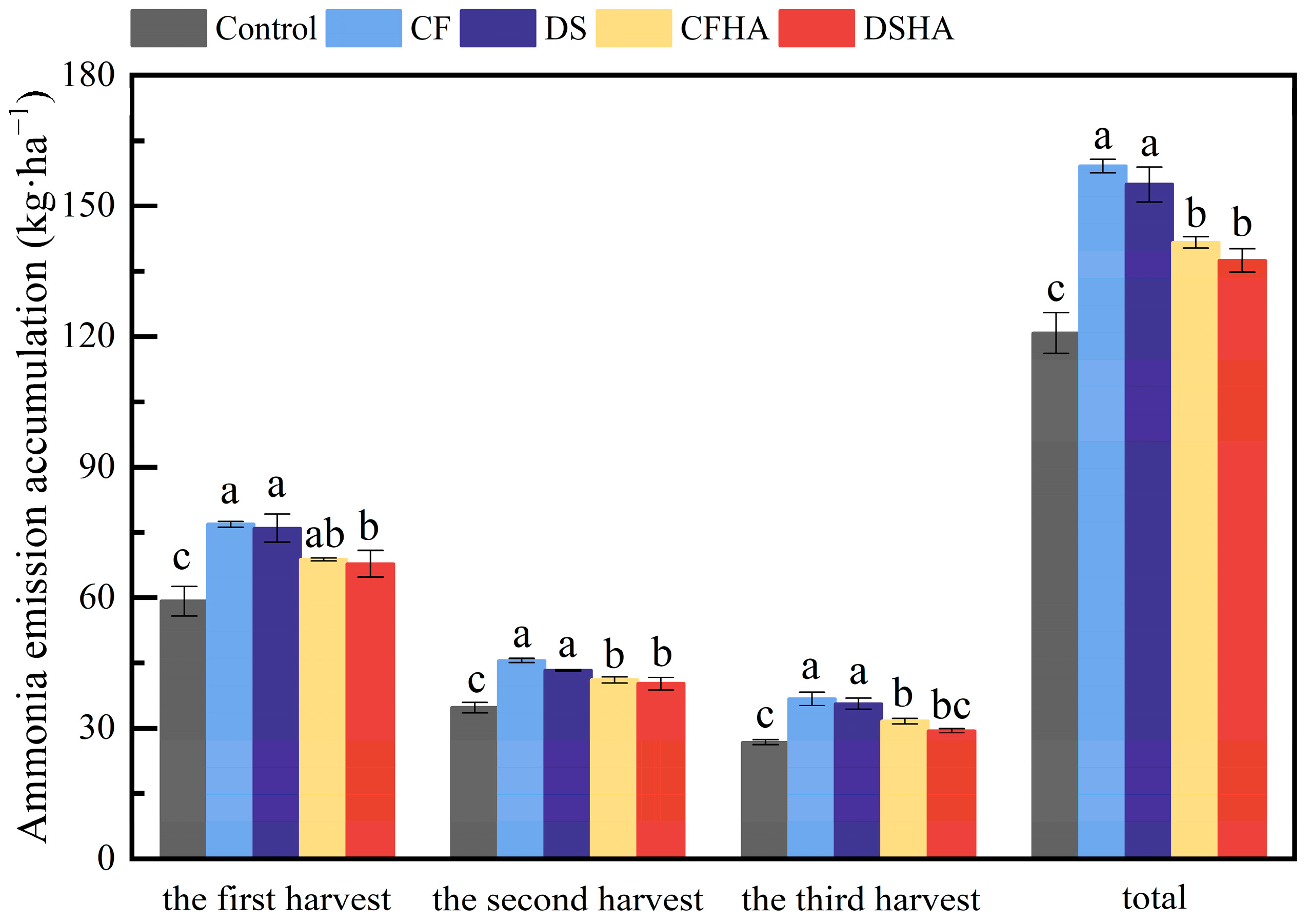
3.7. Ammonia Emissions
3.8. Nitrogen Leaching
4. Discussion
4.1. The Impact of Applying Dairy Slurry with Humic Acids on the Production of Alfalfa
4.2. The Impact of Applying Dairy Slurry with Humic Acids on the Nitrogen Loss
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Özköse, A. Effect of environment × cultivar interaction on protein and mineral contents of Alfalfa (Medicago sativa L.) in Central Anatolia, Turkey. Sains Malays. 2018, 47, 551–562. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, J. Creating modern technological system for grass product processing to guarantee macroscopic food security. Bull. Chin. Acad. Sci. 2021, 36, 675–684. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Y. China’s alfalfa market and imports: Development, trends, and potential impacts of the US–China trade dispute and retaliations. J. Integr. Agric. 2020, 19, 1149–1158. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Shi, S.; Nan, L.; Smith, K.F. The current status, problems, and prospects of alfalfa (Medicago sativa L.) breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef]
- Wan, W.; Li, Y.; Li, H. Yield and quality of alfalfa (Medicago sativa L.) in response to fertilizer application in China: A meta-analysis. Front. Plant Sci. 2022, 13, 1051725. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Gao, J.; Wu, S.; Liu, Y. Evaluation of the effectiveness of irrigation methods and fertilization strategies for alfalfa: A meta-analysis. J. Agron. Crop Sci. 2023, 6, 788–801. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Shen, Y.; Zhang, Q.; Li, Y. Fertilization increased alfalfa yield and nutritive value in northern China: A Meta-analysis. Pratacultural Sci. 2023, 40, 208–217. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Q.; Chu, Y.; Yin, Y.; Ying, H.; Zhang, F.; Cui, Z. Comprehensive assessment of the utilization of manure in China’s croplands based on national farmer survey data. Sci. Data 2023, 10, 223. [Google Scholar] [CrossRef]
- Chadwick, D.; Wei, J.; Yan’an, T.; Guanghui, Y.; Qirong, S.; Qing, C. Improving manure nutrient management towards sustainable agricultural intensification in China. Agric. Ecosyst. Environ. 2015, 209, 34–46. [Google Scholar] [CrossRef]
- Pain, B.; Menzi, H. Glossary of Terms on Livestock and Manure Management, 2nd ed.; Ramiran: Hamburg, Germany, 2011; p. 80. [Google Scholar]
