Inhibition of Botrytis cinerea and Escherichia coli by Lactic Acid Bacteria on Leafy Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Testing of LAB Antagonism against Plant Pathogenic Fungi
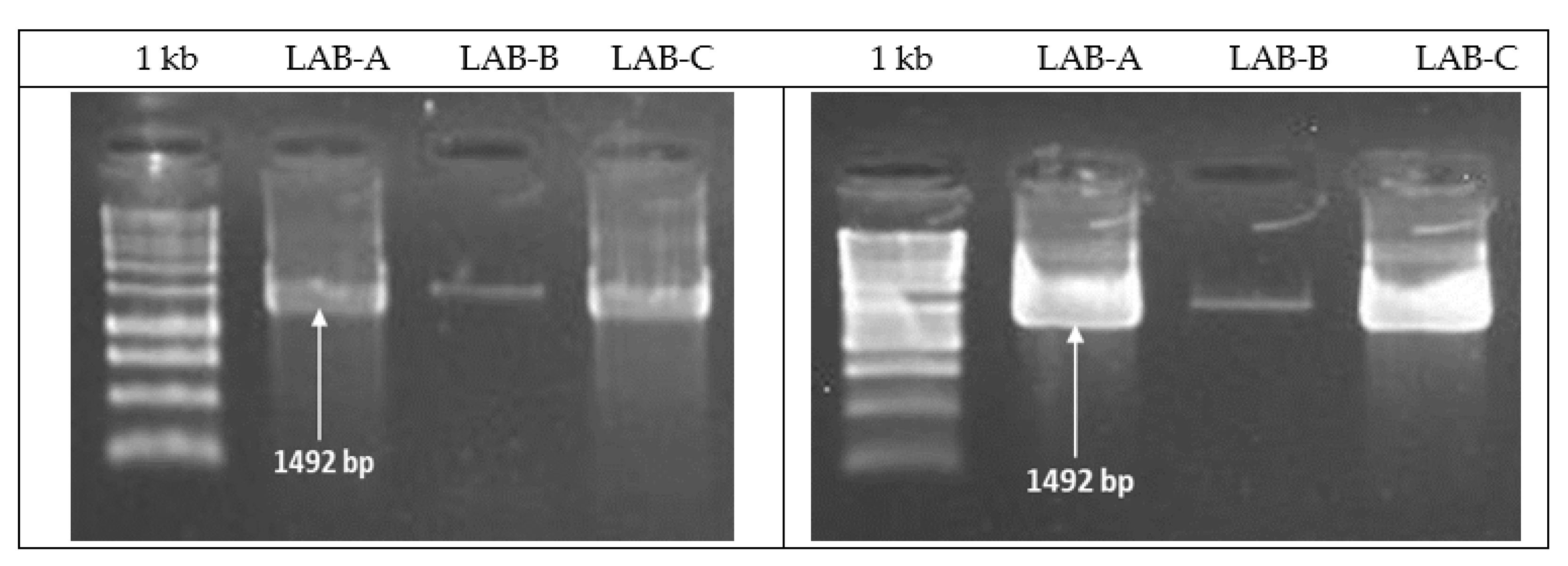
2.2. Identification of LAB Bacterial Isolates Based on 16S rRNA Gene Fragment Sequence Analysis
2.3. Inhibition of B. cinerea via LAB Culture Supernatant
2.4. Inhibition of E. coli Growth via LAB Culture Supernatant
2.5. Determination of the LAB Survival on Spinach and Lettuce Leaves Contaminated with Fungi
2.6. Determination of B. cinerea Inhibition on Lettuce Leaves Coated with LAB
2.7. Determination of E. coli Inhibition on Spinach Leaves Coated with LAB
2.8. The Effect of LAB Strains on the Growth of Spinach and Lettuce Plants
2.9. Statistical Analysis
3. Results
3.1. LAB Antagonism against Plant Pathogenic Fungi
3.2. Identification of LAB Bacterial Isolates Based on 16S rRNA Gene Fragment Sequence Analysis
