Wolbachia Transinfection and Effect on the Biological Traits of Matsumuratettix hiroglyphicus (Matsumura), the Leafhopper Vector of Sugarcane White Leaf Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Mass Rearing of the Leafhoppers
2.2. Preparation of Wolbachia Source
2.3. Microinjection for Wolbachia Transinfection
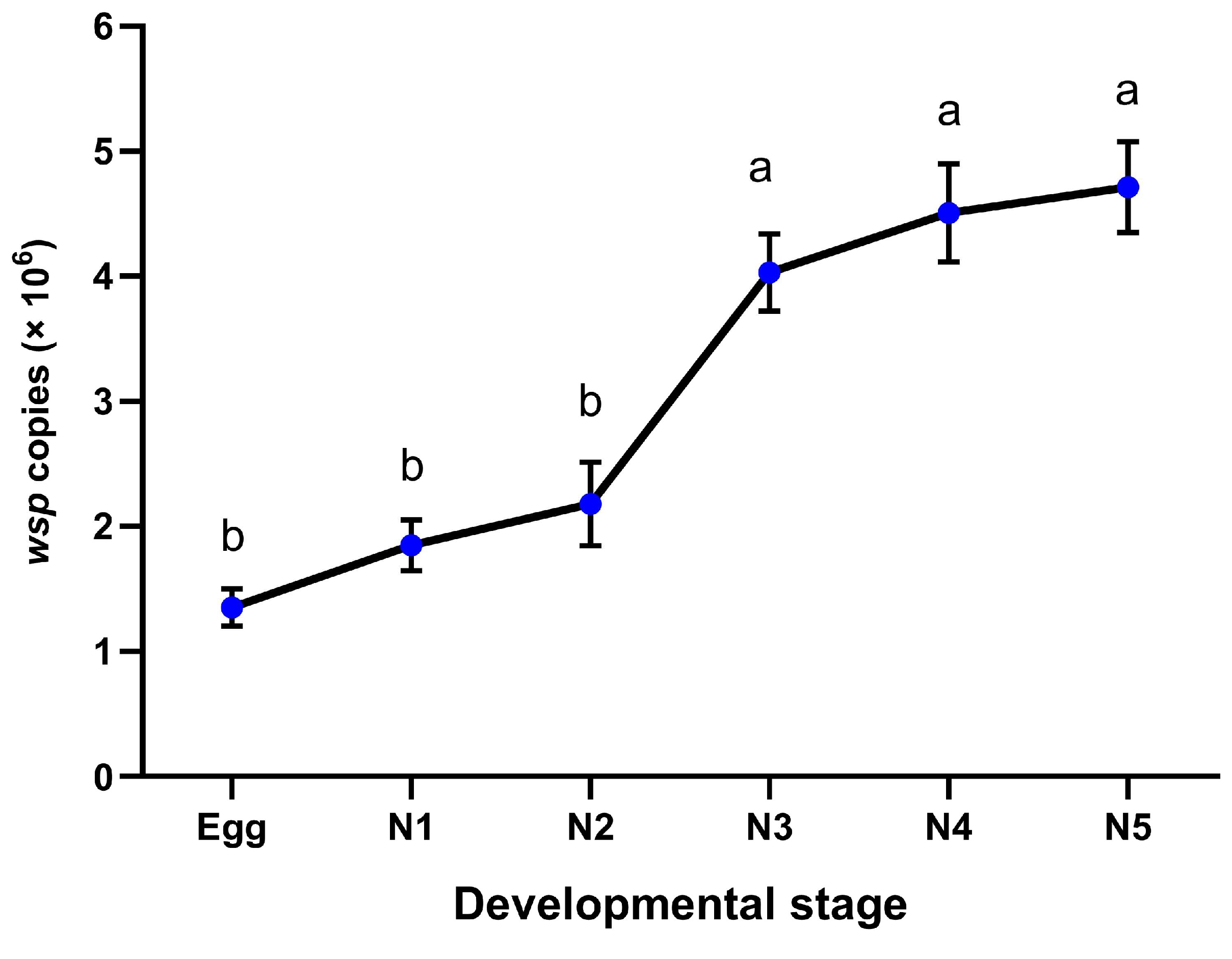
2.4. Wolbachia Infection Dynamics
2.5. DNA Extraction and PCR Amplification
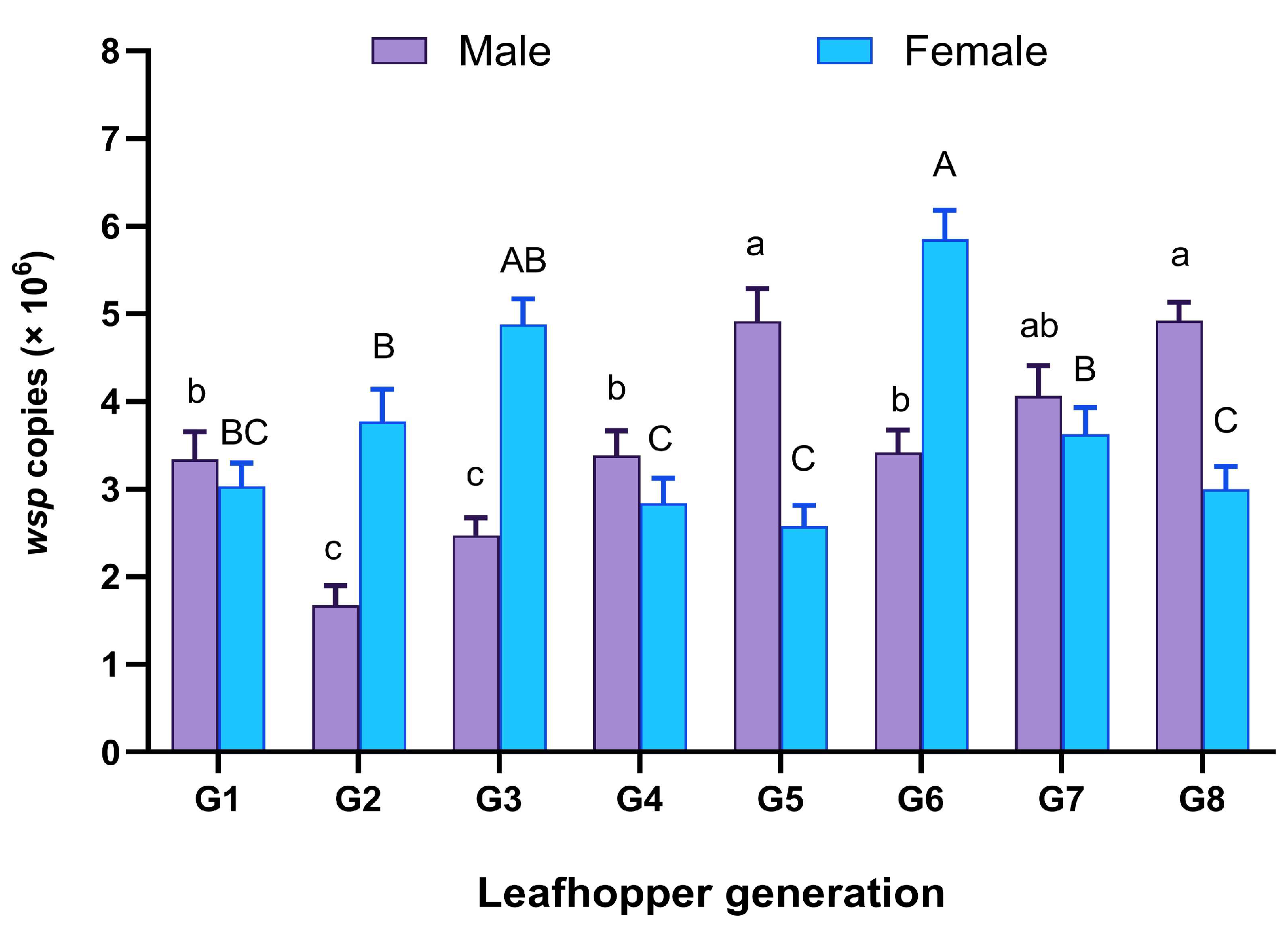
2.6. Wolbachia Quantification
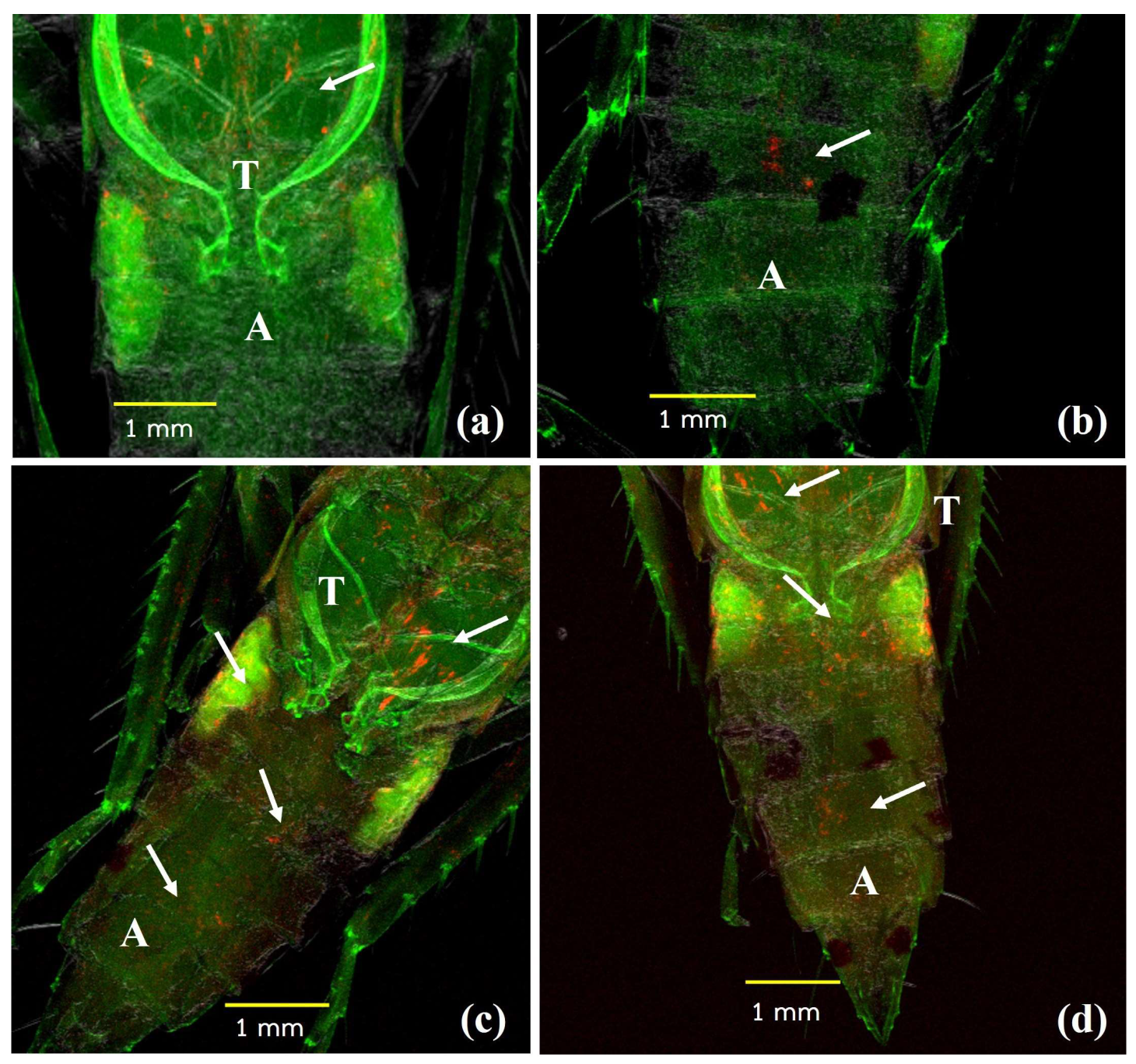
2.7. Wolbachia Distribution in Dissected Organs
2.8. Fluorescence In Situ Hybridization (FISH)
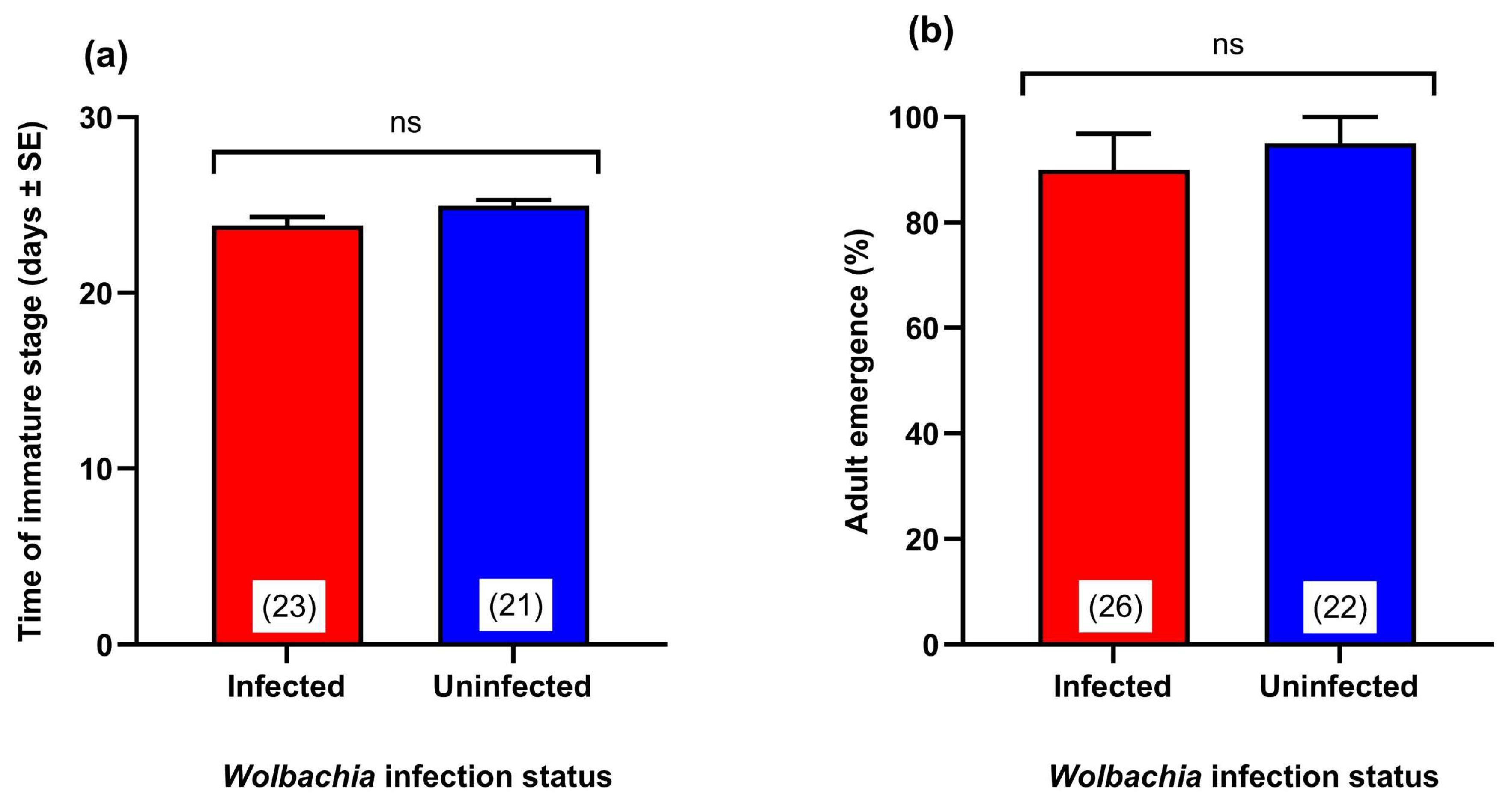
2.9. Determining the Effect of Wolbachia Transinfection on Development Time and Adult Emergence