- Zhang, K.; Du, L.; Du, H.; Shen, S. Application of livestock and poultry waste to agricultural land: A review. J. Agric. Environ. Sci. 2021, 11, 2472–2481+2591. (In Chinese) [Google Scholar] [CrossRef]
- Dong, H.; Zuo, L.; Wei, S.; Zhu, Z.; Yin, F. Establish manure nutrient management plan to promote green development of integrated crop-livestock production system. Bull. Chin. Acad. Sci. 2019, 34, 180–189. (In Chinese) [Google Scholar] [CrossRef]
- Lourenzi, C.; Ceretta, C.; Brunetto, G.; Girotto, E.; Tiecher, T.; Vieira, R.; Cancian, A.; Ferreira, P. Pig slurry and nutrient accumulation and dry matter and grain yield in various crops. Rev. Bras. De Cienc. Do Solo 2014, 38, 949–958. [Google Scholar] [CrossRef]
- Du, Y.; Cui, B.; Wang, Z.; Sun, J.; Niu, W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Stoltz, E.; Nadeau, E. Effects of intercropping on yield, weed incidence, forage quality and soil residual N in organically grown forage maize (Zea mays L.) and faba bean (Vicia faba L.). Field Crop. Res. 2014, 169, 21–29. [Google Scholar] [CrossRef]
- De, C.; Ceretta, C.; Ferreira, P.; Lorensini, F.; Lourenzi, C.; Vidal, R.; Tassinari, A.; Brunetto, G. Effects of pig slurry application and crops on phosphorus content in soil and the chemical species in solution. Rev. Bras. De Cienc. Do Solo 2015, 39, 774–787. [Google Scholar] [CrossRef]
- Park, S.; Lee, B.; Cho, W.; Kim, T. Comparative nitrogen use efficiency of urea and pig slurry for regrowth yield and nutritive value in perennial ryegrass sward. Asian Australas. J. Anim. 2017, 30, 514. [Google Scholar] [CrossRef]
- Shi, Y.; Ziadi, N.; Hamel, C.; Bittman, S.; Hunt, D.; Lalande, R.; Shang, J. Soil microbial biomass, activity, and community composition as affected by dairy manure slurry applications in grassland production. Appl. Soil Ecol. 2018, 125, 97–107. [Google Scholar] [CrossRef]
- Shakoor, A.; Bosch-Serra, À.D.; Alberdi, J.R.O.; Herrero, C. Seven years of pig slurry fertilization: Impacts on soil chemical properties and the element content of winter barley plants. Environ. Sci. Pollut. Res. 2022, 29, 74655–74668. [Google Scholar] [CrossRef]
- Abubaker, J.; Alaswd, A.; Mohammed, N.S.; Zeadani, H.E.; Khalifa, M. Alfalfa (Medicago sativa L.) growth and yield in desert soil fertilized with raw and anaerobically digested cattle manure. J. Plant Nutr. 2021, 45, 992–1003. [Google Scholar] [CrossRef]
