The Antifungal and Inhibitory Effects of Massoia Essential Oil and C10 Massoia Lactone on Mycotoxin Production in Fusarium graminearum KACC 41047
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fungal Strain and Culture Conditions
2.3. Disc Diffusion Assay
2.4. Agar Dilution Assay
2.5. Mycelial Growth Assay
2.6. Mycotoxin Analysis Using LC–MS/MS
2.7. RNA Extraction and RT-qPCR
2.8. Statistical Analyses
3. Results
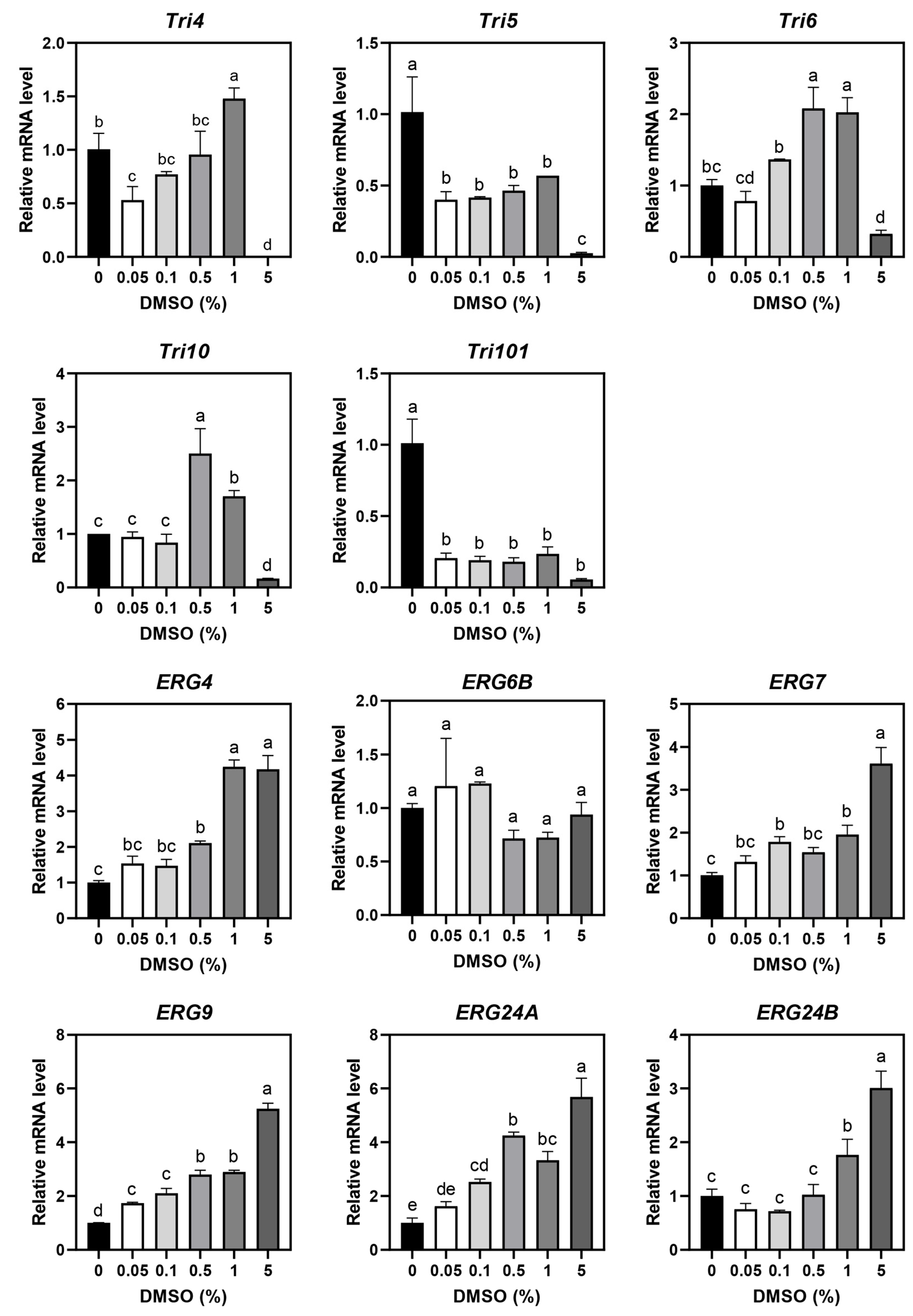
3.1. DMSO Effects on F. graminearum
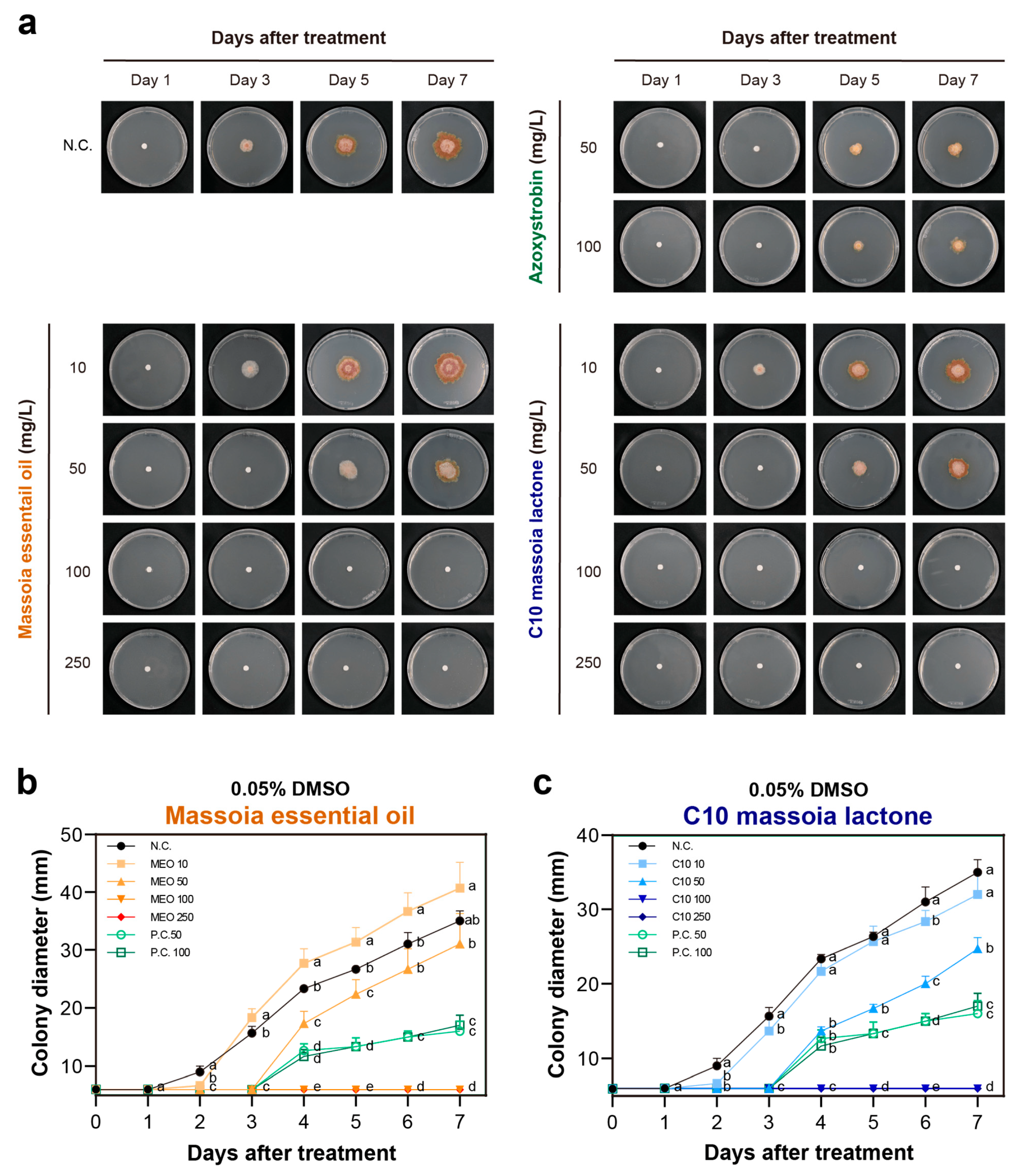
3.2. The Antifungal Activities of MEO and C10 against F. graminearum
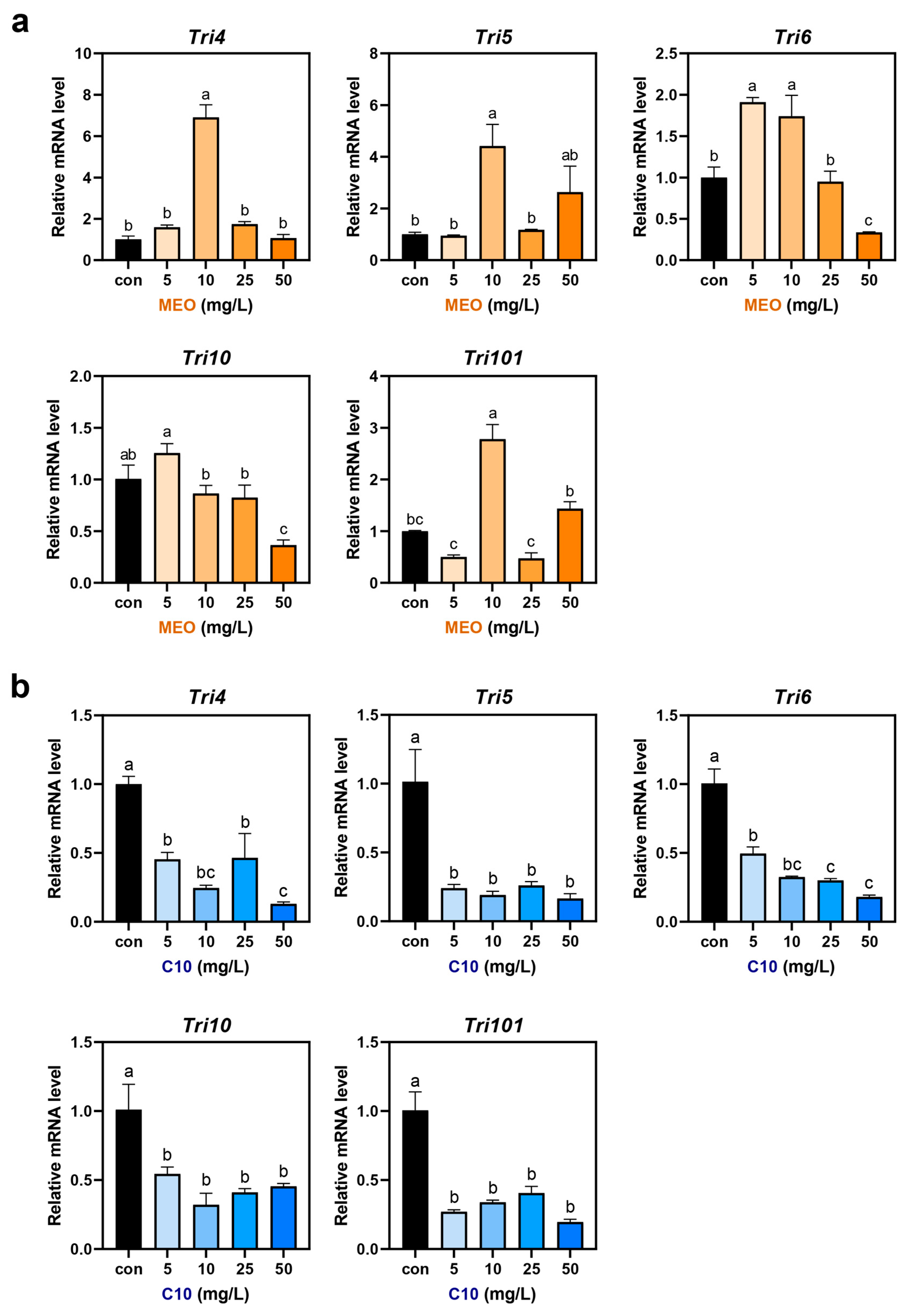
3.3. Mycotoxin Production and Gene Expression in MEO- and C10-Treated F. graminearum
4. Discussion
4.1. DMSO as an Antifungal Agent Solvent
4.2. MEO and C10 as Antifungal Agents against F. graminearum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, G.; Shaner, G. Scab of wheat; prospects of control. Plant Dis. 1994, 78, 760–766. [Google Scholar]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Scanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Chhabra, B.; Raza, A.; Yang, S.H.; Sandhu, K.S. Important wheat diseases in the US and their management in the 21st century. Front. Plant Sci. 2023, 13, 1010191. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Dahl, B.; Nganje, W. Economic costs of fusarium head blight, scab and deoxynivalenol. World Mycotoxin J. 2018, 11, 291–302. [Google Scholar] [CrossRef]
- Shim, H.K.; Gang, I.J. Development of monitoring and management technology for Fusarium head blight. Natl. Inst. Crop Sci. 2018, 2, 1593–1666. [Google Scholar]
- Jung, J.Y.; Kim, J.H.; Baek, M.; Cho, C.; Cho, J.; Kim, J.; Pavan, W.; Kim, K.H. Adapting to the projected epidemics of Fusarium head blight of wheat in Korea under climate changes scenarios. Front. Plant Dis. 2022, 13, 1040752. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Moonjely, S.; Ebert, M.; Paton-Glassbrook, D.; Noel, Z.A.; Roze, L.; Shay, R.; Watkins, T.; Trail, F. Update on the state of research to manage Fusarium head blight. Fungal Genet. Biol. 2023, 169, 103829. [Google Scholar] [CrossRef]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crop. Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Pazdiora, P.C.; da Rosa Dorneles, K.; Morello, T.N.; Nicholson, P.; Dallagnol, L.J. Silicon soil amendment as a complement to manage tan spot and Fusarium head blight in wheat. Agron. Sustain. Dev. 2021, 41, 21. [Google Scholar] [CrossRef]
- Anderson, N.R.; Freije, A.N.; Bergstrom, G.C.; Bradley, C.A.; Cowger, C.; Faske, T.; Hollier, C.; Kleczewski, N.; Padgett, G.B.; Paul, P.; et al. Sensitivity of Fusarium graminearum to metconazole and tebuconazole fungicides before and after widespread use in wheat in the United States. Plant Health Prog. 2020, 21, 85–90. [Google Scholar] [CrossRef]
- Paul, P.A.; Bradley, C.A.; Madden, L.V.; Dalla Lana, F.; Bergstrom, G.C.; Dill-Macky, R.; Esker, P.D.; Wise, K.A.; McMullen, M.; Grybauskas, A.; et al. Meta-analysis of the effects of QoI and DMI fungicide combinations on Fusarium head blight and deoxynivalenol in wheat. Plant Dis. 2018, 102, 2602–2615. [Google Scholar] [CrossRef]
- Jung, B.; Li, T.; Ji, S.; Lee, J. Efficacy of diphenyleneiodonium chloride (DPIC) against diverse plant pathogens. Mycobiology 2019, 47, 105–111. [Google Scholar] [CrossRef]
