Response of Yam Yield and Soil Microbial Communities to Soil Fumigation and Substrate Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Experimental Design
2.2. Soil Samples
2.3. Soil pH and Nutrient Contents
2.4. Soil Pathogens
2.5. Yam Growth and Yield
2.6. Soil Bacterial Community Analysis
2.7. Data Analysis
3. Results
3.1. Changes in Soil pH and Nutrients
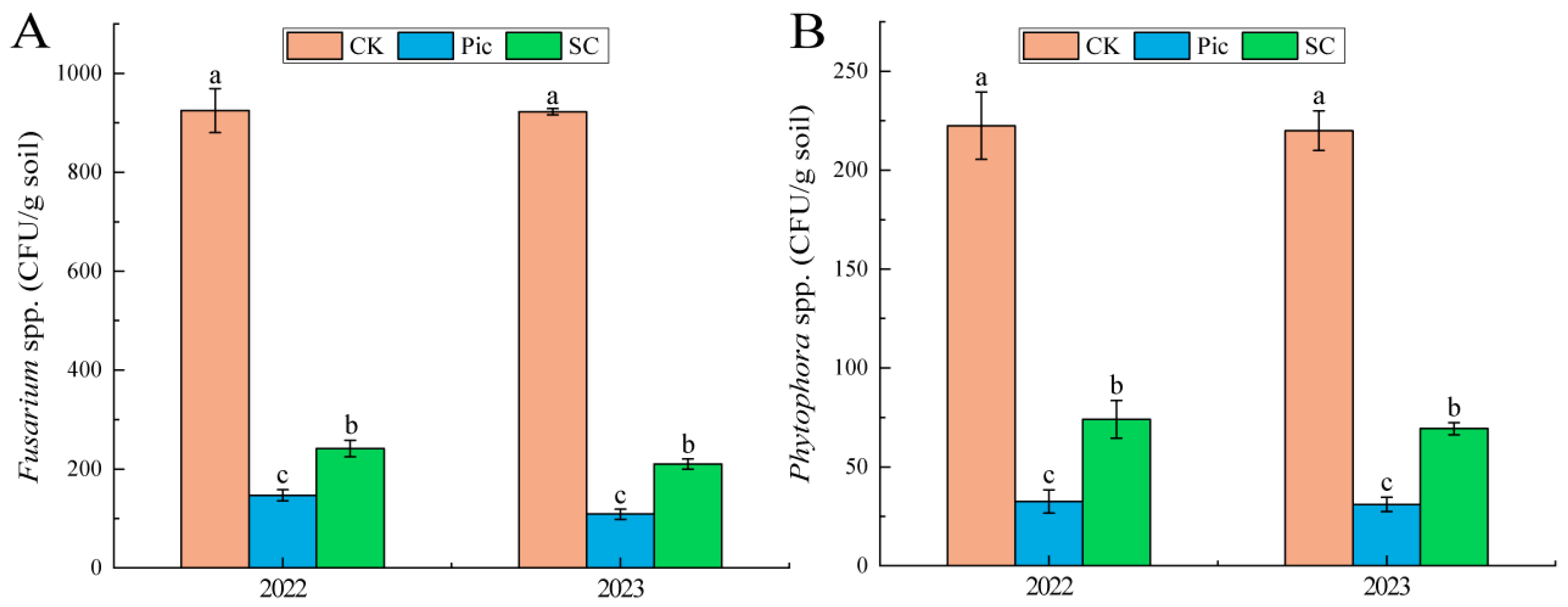
3.2. Changes in Soil Pathogens
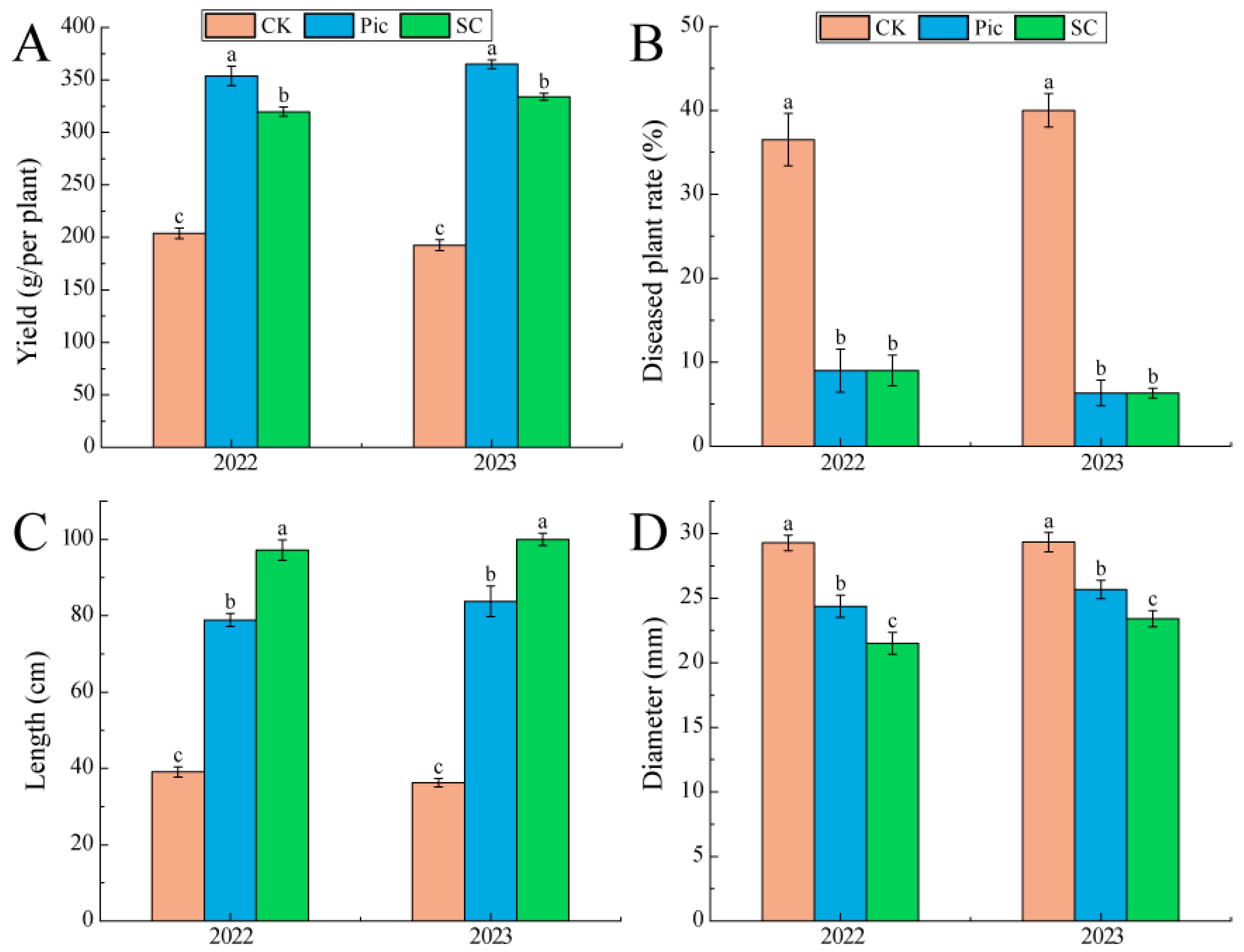
3.3. Changes in Growth Indicators and Yield of Yam
3.4. Changes in Soil Bacterial Community
3.4.1. Alpha Diversity
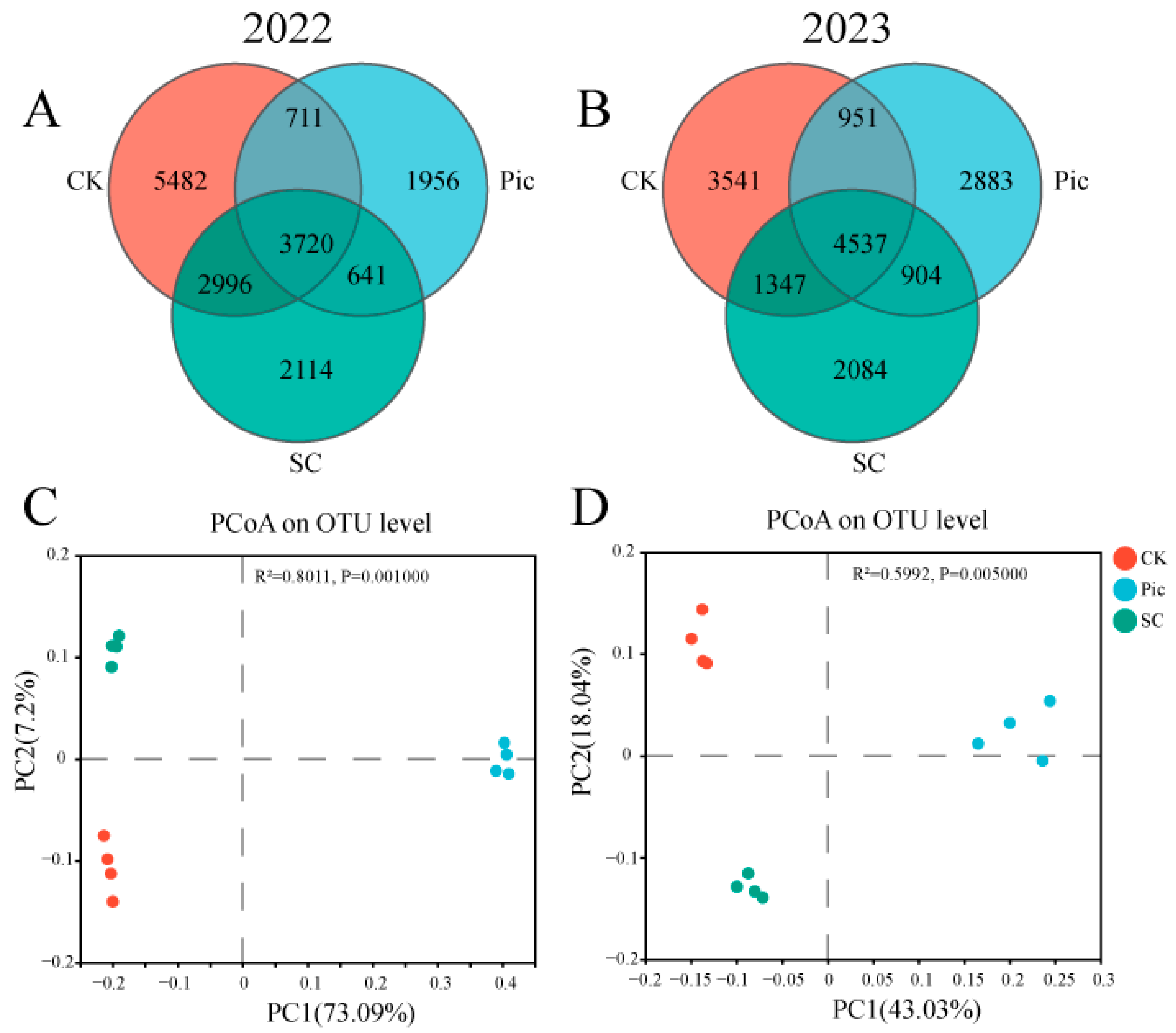
3.4.2. Venn Diagram and Beta Diversity
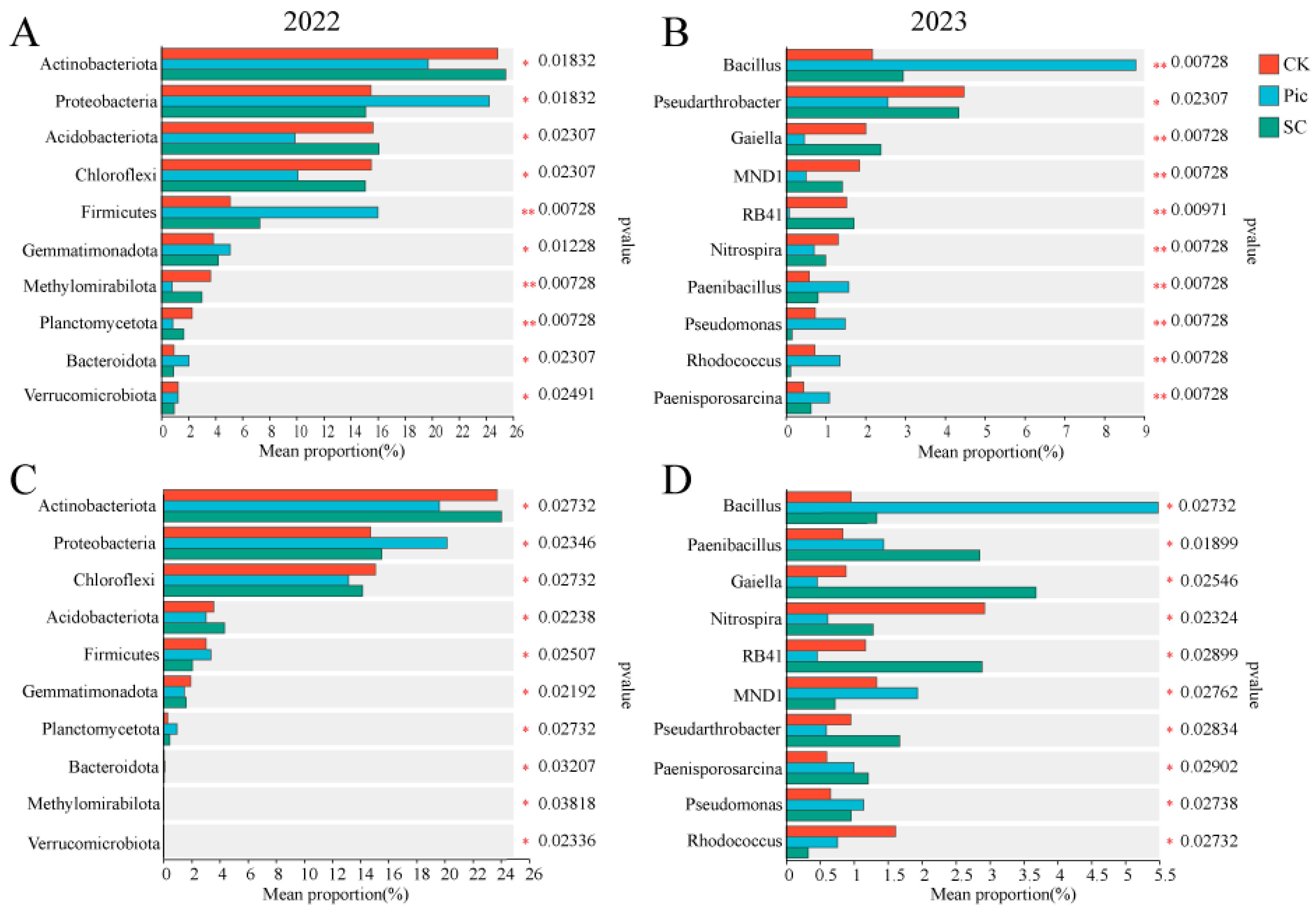
3.4.3. Species Composition
3.4.4. Biomarkers Analysis
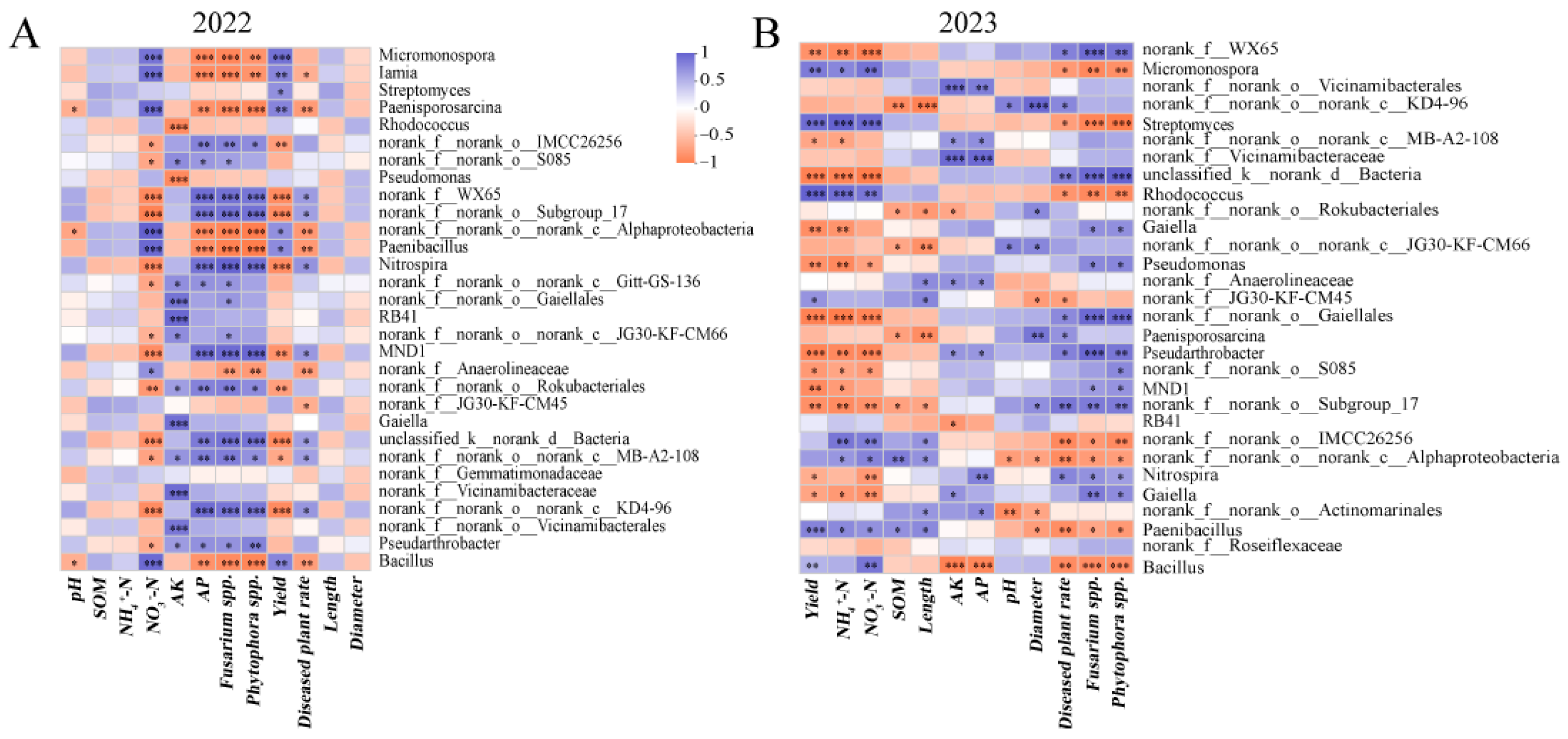
3.4.5. Correlation between Soil Microorganisms, Environmental Factors and Yam Growth
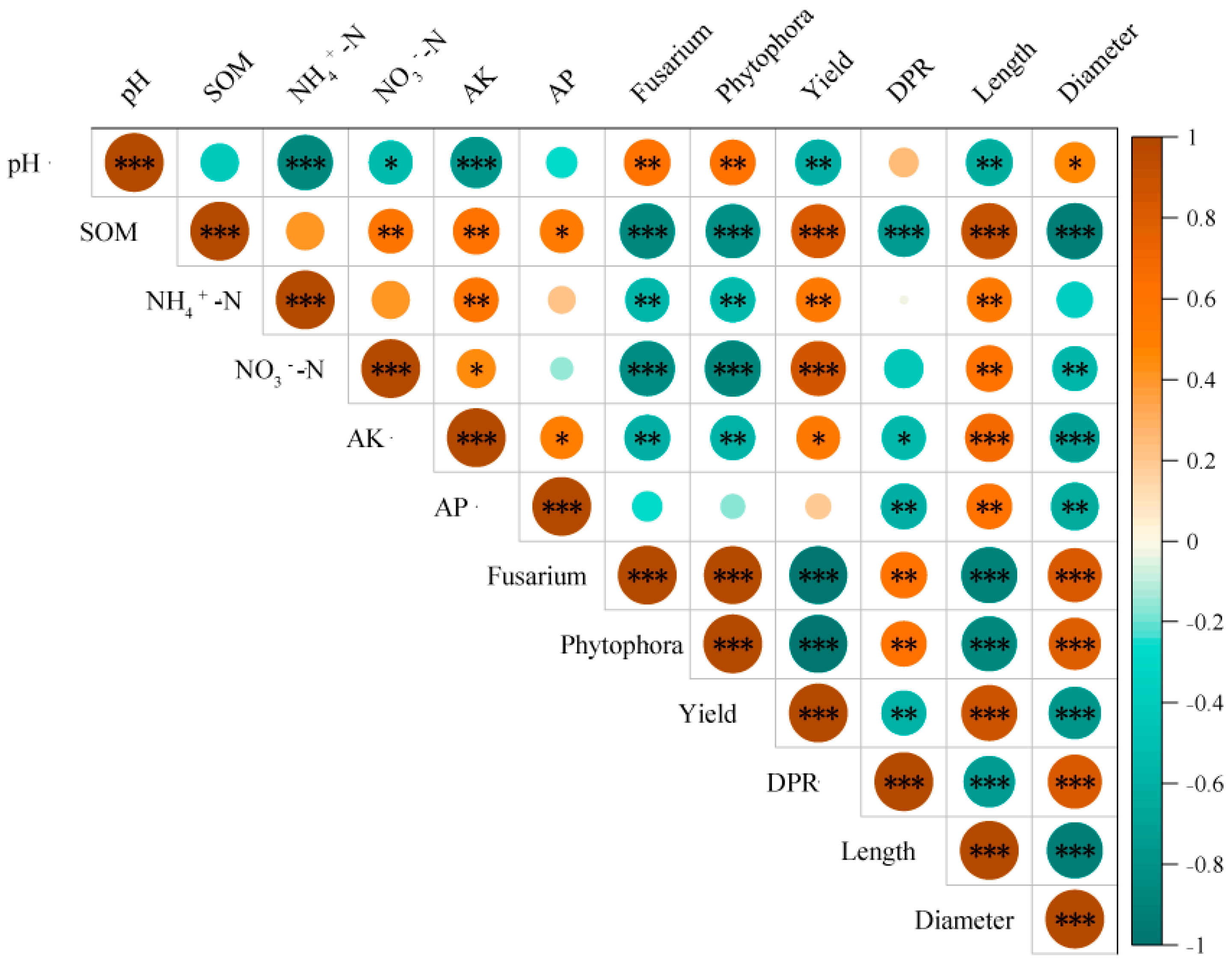
3.5. Correlation Analysis of Yam Plant Growth and Soil Environmental Factors
4. Discussion
4.1. Effects of Soil Fumigation and Substrate Cultivation on Soil pH and Nutrients
4.2. Effects of Soil Fumigation and Substrate Cultivation on Soil Pathogens
4.3. Effects of Soil Fumigation and Substrate Cultivation on Yam Growth
4.4. Effects of Soil Fumigation and Substrate Cultivation on Soil Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, S.; Zhang, W.; Chang, G.; Guo, L.; Li, X.; Gao, W. Prospects of yam (Dioscorea) polysaccharides: Structural features, bioactivities and applications. Food Chem. 2024, 446, 138897. [Google Scholar] [CrossRef]
- Abdulsalam, S.; Peng, H.; Yao, Y.; Fan, L.; Jiang, R.; Shao, H.; Zhang, Y.; Huang, W.; Kong, L.; Peng, D. Prevalence and Molecular Diversity of Plant-Parasitic Nematodes of Yam (Dioscorea spp.) in China, with Focus on Merlinius spp. Biology 2021, 12, 1299. [Google Scholar] [CrossRef]