- Lin, F.; Liu, X.; Tong, C.; Wu, Y. Characteristics of light energy utilization of intercropping alfalfa/Gramineae forage based on yield effect. Chin. J. Appl. Ecol. 2020, 31, 2963–2976. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Q.; Zhang, Z.; Ci, D.; Zhang, J.; Xu, Y.; Guo, Q.; Xu, M.; He, K. Effect of Reducing Nitrogen Fertilization and Adding Organic Fertilizer on Net Photosynthetic Rate, Root Nodules and Yield in Peanut. Plants 2023, 12, 2902. [Google Scholar] [CrossRef]
- Vasileva, V.; Kostov, O. Effect of mineral and organic fertilization on alfalfa forage and soil fertility. Emir. J. Food Agric. 2015, 27, 678–686. [Google Scholar] [CrossRef]
- Huddell, A.; Galford, G.; Tully, K.; Crowley, C.; Palm, C.; Neill, C.; Menge, D. Meta-analysis on the potential for increasing nitrogen losses from intensifying tropical agriculture. Glob. Change Biol. 2020, 26, 1668–1680. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, Y.; He, T.; Liu, R.; Yi, M.; Yang, Z. Nitrogen leaching losses following biogas slurry irrigation to purple soil of the Three Gorges Reservoir Area. Environ. Sci. Pollut. Res. 2015, 25, 29096–29103. [Google Scholar] [CrossRef]
- Gronwald, M.; Helfrich, M.; Don, A.; Fuß, R.; Well, R.; Flessa, H. Application of hydrochar and pyrochar to manure is not effective for mitigation of ammonia emissions from cattle slurry and poultry manure. Biol. Fertil. Soils 2018, 54, 451–465. [Google Scholar] [CrossRef]
- Pedersen, J.; Nyord, T.; Feilberg, A.; Labouriau, R.; Hunt, D.; Bittman, S. Effect of reduced exposed surface area and enhanced infiltration on ammonia emission from untreated and separated cattle slurry. Biosyst. Eng. 2021, 211, 141–151. [Google Scholar] [CrossRef]
- Hou, R.; Li, T.; Fu, Q.; Liu, D.; Li, M.; Zhou, Z.; Li, Q.; Zhao, H.; Yu, P.; Yan, J. The effect on soil nitrogen mineralization resulting from biochar and straw regulation in seasonally frozen agricultural ecosystem. J. Clean. Prod. 2020, 255, 120302. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China, 2017, Bulletin of the Second National Pollution Source Census. Available online: https://www.mee.gov.cn/home/ztbd/rdzl/wrypc/zlxz/202006/t20200616_784745.html (accessed on 16 June 2024).