- Harčárová, M.; Čonková, E.; Proškovcová, M.; Váczi, P.; Marcinčáková, D.; Bujňák, L. Comparison of antifungal activity of selected essential oils against Fusarium graminearum in vitro. Ann. Agric. Environ. Med. 2021, 28, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Baek, S.G.; Choi, J.H.; Kim, S.; Kim, J.; Kim, D.W.; Yun, S.H.; Lee, T. Characterization of nivalenol-producing Fusarium asiaticum that causes cereal head blight in Korea. Plant Pathol. J. 2019, 35, 543–552. [Google Scholar] [CrossRef]
- Sydenham, E.W.; Thiel, P.G.; Marasas, W.F.O.; Nieuwenhuis, J.J. Occurrence of deoxynivalenol and nivalenol in Fusarium graminearum infected undergrade wheat in South Africa. J. Agric. Food Chem. 1989, 37, 921–926. [Google Scholar] [CrossRef]
- Haidukowski, M.; Somma, S.; Ghionna, V.; Cimmarusti, M.T.; Masiello, M.; Logrieco, A.F.; Moretti, A. Deoxynivalenol and T-2 toxin as major concerns in durum wheat from Italy. Toxins 2022, 14, 627. [Google Scholar] [CrossRef]
- EU (European Union). Maximum Levels for Certain Contamination in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products. Commission Regulation (EC) No. 1126/2007. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32007R1126 (accessed on 22 May 2024).