- Lu, X.; Meng, J.; Xiao, D.; He, L.; Chen, B.; Zou, C. Polyclonal antibody preparation and establishment of antibody-based method for the detection of coat proteins of Japanese yam mosaic virus. Acta Phytophy Sin. 2024, 1–14. [Google Scholar] [CrossRef]
- Ufondu, H.E.; Maziya-Dixon, B.; Okoyeuzu, C.F.; Okonkwo, T.M.; Okpala, C.O.R. Effects of yam varieties on flour physicochemical characteristics and resultant instant fufu pasting and sensory attributes. Sci. Rep. 2022, 12, 20276. [Google Scholar] [CrossRef]
- Alharazi, W.Z.; McGowen, A.; Rose, P.; Jethwa, P.H. Could consumption of yam (Dioscorea) or its extract be beneficial in controlling glycaemia: A systematic review. Br. J. Nutr. 2022, 128, 613–624. [Google Scholar] [CrossRef]
- Li, X.L.; Shen, Y.; Hu, F.; Zhang, X.X.; Thakur, K.; Rengasamy, K.R.R.; Khan, M.R.; Busquets, R.; Wei, Z.J. Fortification of polysaccharide-based packaging films and coatings with essential oils: A review of their preparation and use in meat preservation. Int. J. Biol. Macromol. 2023, 242, 124767. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea genus (yam)-an appraisal of nutritional and therapeutic potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Yao, J.; Wu, C.; Fan, L.; Kang, M.; Liu, Z.; Huang, Y.; Xu, X.; Yao, Y. Effects of the Long-Term Continuous Cropping of Yongfeng Yam on the Bacterial Community and Function in the Rhizospheric Soil. Microorganisms 2023, 11, 274. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yao, X.; Zhang, P.; Liu, W.; Huang, J.; Sun, H.; Wang, N.; Zhang, X.; Wang, H.; Zhang, H.; et al. Effects of continuous cropping on fungal community diversity and soil metabolites in soybean roots. Microbiol. Spectr. 2023, 11, e0178623. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Ge, A.H.; Liang, Z.H.; Xiang, J.F.; Xiao, J.L.; Zhang, Y.; Liu, Y.R.; Zhang, L.M.; Shen, J.P. Fumigation practice combined with organic fertilizer increase antibiotic resistance in watermelon rhizosphere soil. Sci. Total Environ. 