- Klammler, G.; Fank, J. Determining water and nitrogen balances for beneficial management practices using lysimeters at Wagna test site (Austria). Sci. Total Environ. 2014, 499, 448–462. [Google Scholar] [CrossRef]
- Wang, X.; Di, H.; Cameron, K.; Li, B. Effect of treated farm dairy effluent on E. coli, phosphorus and nitrogen leaching and greenhouse gas emissions: A field lysimeter study. J. Soils Sediments 2019, 19, 2303–2312. [Google Scholar] [CrossRef]
- Rychel, K.; Meurer, K.; Getahun, G.; Bergström, L.; Kirchmann, H.; Kätterer, T. Lysimeter deep N fertilizer placement reduced leaching and improved N use efficiency. Nutr. Cycl. Agroecosyst. 2023, 126, 213–228. [Google Scholar] [CrossRef]
- Gu, L.; Liu, T.; Wang, J.; Liu, P.; Dong, S.; Zhao, B.; So, H.; Zhang, J.; Li, J. Lysimeter study of nitrogen losses and nitrogen use efficiency of Northern Chinese wheat. Field Crop. Res. 2016, 188, 82–95. [Google Scholar] [CrossRef]
- Karimi, R.; Akinremi, W.; Flaten, D. Cropping system and type of pig manure affect nitrate-nitrogen leaching in a sandy loam soil. J. Environ. Qual. 2017, 46, 785–792. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.; Motta, F.; Santana, M. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.; Deng, F.; Cao, Z.; Zheng, G. Production of artificial humic acid from rice straw for fertilizer production and soil improvement. Sci. Total Environ. 2024, 906, 167548. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Surgy, S.; Fraga, I.; Vasconcelos, E.; Coutinho, J. Acid treatment of animal slurries: Potential and limitations. In Proceedings-International Fertiliser Society. Int. Fert. Soc. 2015, 775, 1–24. [Google Scholar] [CrossRef]
- Zhi, Y.; Li, X.; Wang, X.; Jia, M.; Wang, Z. Photosynthesis promotion mechanisms of artificial humic acid depend on plant types: A hydroponic study on C3 and C4 plants. Sci. Total Environ. 2024, 917, 170404. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F. Contribution of piriformospora indica on improving the nutritional quality of greenhouse tomato and its resistance against cu toxicity after humic acid addition to soil. Environ. Sci. Pollut. Res. 2021, 28, 64572–64585. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, W.; Yang, J.; Yao, R.; Wang, X.; Li, W. Effect of Different Fertilization Measures on Soil Salinity and Nutrients in Salt-Affected Soils. Water 2023, 15, 3274. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L. Vermiculite and humic acid improve the quality of green waste compost as a growth medium for Centaurea cyanus L. Environ. Technol. Innov. 2021, 24, 101945. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Wu, Y.; Liu, B.; Tao, L.; Zhan, Y.; Ni, X.; Yang, Y. Better performance of compound fertilizers than bulk-blend fertilizers on reducing ammonia emission and improving wheat productivity. Agric. Ecosyst. Environ. 2022, 335, 108018. [Google Scholar] [CrossRef]
- Fangueiro, D.; Pereira, J.; Bichana, A.; Surgy, S.; Cabral, F.; Coutinho, J. Effects of cattle-slurry treatment by acidification and separation on nitrogen dynamics and global warming potential after surface application to an acidic soil. J. Environ. Manag. 2015, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Technical Regulations for Soil Testing and Fertilization in Grassland Alfalfa. Available online: https://hbba.sacinfo.org.cn/stdDetail/323920d1219b9c18ffd0da6e5444dd6c2fd76044231571a2651be9f927fb16f0 (accessed on 16 June 2024).
- Soil Testing Part 6: Method for Determination of Soil Organic Matter. Available online: https://hbba.sacinfo.org.cn/stdDetail/8c5969f13e0acfbaf685a468a20e6f92b028b4c34fce864c83d6916c47287abe (accessed on 16 June 2024).