2.10. Effect of Wolbachia Transinfection on Adult Survival and Longevity
2.11. Crossing Experiment
2.12. Statistical Analysis
3. Results
3.1. Wolbachia Transinfection
3.2. Wolbachia Infection Frequency in the Transinfected M. hiroglyphicus Samples
3.3. Distribution of Wolbachia in the Transinfected M. hiroglyphicus Samples
3.4. Effect of Wolbachia Transinfection on the Development Time of the Immature Stages
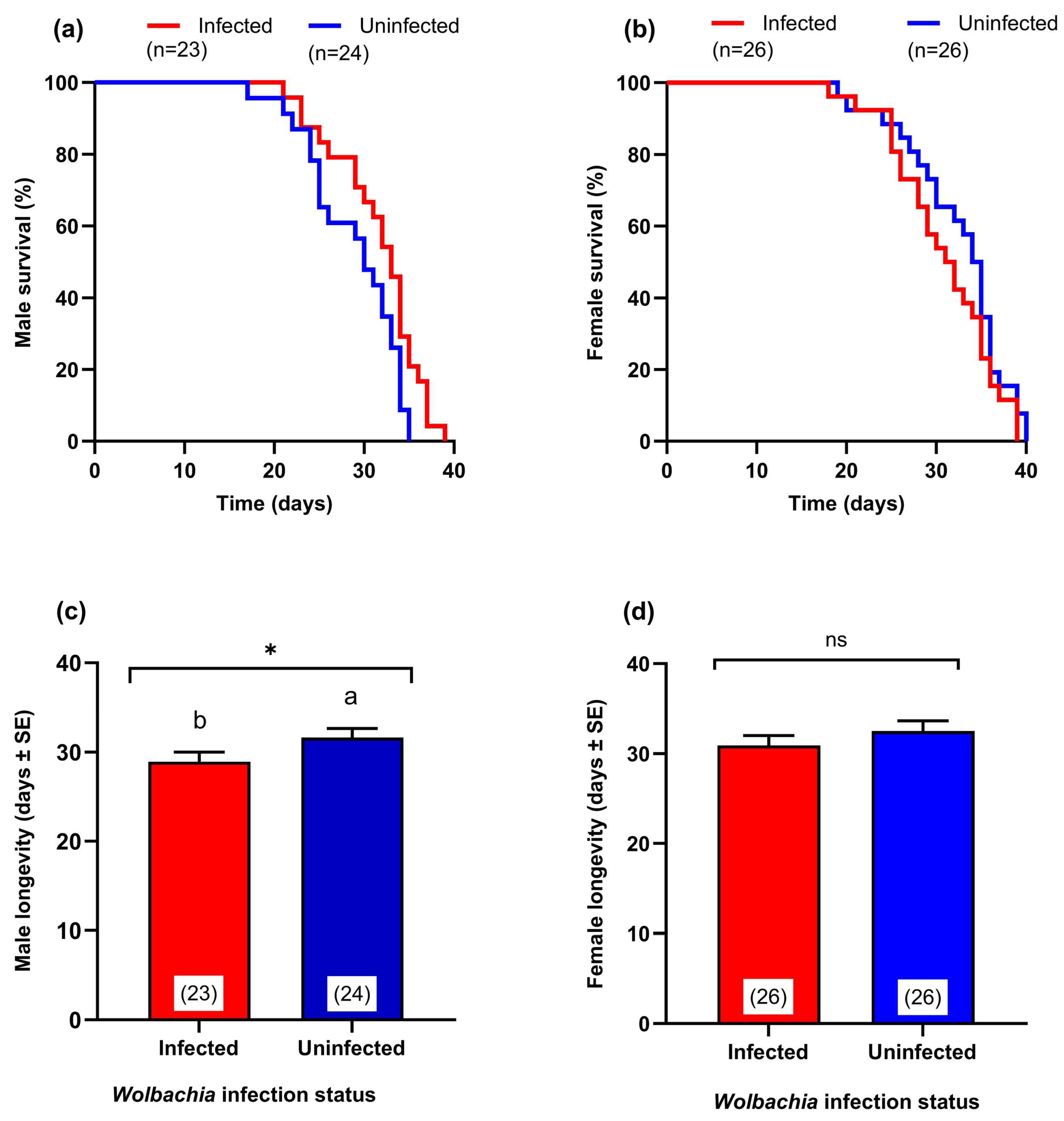
3.5. Effect of Wolbachia Transinfection on Adult Survival
3.6. Effect of Wolbachia Infection on Fecundity and CI Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, A.A.; Ross, P.A.; Rašić, G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015, 8, 751–768. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L. The use of Wolbachia by the world mosquito program to interrupt transmission of Aedes aegypti transmitted viruses. Adv. Exp. Med. Biol. 2018, 1062, 355–360. [Google Scholar] [PubMed]
- Cardona-Salgado, D.; Campo-Duarte, D.E.; Sepulveda-Salcedo, L.S.; Vasilieva, O. Wolbachia-based biocontrol for dengue reduction using dynamic optimization approach. Appl. Math. Modell. 2020, 82, 125–149. [Google Scholar] [CrossRef]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Thi Vo, L.; et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary ecology of Wolbachia releases for disease control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.S.; Failloux, A.B. A review: Wolbachia-based population replacement for mosquito control shares common points with genetically modified control approaches. Pathogens 2020, 9, 404. [Google Scholar] [CrossRef]
- Guo, Y.; Shao, J.; Wu, Y.; Li, Y. Using Wolbachia to control rice planthopper populations: Progress and challenges. Front. Microbiol. 2023, 14, 1244239. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, T.P.; Wang, M.K.; Hong, X.Y. Wolbachia-based strategies for control of agricultural pests. Curr. Opin. Insect Sci. 2023, 57, 101039. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, H.; Chang, P.L.; Mazzoglio, P.J.; Negri, I. Wolbachia is not all about sex: Male feminizing Wolbachia alters the leafhopper Zyginidia pullula transcriptome in a mainly sex-independent manner. Front. Microbiol. 2014, 5, 430. [Google Scholar] [CrossRef] [PubMed]