2022, 805, 150426. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Harkes, P.; van Steenbrugge, J.J.M.; Xu, M.; Geissen, V. Soil microeukaryotic communities and phosphorus-cycling microorganisms respond to chloropicrin fumigation and azoxystrobin application. Sci. Total Environ. 2024, 933, 172871. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.J.; Zhang, D.Q.; Ren, L.R.; Yan, D.D.; Li, Y.; Wang, Q.X.; Cao, A.C. Effects of chloropicrin and dazomet on strawberry soil nutrients, pathogenic bacteria and microbial communities. J. Agric Univ. Hebei 2021, 44, 24–29. [Google Scholar]
- Li, H. Control Effect of Soil Disinfection on the Yam "Paste" Disease and Effects on Soil Environment. Master’s Thesis, Hebei Agricultural University, Hebei, China, 2019. [Google Scholar]
- Pesonen, M.; Vähäkangas, K. Chloropicrin-induced toxicity in the respiratory system. Toxicol. Lett. 2020, 323, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wang, X.; Yang, S.; Chen, H.; Zhao, Y. Addition Pinus massoniana fallen wood improved the growth of Plagiomnium acutum in a substrate cultivation. Sci. Rep. 2022, 12, 17755. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, L.; Zhou, X.; Tian, T.; Xu, S.; Li, D.; Li, C.; Li, Y. Optimizing Straw-Rotting Cultivation for Sustainable Edible Mushroom Production: Composting Spent Mushroom Substrate with Straw Additions. J. Fungi 2023, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques-Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhou, Y.; Wu, Y.; Duan, F.; Sun, F.; Kang, X. Research progress of substrates for soilless cultivation of cucumber. Vegetables 2023, 4, 34–39. [Google Scholar]