- Dhakal, M.; West, C.; Villalobos, C.; Sarturi, J.; Deb, S. Trade-off between nutritive value improvement and crop water use for an alfalfa–grass system. Crop Sci. 2020, 60, 1711–1723. [Google Scholar] [CrossRef]
- Noland, R.; Wells, M.; Coulter, J.; Tiede, T.; Baker, J.; Martinson, K.; Sheaffer, C. Estimating alfalfa yield and nutritive value using remote sensing and air temperature. Field Crop. Res. 2018, 222, 189–196. [Google Scholar] [CrossRef]
- Aung, B.; Gao, R.; Gruber, M.; Yuan, Z.; Sumarah, M.; Hannoufa, A. MsmiR156 affects global gene expression and promotes root regenerative capacity and nitrogen fixation activity in alfalfa. Transgenic Res. 2017, 26, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, M.; Liu, M.; Lu, Y.; Yang, H. Nitrogen and phosphorus fertilizations alter nitrogen, phosphorus and potassium resorption of alfalfa in the Loess Plateau of China. J. Plant Nutr. 2019, 42, 2234–2246. [Google Scholar] [CrossRef]
- McDonald, I.; Baral, R.; Min, D. Effects of alfalfa and alfalfa-grass mixtures with nitrogen fertilization on dry matter yield and forage nutritive value. J. Anim. Sci. Technol. 2021, 63, 305. [Google Scholar] [CrossRef] [PubMed]
- Sradnick, A.; Feller, C. A typological concept to predict the nitrogen release from organic fertilizers in farming systems. Agronomy 2020, 10, 1448. [Google Scholar] [CrossRef]
- Bhogal, A.; Williams, J.; Nicholson, F.; Chadwick, D.; Chambers, K.; Chambers, B. Mineralization of organic nitrogen from farm manure applications. Soil Use Manag. 2016, 32, 32–34. [Google Scholar] [CrossRef]
- Hua, W.; Luo, P.; An, N.; Cai, F.; Zhang, S.; Chen, K.; Yang, J.; Han, X. Manure application increased crop yields by promoting nitrogen use efficiency in the soils of 40-year soybean-maize rotation. Sci. Rep. 2020, 10, 14882. [Google Scholar] [CrossRef]
- Pandey, B.; Chen, L. Technologies to recover nitrogen from livestock manure-A review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef]
- Ransom, C.; Jolley, V.; Blair, T.; Sutton, L.; Hopkins, B. Nitrogen release rates from slow- and controlled-release fertilizers influenced by placement and temperature. PLoS ONE 2020, 15, e0234544. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, S.; Zhang, J.; Zhang, X.; Wang, C. Effects of controlled-release fertilizer on rice grain yield, nitrogen use efficiency, and greenhouse gas emissions in a paddy field with straw incorporation. Field Crop. Res. 2020, 253, 107814. [Google Scholar] [CrossRef]
- Dogan, Y.; Togay, Y.; Togay, N.; Kulaz, H. Effect of humic acid and phosphorus applications on the yield and yield components in lentil (Lens culinaris Medic.). Legume Res. Int. J. 2014, 37, 316–320. [Google Scholar] [CrossRef]
- Mosaad, I.; Serag, A.; Sheta, M. Promote sugar beet cultivation in saline soil by applying humic substances in-soil and mineral nitrogen fertilization. J. Plant Nutr. 2022, 45, 2447–2464. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Xu, S.; Neil, B.; Liu, J. Effect of water soluble humic acid applied to potato foliage on plant growth, photosynthesis characteristics and fresh tuber yield under different water deficits. Sci. Rep. 2020, 10, 7854. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Li, Y.; Koopal, L.; Chai, Y.; Tan, W.; Wu, C.; Tang, X.; Chen, Y. Spectroscopic investigation of conformational changes in urease caused by interaction with humic acid. Colloids Surf. B 2022, 215, 112510. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Li, Z.; Zhang, C.; Wan, C.; Zhang, Y.; Lee, D. Inhibition of urease activity by humic acid extracted from sludge fermentation liquid. Bioresour. Technol. 2019, 290, 121767. [Google Scholar] [CrossRef]