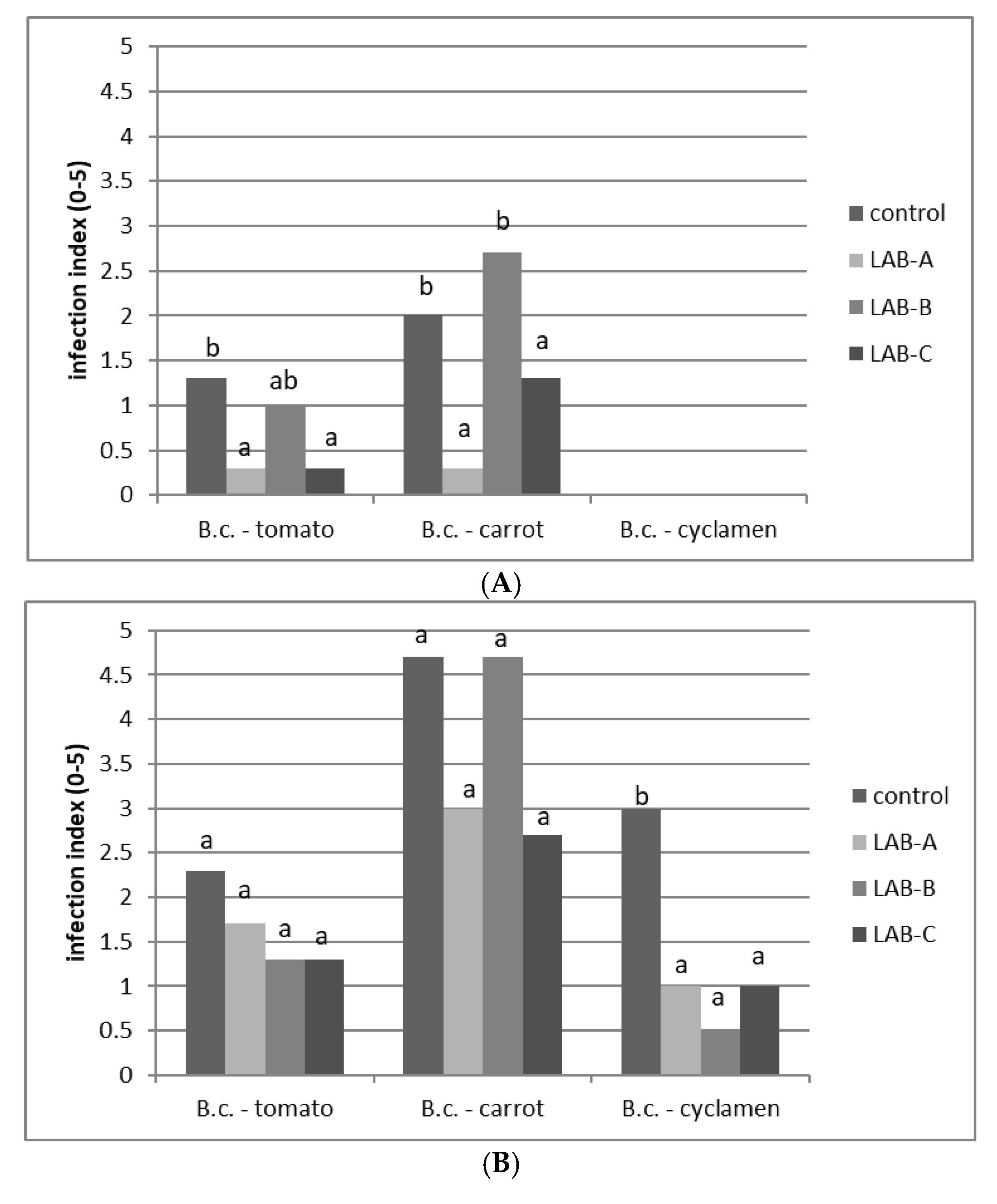
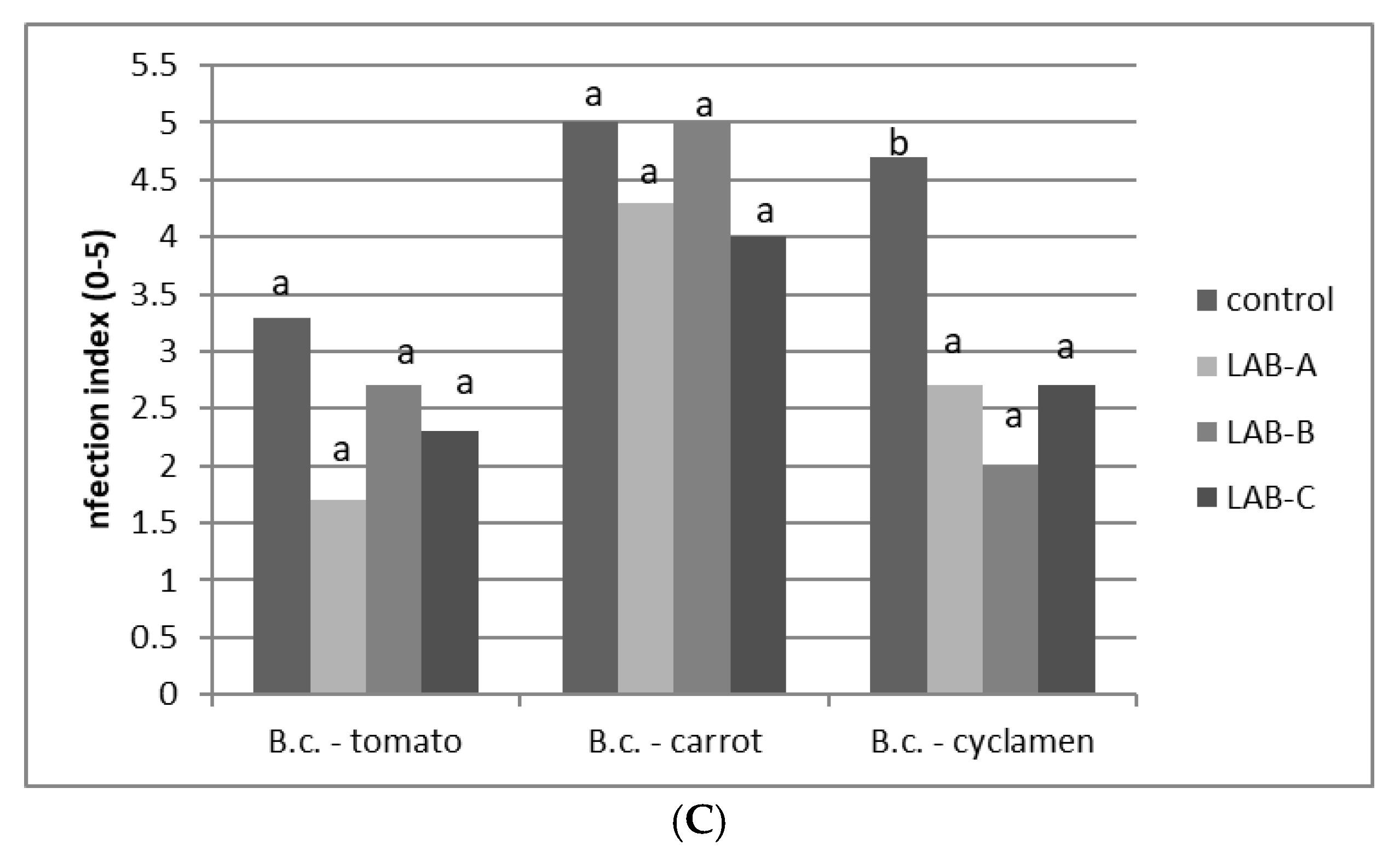
3.3. Inhibition of B. cinerea via LAB Culture Supernatant
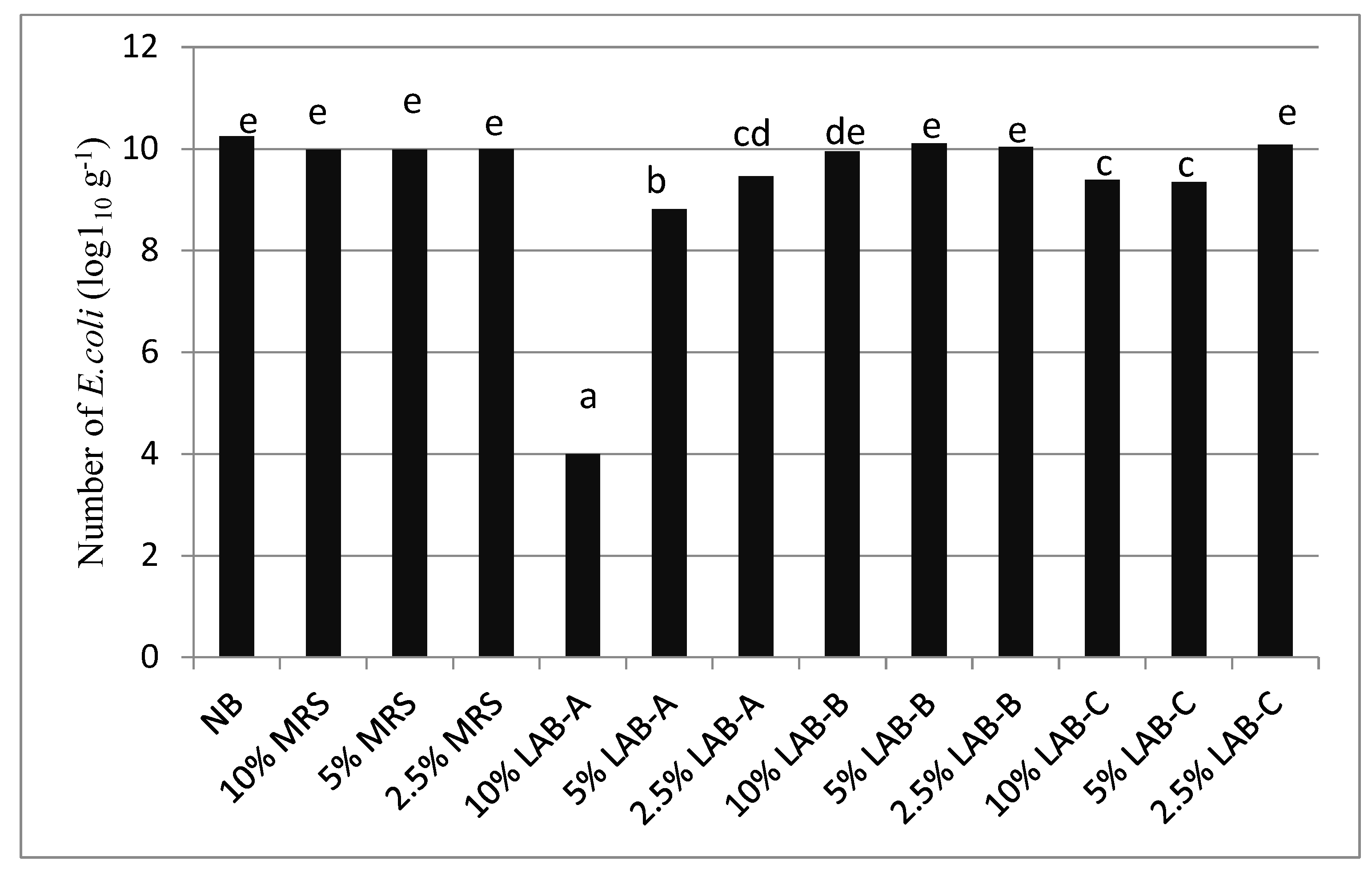
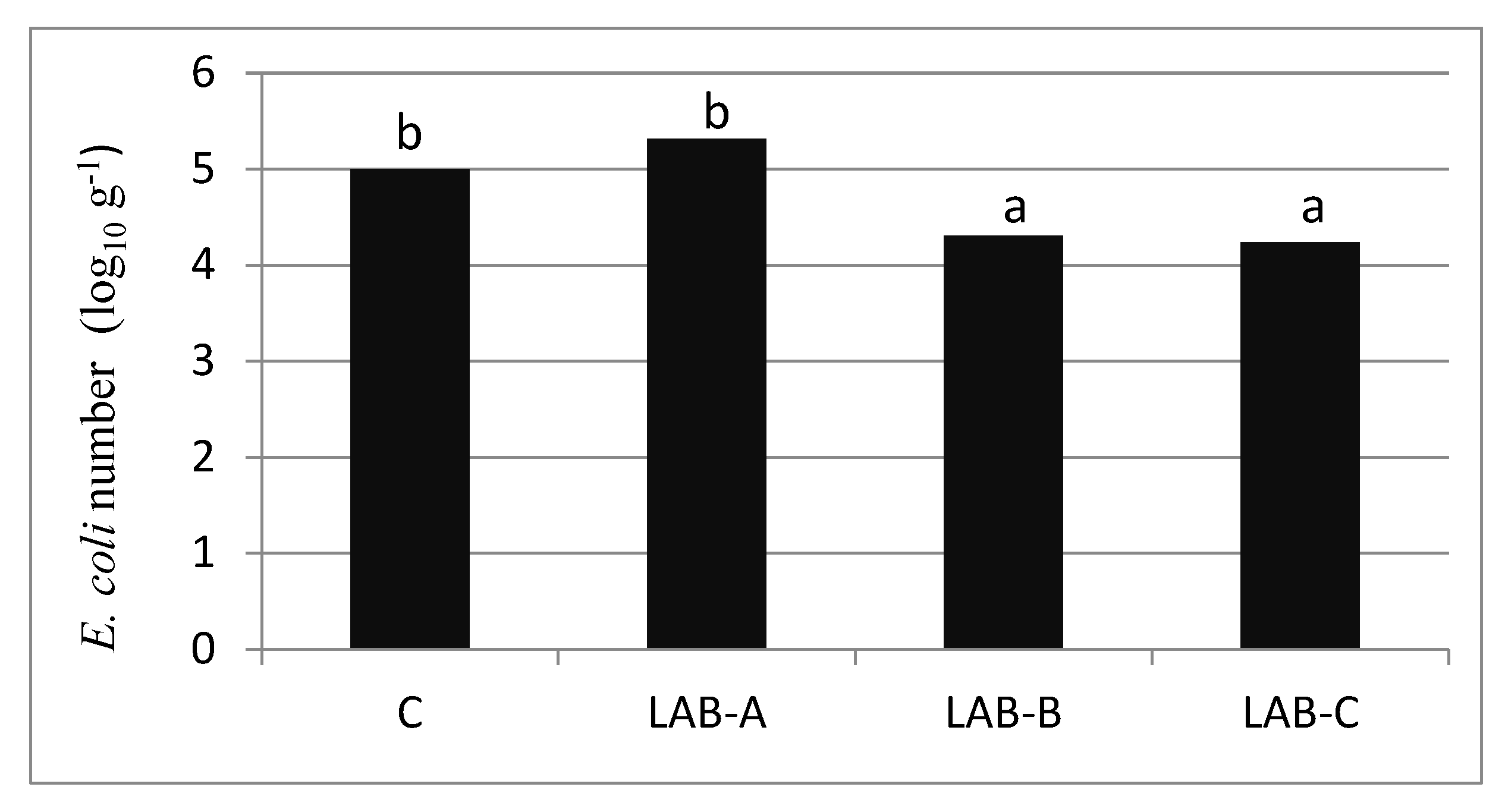
3.4. Inhibition of E. coli Growth via LAB Culture Supernatant
3.5. Survival of LAB Isolates and Fungal Contamination of Coated Spinach and Lettuce Leaves
3.6. B. cinerea Inhibition on Lettuce Leaves Coated with LAB
3.7. E. coli Inhibition on Spinach Leaves Coated with LAB
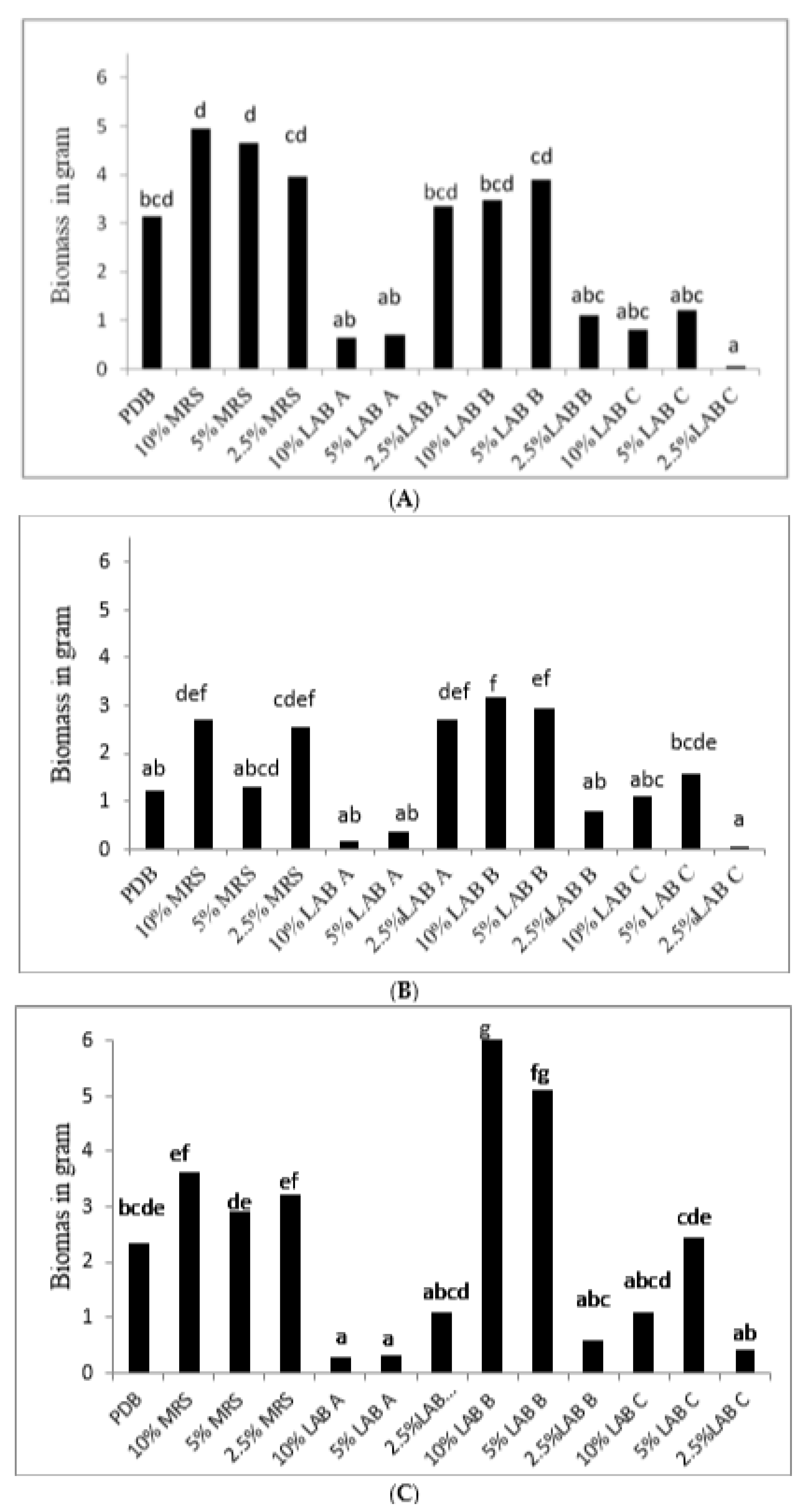
3.8. The Effects of LAB on the Growth of Spinach and Lettuce Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warnings to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–589. [Google Scholar] [CrossRef]
- Julien-Javaux, F.; Gerard, C.; Campagnoli, M.; Zuber, S. Strategies for the safety management of fresh produce from farm to fork. Curr. Opin. Food Sci. 2019, 27, 145–152. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Sobiczewski, P.; Iakimova, E.T. Plant and human pathogenic bacteria exchanging their primary host environments. J. Hortic. Res. 2022, 30, 11–30. [Google Scholar] [CrossRef]
- Arshad, F.; Mehmood, R.; Hussain, S.; Khan, M.A.; Khan, M.S. Lactobacilli as probiotics and their isolation from different sources. Br. J. Res. 2018, 5, 43. [Google Scholar] [CrossRef]
- Bernal-Castro, C.; Espinosa-Poveda, E.; Gutiérrez-Cortés, C.; Díaz-Moreno, C. Vegetable substrates as an alternative for the inclusion of lactic acid bacteria with probiotic potential in food matrices. J. Food Sci. Technol. 2024, 61, 833–846. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apple and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Murray, K.; Wu, F.; Shi, J.; Xue, S.J.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- Marin, V.R.; Ferrarezi, J.H.; Vieira, G.; Sass, D.C. Recent advances in the biocontrol of Xanthomonas spp. World J. Microbiol. Biotechnol. 2019, 35, 72. [Google Scholar] [CrossRef]
- Lopez-Seijas, J.; Garcia-Fraga, B.; Da Silva, A.F.; Sieiro, C. Wine lactic acid bacteria with antibacterial activity as potential biocontrol agents against Fusarium oxysporum f. sp. lycopersici. Agronomy 2020, 10, 31. [Google Scholar] [CrossRef]
- Issaoui, K.I.; Senhaji, N.S.; Zinebi, S.; Haoujar, I.; Amajoud, N.; Abrini, J.; Khay, E.O. Potencial application of bacteriocin produced from lactic acid bacteria. Microbiol. Biotechnol. Lett. 2020, 48, 237–251. [Google Scholar] [CrossRef]
- Daranas, N.; Rosello, G.; Cabrefiga, J.; Donati, I.; Frances, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2018, 174, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Tsuji, G.; Higashiyama, M.; Ogiyama, H.; Umemura, K.; Mitomi, M.; Kubo, Y.; Kosaka, Y. Biological control of bacterial soft rot in Chinese cabbage by Lactobacillus plantarum strain BY under field