- Węgrzyn, A.; Tsurtsumia, A.; Witkowski, S.; Freitas, O.; Figueiredo, S.; Cybińska, J.; Stawiński, W. Vermiculite as a potential functional additive for water treatment bioreactors inhibiting toxic action of heavy metal cations upsetting the microbial balance. J. Hazard Mater. 2022, 433, 128812. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Cheng, H.; Jiang, C.; Zheng, L. Remediation of soil mercury by modified vermiculite-montmorillonite and its effect on the growth of Brassica chinensis L. Molecules 2022, 27, 5340. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Hikono, A.; Saito, T. Effects of zeolite and vermiculite addition on exchangeable radiocaesium in soil with accelerated ageing. J. Environ. Radioact. 2019, 203, 18–24. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere. 2011, 82, 169–178. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Zhang, Y.; Ma, W.; Sun, Z. Nutrient accumulation characteristics and quality differences of mineral nutrients of main yam cultivars in Hebei Province. Plant Nutr. Fert. Sci. 2022, 28, 1734–1744. [Google Scholar]
- Chen, S.; Sun, Y.; Wei, Y.; Li, H.; Yang, S. Different rhizosphere soil microbes are recruited by tomatoes with different fruit color phenotypes. BMC Microbiol. 2022, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.P.R.; Oliveira, A.P.; Cruz, J.M.F.L.; Silva, L.D.R.; Sousa, V.F.O.; Farias, O.R.; Nunes, J.C.; Nascimento, I.R.S.; Martins, J.V.S. Nitrogen and potassium synergism influences the yield and quality of Dioscorea cayennensis. Braz. J. Biol. 2022, 82, e263916. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Wen, D.; Gao, P.; Yan, D.; Yang, N. Biodegradable PBAT microplastics adversely affect pakchoi (Brassica chinensis L.) growth and the rhizosphere ecology: Focusing on rhizosphere microbial community composition, element metabolic potential, and root exudates. Sci. Total Environ. 2024, 912, 169048. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Li, W.; Zhang, D.; Fang, W.; Li, Y.; Wang, Q.; Cao, A.; Yan, D. Long-term effects of chloropicrin fumigation on soil microbe recovery and growth promotion of Panax notoginseng. Front. Microbiol. 