- Aon, M.; Cabello, M.; Sarena, D.; Colaneri, A.; Franco, M.; Burgos, J.; Cortassa, S. Spatio-temporal patterns of soil microbial and enzymatic activities in an agricultural soil. Appl. Soil Ecol. 2001, 18, 239–254. [Google Scholar] [CrossRef]
- Fu, L.; Wang, G.; Du, H.; Zhang, K. Effect of slurry application on soil ammonia volatilization and response factors. J. Agric. Resour. Environ. 2020, 06, 931–938. (In Chinese) [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and greenhouse gas emissions from slurry storage-A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Zhou, M.; Ying, S.; Chen, J.; Jiang, P.; Teng, Y. Effects of biochar-based fertilizer on nitrogen use efficiency and nitrogen losses via leaching and ammonia volatilization from an open vegetable field. Environ. Sci. Pollut. Res. 2021, 28, 65188–65199. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Song, F.; Song, X.; Guo, X.; Lu, Y.; Chen, S.; Zhu, F.; Zhang, N.; Zou, J.; Zhang, P. Effects of different types of humic acid isolated from coal on soil NH3 volatilization and CO2 emissions. Environ. Res. 2021, 194, 110711. [Google Scholar] [CrossRef] [PubMed]
- Jatana, B.S.; Gami, S.K.; Ketterings, Q.M. Assessing the Impact of Mixing Acid Whey With Dairy Manure On pH and Nitrogen Dynamics During Manure Handling. J. Soil Sci. Plant Nutr. 2023, 23, 2878–2890. [Google Scholar] [CrossRef]
- Gurgel, G.; Ferrari, A.; Fontana, A.; Polidoro, J.; Coelho, L.; Zonta, E. Ammonia volatilization from mixed mineral fertilizers containing urea. Pesqui. Agropecu. Bras. 2016, 51, 1686–1694. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, H.; Gao, W.; Li, M. The effects of humic acid urea and polyaspartic acid urea on reducing nitrogen loss compared with urea. J. Sci. Food Agric. 2020, 100, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, C.; Seddaiu, G.; Ledda, L.; Cappai, C.; Doro, L.; Carletti, A.; Roggero, P. Replacing organic with mineral N fertilization does not reduce nitrate leaching in double crop forage systems under Mediterranean conditions. Agric. Ecosyst. Environ. 2016, 219, 83–92. [Google Scholar] [CrossRef]
- Du, H.; Gao, W.; Li, J.; Shen, S.; Wang, F.; Fu, L.; Zhang, K. Effects of digested biogas slurry application mixed with irrigation water on nitrate leaching during wheat-maize rotation in the North China Plain. Agric. Water Manag. 2019, 213, 882–893. [Google Scholar] [CrossRef]
- Kim, S.; Heo, W.; Lee, S.; Han, B.; Lee, H.; Kim, Y. Characterisitcs of Saccharomyces boulardii for reducing ammonia emission from livestock manure. Appl. Biol. Chem. 2021, 64, 30. [Google Scholar] [CrossRef]
- Kemmitt, S.; Wright, D.; Jones, D. Soil acidification used as a management strategy to reduce nitrate losses from agricultural land. Soil Biol. Biochem. 2005, 37, 867–875. [Google Scholar] [CrossRef]
- Shahraki, Z.; Mao, X.; Waugh, S.; Lotfikatouli, S.; Walker, H.; Gobler, C.; Wanlass, J. Potential release of legacy nitrogen from soil surrounding onsite wastewater leaching pools. Water Res. 2020, 169, 115241. [Google Scholar] [CrossRef]
- Elgharably, A.; Benes, S. Alfalfa biomass yield and nitrogen fixation in response to applied mineral nitrogen under saline soil conditions. J. Soil Sci. Plant Nutr. 2021, 21, 744–755. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, B.; Lam, S.; Bai, E.; Hou, P.; Chen, D. Predicting the ratio of nitrification to immobilization to reflect the potential risk of nitrogen loss worldwide. Environ. Sci. Technol. 2021, 55, 7721–7730. [Google Scholar] [CrossRef]