conditions. Biol. Control 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Asare, P.T.; Greppi, A.; Stettler, M.; Schwab, C.; Stevens, M.J.A.; Lacroix, C. Decontamination of minimally-processed fresh lettuce using reuterin produced by Lactobacillus reuteri. Front. Microbiol. 2018, 9, 1421. [Google Scholar] [CrossRef]
- Budryn, G.; Klewicka, E.; Grzelczyk, J.; Gałązka-Czarnecka, I.; Mostowski, R. Lactic acid fermentation of legume seed sprouts as a method of increasing the content of isoflavones and reducing microbial contamination. Food Chem. 2019, 285, 478–484. [Google Scholar] [CrossRef]
- Yin, H.B.; Chen, C.H.; Gu, G.; Nou, X.; Patel, J. Pre-harvest biocontrol of Listeria and Escherichia coli O157 on lettuce and spinach by lactic acid bacteria. Int. J. Food Microbiol. 2023, 387, 110051. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The destructive fungal pathogen Botrytis cinerea—Insights from genes studies with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.W.; Zhang, Z.X.; Chai, A.L.; Shi, Y.X.; Li, B.J. Grey mould of leaf mustard caused by Botrytis cinerea, a new disease in China. Austral. Plant Dis. Notes 2016, 11, 23. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shipping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Garfinkel, A.R. The history of Botrytis taxonomy, the rise of phylogenetics, and implications for species recognition. Phytopathology 2021, 111, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Martins, A.D.; Silva, C.M.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqua, M.; Doria, J. Endophytic bacteria from strawberry plants control gray mold in fruits via production of antifungal compounds against Botrytis cinerea L. Microbial. Res. 2021, 251, 126793. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation No2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R2073:20130701:EN:PDF (accessed on 14 July 2024).