- Lee, T.; Baek, S.G.; Kim, S.; Paek, J.S.; Choi, J.; Choi, J.H.; Jang, Y.J.; Kim, J. Trends in mycotoxin contamination of cereals and cereal products in Korea. Res. Plant Dis. 2022, 28, 179–194. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.J.; Kim, K.; Kim, T.O.; Lee, S.E. Antifungal and antiaflatoxigenic activities of massoia oil and C10 massoia lactone against aflatoxin-producing Aspergillus flavus. Toxins 2023, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Hazen, K.C. Influence of DMSO on antifungal activity during susceptibility testing in vitro. Diagn. Microbiol. Infect. Dis. 2013, 75, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Pączka, A.; Mołon, M.; Bartosz, G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Kasparyan, G.; Poojari, C.; Rog, T.; Hub, J.S. Cooperative effects of an antifungal moiety and DMSO on pore formation over lipid membranes revealed by free energy calculations. J. Phys. Chem. B 2020, 124, 8811–8821. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A. The effect of dimethyl sulfoxide (DMSO) on the growth of dermatophytes. Jap. J. Med. Mycol. 2006, 47, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.H.; Lippi, Y.; Naylies, C.; Jamin, E.L.; Genthon, C.; Bailly, S.; Oswald, I.P.; Bailly, J.-D.; Puel, O. The solvent dimethyl sulfoxide affects physiology, transcriptome and secondary Metabolism of Aspergillus flavus. J. Fungi 2021, 7, 1055. [Google Scholar] [CrossRef]
- Romoli, J.C.Z.; Silva, M.V.; Pante, G.C.; Hoeltgebaum, D.; Castro, J.C.; da Rocha, G.H.O.; Capoci, I.R.G.; Nerilo, S.B.; Mossini, S.A.G.; da Gloria, E.M.; et al. Anti-mycotoxigenic and antifungal activity of ginger, turmeric, thyme and rosemary essential oils in deoxynivalenol (DON) and zearalenone (ZEA) producing Fusarium graminearum. Food Addit. Contam. A 2022, 39, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Maeri, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against F. graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef]
- Seo, S.M.; Lee, J.W.; Shin, J.; Tak, J.H.; Hyun, J.; Park, I.K. Development of cellulose nanocrystal-stabilized pickering emulsions of massoia and nutmeg essential oils for the control of Aedes abbopictus. Sci. Rep. 2022, 11, 12038. [Google Scholar] [CrossRef]
- Popiel, D.; Dawidziuk, A.; Koczyk, G.; Mackowiak, A.; Marcinkowska, K. Multiple facets of response to fungicides—The influence of azole treatment on expression of key mycotoxin biosynthetic genes and candidate resistance factors in the control of resistant Fusarium strains. Eur. J. Plant Pathol. 2017, 147, 773–785. [Google Scholar] [CrossRef]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef]
- Fan, J.; Urban, M.; Parker, J.E.; Brewer, H.C.; Kelly, S.L.; Hammond-Kosack, K.E.; Fraaije, B.A.; Liu, X.; Cools, H.J. Characterization of the sterol 14a-demethylase of Fusarium graminearum identifies a novel genus-specific CYP51 function. New Phytol. 2013, 198, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Rybak, J.M.; Martin-Vincente, A.; Guruceaga, X.; Thorn, H.I.; Nywening, A.V.; Ge, W.; Parker, J.E.; Kelly, S.L.; Rogers, P.D.; et al. The sterol C-24 methyltranferase encoding gene, erg6, is essential for viability of Aspergillus species. Nat. Commun. 2024, 15, 4261. [Google Scholar] [CrossRef]