2023, 14, 1225944. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.H.; Liu, D.Q.; Wang, L.M.; Yang, Y.; Cui, X.M. Effects of soil fumigant chloropicrin on the growth development and soil physico-chemical properties of continuous cropping Panax notoginseng. Mod. Chin. Med. 2018, 20, 842–849. [Google Scholar]
- Yang, Y.Q.; Fan, L.J.; Liu, Q.Z.; Li, W.H.; Song, Z.X. Effects of dazomet and chloropicrin on the soil nematode communities and nutrient content of replanted strawberry. Acta Hortic. Sin. 2018, 45, 725–733. [Google Scholar]
- Pu, R.; Wang, P.; Guo, L.; Li, M.; Cui, X.; Wang, C.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotoxicol. Environ. Saf. 2022, 231, 113188. [Google Scholar] [CrossRef] [PubMed]
- Cechin, I.; da Silva, L.P.; Ferreira, E.T.; Barrochelo, S.C.; de Melo, F.P.S.R.; Dokkedal, A.L.; Saldanha, L.L. Physiological responses of Amaranthus cruentus L. to drought stress under sufficient- and deficient-nitrogen conditions. PLoS ONE 2022, 17, e0270849. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Song, Z.; Ren, L.; Hao, B.; Zhu, J.; Fang, W.; Yan, D.; Li, Y.; et al. Chloropicrin alternated with dazomet improved the soil's physicochemical properties, changed microbial communities and increased strawberry yield. Ecotoxicol. Environ. Saf. 2021, 220, 112362. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yan, N.; Miao, X.; Li, Q.; Chen, C. Different Responses of Soil Environmental Factors, Soil Bacterial Community, and Root Performance to Reductive Soil Disinfestation and Soil Fumigant Chloropicrin. Front. Microbiol. 2021, 12, 796191. [Google Scholar] [CrossRef] [PubMed]