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef]
- Elias, S.O.; Noronha, T.B.; Tondo, E.C. Salmonella spp. and Escherichia coli O157:H7 prevalence and levels on lettuce: A systemic review and meta-analysis. Food Microbiol. 2019, 84, 103217. [Google Scholar] [CrossRef]
- Azimirad, M.; Nadalian, B.; Alavifard, H.; Panirani, S.N.; Bonab, S.M.V.; Azimirad, F.; Gholami, F.; Jabbari, P.; Yadegar, A.; Busani, L.; et al. Microbiological survey and occurrence of bacterial foodborne pathogens in raw and ready-to-eat green leafy vegetables marked in Tehran, Iran. Int. J. Hyg. Environ. Health 2021, 237, 113824. [Google Scholar] [CrossRef] [PubMed]
- Mootian, G.; Wu, W.H.; Matthews, K.R. Transfer of Escherichia coli O157:H7 from soil, water, and manure contaminated with low numbers of the pathogen to lettuce plants. J. Food Protect. 2009, 72, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Hruby, C.E.; Soupir, M.L.; Moorman, T.B.; Pederson, C.; Kanwar, R. Salmonella and fecal indicator bacteria survival in soils amended with poultry manure. Water Air Soil Pollut. 2018, 229, 32. [Google Scholar] [CrossRef]
- Decol, L.T.; Casarin, L.S.; Hessel, C.T.; Batista, A.C.F.; Allende, A.; Tondo, E.C. Microbial quality of irrigation water used in leafy green production in Southern Brazil and its relationship with produce safety. Food Microbiol. 2017, 65, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how ? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
- Liu, C.; Hofstra, N.; Franz, E. Impacts of climate change on the microbial safety of pre-harvest leafy green vegetables as indicated by Escherichia coli O157 and Salmonella spp. Int. J. Food Microbiol. 2013, 163, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Kowalska, B.; Smolińska, U.; Maciorowski, R.; Oskiera, M.; Michalska, A. Microbial quality of organic and conventional vegetables from Polish farms. Int. J. Food Microbiol. 2018, 286, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, L.; Ximenes, E.; Ku, S.; Ladisch, M. Foodborne pathogens in horticultural production systems: Ecology and mitigation. Sci. Hortic. 2018, 236, 192–206. [Google Scholar] [CrossRef]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Linden, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The hurdle approach—A holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy vegetables. A review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, B.; Szczech, M. Differences in microbiological quality of leafy green vegetables. Ann. Agric. Environ. Med. 2022, 29, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Uhlig, E.; Kjellström, A.; Nurminen, N.; Olsson, C.; Oscarsson, E.; Canaviri-Paz, P.; Mogren, L.; Alsanius, B.; Molin, G.; Håkansson, A. Use of bacterial strains antagonistic to Escherichia coli for biocontrol of spinach: A field trial. Innov. Food Sci. Emerg. Technol. 2021, 74, 102862. [Google Scholar] [CrossRef]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; Romero-Peña, M.; et al. Conventional and non-conventional disinfection methods to present microbial contamination in minimally processed fruits and vegetables. LWT Food Sci. Technol. 2022, 165, 113714. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ristić, D.; Vučurović, I.; Aleksić, G.; Nikolić, B.; Durović, S.; Starović, M. Application of different combinations of lactic acid, phototrophic bacteria and yeast mixtures in control of seed and seedlings pathogens of tomato and pepper. Pestic. Phytomed. 2021, 36, 73–82. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of lactic acid bacteria for the bio-control of Botrytis cinerea and the potential of Lactiplantibacillus plantarum for eco-friendly preservation of fresh-cut kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef]
- Haidar, R.; Fermaud, M.; Calvo-Garrido, C.; Roudet, J.; Deschamps, A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 55, 13–34. [Google Scholar] [CrossRef]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-Lόpez, D.; Vázquez-Ovando, A. Antifungal lactic acid bacteria isolated from fermented beverages with activity against Colletotrichum gleosporioides. Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- Manjarres Melo, J.J.; Alvarez, A.; Ramirez, C.; Bolivar, G. Antagonistic activity of lactic acid bacteria against phytopathogenic fungi isolated from cherry tomato (Solanum lycopersicum var. cerasiforme). Curr. Microbiol. 2021, 78, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal attributes of Lactobacillus plantarum MYS6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS ONE 2016, 11, e155122. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Plotto, A.; Atares, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coating to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Dubourg, G.; Elsawi, Z.; Raoult, D. Assessment of the in vitro antimicrobial activity of Lactobacillus species for identifying new potential antibiotics. Int. J. Antimicrob. Agents 2015, 46, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Złotek, U.; Baraniak, B. Lactobacillus plantarum 299V improves the microbiological quality of legume sprouts and effectively survives in these carriers during cold storage and in vitro digestion. PLoS ONE 2018, 13, e0207793. [Google Scholar] [CrossRef]