| Depth (cm) | SOM (g·kg−1) | TN (mg·kg−1) | NH4+-N (mg·kg−1) | NO3−-N (mg·kg−1) | Olsen-P (mg·kg−1) | Ni (mg·kg−1) | As (mg·kg−1) | Cu (mg·kg−1) | Zn (mg·kg−1) | Cr (mg·kg−1) | Cd (mg·kg−1) | Pb (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | 13.42 | 989.37 | 5.68 | 28.34 | 10.10 | 21.95 | 35.52 | 11.41 | 29.28 | 34.24 | 0.99 | 21.25 |
| 20–40 | 8.91 | 658.09 | 4.08 | 12.13 | 3.45 | 17.33 | 36.64 | 7.38 | 22.17 | 30.89 | 0.91 | 17.28 |
| 40–60 | 5.73 | 489.43 | 3.59 | 11.09 | 0.89 | 17.95 | 37.18 | 7.74 | 20.14 | 32.05 | 0.84 | 16.94 |
| Treatment | Alfalfa Yield of Each Harvest (kg·ha−1) | Total Yield (kg·ha−1) | Average Yield (kg·ha−1) | Average Increase Rate (%) | ||
|---|---|---|---|---|---|---|
| First Harvest | Second Harvest | Third Harvest | ||||
| Control | 9065.47 ± 107.15 b | 7242.19 ± 168.30 e | 7532.49 ± 63.54 c | 23,840.14 ± 139.26 c | 7946.71 c | - |
| CF | 11,031.35 ± 161.59 a | 9284.46 ± 221.04 d | 10,399.82 ± 152.11 b | 30,715.63 ± 336.64 b | 10,238.54 b | 28.84% b |
| DS | 11,072.09 ± 186.99 a | 9814.13 ± 89.72 c | 10,552.61 ± 195.40 b | 31,438.83 ± 226.45 b | 10,479.61 b | 31.87% b |
| CFHA | 12,263.84 ± 775.02 a | 10,282.68 ± 93.86 b | 12,218.01 ± 600.21 a | 34,764.53 ± 1450.85 a | 11,588.18 a | 45.82% a |
| DSHA | 12,284.22 ± 717.46 a | 11,117.93 ± 59.61 a | 11,591.57 ± 231.53 a | 34,993.72 ± 924.34 a | 11,664.57 a | 46.78% a |
| Treatment | pH | Urease Activity (mg·g−1·d−1) | ||||
|---|---|---|---|---|---|---|
| First Harvest | Second Harvest | Third Harvest | First Harvest | Second Harvest | Third Harvest | |
| Control | 8.83 ± 0.05 a | 8.43 ± 0.07 a | 8.17 ± 0.02 a | 1.57 ± 0.08 b | 1.67 ± 0.04 a | 1.74 ± 0.05 b |
| CF | 8.79 ± 0.02 a | 8.24 ± 0.04 b | 8.17 ± 0.03 a | 1.82 ± 0.07 a | 1.57 ± 0.02 a | 1.67 ± 0.07 b |
| DS | 8.83 ± 0.03 a | 8.17 ± 0.00 b | 8.14 ± 0.07 a | 1.87 ± 0.04 a | 1.53 ± 0.09 ab | 1.70 ± 0.05 b |
| CFHA | 8.80 ± 0.03 a | 7.91 ± 0.05 c | 8.06 ± 0.04 a | 1.79 ± 0.11 a | 1.40 ± 0.01 b | 1.93 ± 0.03 a |
| DSHA | 8.78 ± 0.04 a | 7.75 ± 0.05 d | 8.10 ± 0.03 a | 1.78 ± 0.02 ab | 1.69 ± 0.07 a | 1.68 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Shi, H.; Sindhøj, E.; Wang, G.; Liu, F.; Gao, X.; Du, H.; Du, L.; Zhang, K. Humic Acids Combined with Dairy Slurry as Fertilizer Can Increase Alfalfa Yield and Reduce Nitrogen Losses. Agriculture 2024, 14, 1208. https://doi.org/10.3390/agriculture14081208
Huang Y, Shi H, Sindhøj E, Wang G, Liu F, Gao X, Du H, Du L, Zhang K. Humic Acids Combined with Dairy Slurry as Fertilizer Can Increase Alfalfa Yield and Reduce Nitrogen Losses. Agriculture. 2024; 14(8):1208. https://doi.org/10.3390/agriculture14081208
Chicago/Turabian StyleHuang, Yanqin, Huixian Shi, Erik Sindhøj, Guiyun Wang, Fuyuan Liu, Xingliang Gao, Huiying Du, Lianzhu Du, and Keqiang Zhang. 2024. "Humic Acids Combined with Dairy Slurry as Fertilizer Can Increase Alfalfa Yield and Reduce Nitrogen Losses" Agriculture 14, no. 8: 1208. https://doi.org/10.3390/agriculture14081208
APA StyleHuang, Y., Shi, H., Sindhøj, E., Wang, G., Liu, F., Gao, X., Du, H., Du, L., & Zhang, K. (2024). Humic Acids Combined with Dairy Slurry as Fertilizer Can Increase Alfalfa Yield and Reduce Nitrogen Losses. Agriculture, 14(8), 1208. https://doi.org/10.3390/agriculture14081208