- He, S.Q.; Chen, Z.K.; Meng, X.H.; Liu, W.T.; Ren, C.B.; Wu, J.; Zhu, C.S. Preparation and characterization of PA6/antibacterial vermiculite composites. Acta Polym. Sin. 2011, 3, 287–293. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, C.Y.; Geng, A.H.; Wei, R.Q.; Wang, Z.C.; Yang, H.; Sun, X.X.; Chen, J.P.; Wang, Y.H.; Wang, W.; et al. Effects of different blends of vinegar residue substrates on seedling and growth of facility vegetables and soil-borne diseases. Shanghai Veg. 2017, 2, 62–66. [Google Scholar]
- Subbarao, K.V.; Hubbard, J.C.; Koike, S.T. Evaluation of broccoli residue incorporation into field soil for verticillium wilt control in cauliflower. Plant Dis. 1999, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Land, C.J.; Vallad, G.E.; Boyd, N.S. Tomato tolerance and pest control following fumigation with different ratios of dimethyl disulfide and chloropicrin. Pest. Manag. Sci. 2019, 75, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C. The Formula Screening of Pepper Seedlings and Cultivation Substratesin Solar Greenhouse with Vermiculite as the Main Ingredient. Master’s Thesis, Tarim University, Xinjiang, China, 2022. [Google Scholar]
- Sun, B.; Li, J.Q.; Wang, S.; Xu, Y.G. Effect of different substrate cultivation on growth, yield and quality of blueberries. Agric. Eng. Tech. 2024, 44, 39–41. [Google Scholar]
- De, A.; Mridha, D.; Roychowdhury, T.; Bandyopadhyay, B.; Panja, A.S. Substrate level optimization for better yield of oyster mushroom (Pleurotus ostreatus) production, using different ratio of rice straw and sugarcane bagasse. World J. Microbiol. Biotechnol. 2023, 39, 270. [Google Scholar] [CrossRef]
- Ondrasek, G.; Bakić Begić, H.; Zovko, M.; Filipović, L.; Meriño-Gergichevich, C.; Savić, R.; Rengel, Z. Biogeochemistry of soil organic matter in agroecosystems & environmental implications. Sci. Total Environ. 2019, 658, 1559–1573. [Google Scholar]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research Progress of Soil Microorganisms in Response to Heavy Metals in Rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef]