- Alegre, I.; Vinas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Rößle, C.; Auty, M.A.E.; Brunton, N.; Gormley, R.T.; Butler, F. Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innov. Food Sci. Emerg. Technol. 2010, 11, 203–209. [Google Scholar] [CrossRef]
- Strafella, S.; Simpson, D.J.; Khanghahi, M.Y.; De Angelis, M.; Gänzle, M.; Minervini, F.; Crecchio, C. Comparative genomics and in vitro plant growth promotion and biocontrol traits of lactic acid bacteria from the wheat rhizosphere. Microorganisms 2021, 9, 78. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Stringheta, P.C.; Lago-Vanzela, E.S.; Oliveira, P.M.; Martins, M.L. Fresh cut fruit salads as a promising vehicle for Lactobacillus acidophilus and Lactobacillus plantarum. Int. Food Res. J. 2017, 24, 2185–2192. [Google Scholar]
- Konappa, N.M.; Maria, M.; Uzma, F.; Krishnamurthy, S.; Nayaka, S.C.; Niranjana, S.R.; Chowdappa, S. Lactic acid bacteria mediated induction of defense enzymes to enhance the resistance in tomato against Ralstonia solanacearum causing bacterial wilt. Sci. Hortic. 2016, 207, 183–192. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent development in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Tech. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of lactic acid bacteria (LAB) in sustainable agriculture: Advantages and limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
| Tested Fungi | PIRG (%) | ||
|---|---|---|---|
| LAB-A | LAB-B | LAB-C | |
| Alternaria sp. | 7.0 b | 0.0 a | 10.5 c |
| B. cinerea from carrots | 12.1 c | 4.4 b | 16.5 c |
| B. cinerea from tomatoes | 15.8 c | 4.7 b | 11.5 c |
| B. cinerea from cyclamens | 5.7 b | 0.7 a | 14.1 c |
| F. oxysporum | 0.0 a | 3.0 b | 0.0 a |
| Penicillium sp. | 0.0 a | 28.0 c | 0.0 a |
| Phialophora sp. | 54.6 d | 18.9 c | 54.6 d |
| R. solani | 33.3 d | 0.0 a | 15.0 c |
| S. sclerotiorum | 7.0 b | 0.0 a | 3.0 b |
| Isolate | Sequence Length (bp) | Genus/Species with the Highest Similarity | Sequence Number NCBI | Identity (%) | Identification |
|---|---|---|---|---|---|
| LAB-A | 405 | Lactiplantibacillus pingfangensis | No_179289.1 | 99.26 | Lactiplantibacillus sp. |
| Lactiplantibacillus plantarum | No_113338.1 | 99.26 | |||
| Lactiplantibacillus pentosus | No_029133.1 | 99.26 | |||
| LAB-B | 501 | Levilactobacillus brevis | No_044704.2 | 94.64 | Levilactobacillus sp. |
| Levilactobacillus angrenensis | No_180286.1 | 93.01 | |||
| Levilactobacillus yonginensis | No_109452.1 | 93.01 | |||
| LAB-C | 467 | Levilactobacillus brevis | No_116238.1 | 98.93 | Levilactobacillus sp. |
| Levilactobacillus fujinensis | No_180290.1 | 97.64 | |||
| Levilactobacillus tangyuanensis | No_180287.1 | 97.64 |
| Treatment | Lactic Acid Bacteria | Moulds | ||
|---|---|---|---|---|
| The Density of Microorganisms (log10 g−1) | ||||
| Spinach | Lettuce | Spinach | Lettuce | |
| Control (untreated by LAB) | 3.12 a | 2.28 a | 4.00 b | 4.76 b |
| LAB-A | 5.23 b | 5.57 b | 3.40 a | 3.99 a |
| LAB-B | 5.77 b | 6.72 b | 3.67 a | 3.48 a |
| LAB-C | 5.94 b | 6.40 b | 4.04 b | 4.76 b |
| Treatment | Weight of One Plant (g) | Number of LAB (log10 cfu g−1) | ||
|---|---|---|---|---|
| Spinach | Lettuce | Spinach | Lettuce | |
| control | 8.2 a | 6.4 a | 0.00 a | 0.00 a |
| LAB-A | 11.1 c | 7.3 a | 2.85 b | 4.81 c |
| LAB-B | 9.9 b | 7.4 a | 3.21 c | 2.60 b |
| LAB-C | 10.4 bc | 6.6 a | 3.84 c | 4.92 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, B.; Szczech, M.; Lisek, A. Inhibition of Botrytis cinerea and Escherichia coli by Lactic Acid Bacteria on Leafy Vegetables. Agriculture 2024, 14, 1228. https://doi.org/10.3390/agriculture14081228
Kowalska B, Szczech M, Lisek A. Inhibition of Botrytis cinerea and Escherichia coli by Lactic Acid Bacteria on Leafy Vegetables. Agriculture. 2024; 14(8):1228. https://doi.org/10.3390/agriculture14081228
Chicago/Turabian StyleKowalska, Beata, Magdalena Szczech, and Anna Lisek. 2024. "Inhibition of Botrytis cinerea and Escherichia coli by Lactic Acid Bacteria on Leafy Vegetables" Agriculture 14, no. 8: 1228. https://doi.org/10.3390/agriculture14081228