- De Backer, M.D.; Ilyina, T.; Ma, X.J.; Vandoninck, S.; Luyten, W.H.M.; Vanden Bossche, H. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 2001, 45, 1660–1670. [Google Scholar] [CrossRef]
- Heidtmann-Bemvenuti, R.; Tralamazza, S.M.; Ferreira, C.F.J.; Correa, B.; Badiale-Furlong, E. Effect of natural compounds on Fusarium graminearum complex. J. Sci. Food Agric. 2016, 96, 3998–4008. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hou, G.; Ji, F.; Wang, D. Antifungal activity and metabolomics analysis of Piper sarmentosum extracts against Fusarium graminearum. J. Appl. Microbiol. 2023, 134, lxad019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. A recent overview on the biological and pharmacological activities of ferulic acid. EXCLI J. 2019, 18, 132–138. [Google Scholar] [PubMed]
- Yan, H.; Meng, X.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Antifungal activity and inhibitory mechanisms of ferulic acid against the growth of Fusarium graminearum. Food Biosci. 2023, 52, 102414. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, C.; Kim, T.O.; Lee, S.E. Developmental toxicity of C10 massoia lactone, the main constituent of Cryptorya massoia, on zebrafish (Danio rerio) embryos. Appl. Sci. 2024, 14, 538. [Google Scholar] [CrossRef]
- Montibus, M.; Ducos, C.; Bonnin-Verdal, M.-N.; Bormann, J.; Ponts, N. The bZIP Transcription Factor Fgap1 Mediates Oxidative Stress Response and Trichothecene Biosynthesis but Not Virulence in Fusarium graminearum. PLoS ONE 2013, 8, e83377. [Google Scholar]
- Boutigny, A.L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar]
- Wang, L.Q.; Wu, K.T.; Yang, P.; Hou, F.; Rajput, S.A.; Qi, D.S.; Wang, S. Transcriptomics reveals the effect of thymol on the growth and toxin production of Fusarium graminearum. Toxins 2022, 14, 142. [Google Scholar]
- Liu, X.; Jiang, J.; Yin, Y.; Ma, Z. Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum. Mol. Plant Pathol. 2013, 14, 71–83. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, S.-E. The Antifungal and Inhibitory Effects of Massoia Essential Oil and C10 Massoia Lactone on Mycotoxin Production in Fusarium graminearum KACC 41047. Agriculture 2024, 14, 1216. https://doi.org/10.3390/agriculture14081216
Lee J, Lee S-E. The Antifungal and Inhibitory Effects of Massoia Essential Oil and C10 Massoia Lactone on Mycotoxin Production in Fusarium graminearum KACC 41047. Agriculture. 2024; 14(8):1216. https://doi.org/10.3390/agriculture14081216
Chicago/Turabian StyleLee, Jieun, and Sung-Eun Lee. 2024. "The Antifungal and Inhibitory Effects of Massoia Essential Oil and C10 Massoia Lactone on Mycotoxin Production in Fusarium graminearum KACC 41047" Agriculture 14, no. 8: 1216. https://doi.org/10.3390/agriculture14081216