- Adomako, M.O.; Roiloa, S.; Yu, F.H. Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Boyd, N.S.; Strauss, S.L. Impact of fumigants on non-target soil microorganisms: A review. J. Hazard. Mater. 2022, 427, 128149. [Google Scholar] [CrossRef]
- Cantabella, D.; Teixidó, N.; Segarra, G.; Torres, R.; Casanovas, M.; Dolcet-Sanjuan, R. Rhizosphere microorganisms enhance in vitro root and plantlet development of Pyrus and Prunus rootstocks. Planta 2021, 253, 78. [Google Scholar] [CrossRef]
- Gu, H.; Yan, J.; Liu, Y.; Yu, X.; Feng, Y.; Yang, X.; Lam, S.S.; Naushad, M.; Li, C.; Sonne, C. Autochthonous bioaugmentation accelerates phenanthrene degradation in acclimated soil. Environ. Res. 2023, 224, 115543. [Google Scholar] [CrossRef]
- Xu, M.P.; Wang, J.Y.; Zhu, Y.F.; Han, X.H.; Ren, C.J.; Yang, G.H. Plant Biomass and Soil Nutrients Mainly Explain the Variation of Soil Microbial Communities During Secondary Succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Xia, P.; Xun, L.; Liang, Z. Impact of continuous Panax notoginseng plantation on soil microbial and biochemical properties. Sci. Rep. 2019, 9, 13205. [Google Scholar] [CrossRef]
- Li, F.; Zhou, F.; Zhang, G.; Zhou, H.; Wu, X.; Wu, J.; Zhang, X. Impacts of growth-promoting bacteria on root bacteria community of tomato in substrate culture. Microbiol. China 2022, 49, 583–597. [Google Scholar]
- Lyng, M.; Kovács, Á.T. Frenemies of the soil: Bacillus and Pseudomonas interspecies interactions. Trends Microbiol. 2023, 31, 845–857. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Y.; Yao, X.; Wang, J.; Zheng, P.; Xi, C.; Hu, B. Dominance of comammox Nitrospira in soil nitrification. Sci. Total Environ. 2021, 780, 146558. [Google Scholar] [CrossRef]
- Hsu, P.L.; Di, H.J.; Cameron, K.; Podolyan, A.; Chau, H.; Luo, J.; Miller, B.; Carrick, S.; Johnstone, P.; Ferguson, S.; et al. Comammox Nitrospira Clade B is the most abundant complete ammonia oxidizer in a dairy pasture soil and inhibited by dicyandiamide and high ammonium concentrations. Front. Microbiol. 2022, 13, 1048735. [Google Scholar] [CrossRef] [PubMed]


| Year | Treatment | pH | SOM (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | AK (mg/kg) | AP (mg/kg) |
|---|---|---|---|---|---|---|---|
| 2022 | CK | 8.73 a | 10.10 c | 2.91 b | 12.46 c | 157.37 a | 26.13 b |
| Pic | 8.42 b | 12.38 b | 3.10 a | 16.96 a | 111.28 c | 21.25 c | |
| SC | 8.40 b | 13.50 a | 3.25 a | 14.53 b | 151.02 b | 50.50 a | |
| 2023 | CK | 8.34 a | 9.67 b | 3.24 c | 13.04 c | 125.70 b | 25.17 b |
| Pic | 8.17 b | 12.13 a | 3.46 b | 16.47 a | 108.16 c | 21.67 c | |
| SC | 8.12 b | 12.27 a | 3.63 a | 14.89 b | 136.28 a | 49.40 a |
| Year | Treatment | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| 2022 | CK | 7.35 a | 0.0030 b | 8924.4 a | 10012.4 a |
| Pic | 6.45 c | 0.0064 a | 5121.8 c | 5336.0 c | |
| SC | 7.19 b | 0.0035 b | 7238.3 b | 7423.5 b | |
| 2023 | CK | 7.38 a | 0.0017 b | 7964.0 a | 7971.1 a |
| Pic | 7.04 c | 0.0026 a | 5380.6 c | 5473.2 c | |
| SC | 7.28 b | 0.0025 a | 7346.7 b | 7408.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Hao, Z.; Song, Y.; Gao, L.; He, F.; Li, Q.; Cao, A. Response of Yam Yield and Soil Microbial Communities to Soil Fumigation and Substrate Cultivation. Agriculture 2024, 14, 1231. https://doi.org/10.3390/agriculture14081231
Jin X, Hao Z, Song Y, Gao L, He F, Li Q, Cao A. Response of Yam Yield and Soil Microbial Communities to Soil Fumigation and Substrate Cultivation. Agriculture. 2024; 14(8):1231. https://doi.org/10.3390/agriculture14081231
Chicago/Turabian StyleJin, Xi, Zheng Hao, Yelong Song, Lan Gao, Fuqiang He, Qingjie Li, and Aocheng Cao. 2024. "Response of Yam Yield and Soil Microbial Communities to Soil Fumigation and Substrate Cultivation" Agriculture 14, no. 8: 1231. https://doi.org/10.3390/agriculture14081231