- He, L.F.; Feng, D.D.; Li, P.; Zhou, Z.S.; Xu, Z.F. Reproductive modes and daily fecundity of Aenasius bambawalei (Hymenoptera: Encyrtidae), a parasitoid of Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Fla Entomol. 2015, 98, 358–360. [Google Scholar] [CrossRef]
- LePage, D.P.; Metcalf, J.A.; Bordenstein, S.R.; On, J.; Perlmutter, J.I.; Shropshire, J.D.; Layton, E.M.; Funkhouser-Jones, L.J.; Beckmann, J.F.; Bordenstein, S.R. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 2017, 543, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Fukatsu, T.; Lemaitre, B. Common and unique strategies of male killing evolved in two distinct Drosophila symbionts. Proc. Biol. Sci. 2018, 285, 20172167. [Google Scholar]
- Sumida, Y.; Katsuki, M.; Okada, K.; Okayama, K.; Lewis, Z. Wolbachia induces costs to life-history and reproductive traits in the moth, Ephestia kuehniella. J. Stored Prod. Res. 2017, 71, 93–98. [Google Scholar] [CrossRef]
- Guo, Y.; Hoffmann, A.A.; Xu, Q.; Mo, W.; Huang, J.; Gong, T.; Ju, F.; Hong, Y. Vertical transmission of Wolbachia is associated with host vitellogenin in Laodelphax striatellus. Front. Microbiol. 2018, 9, 2016. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. Camb. Philos. Soc. 2015, 90, 89–111. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.C.; Sucupira, P.H.F.; Ferreira, A.G.A.; Moreira, L.A. Wolbachia as translational science: Controlling mosquito-borne pathogens. Trends Parasitol. 2021, 37, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.; Louis, F.; Failloux, A.B.; Sinkins, S.P. Wolbachia transinfections in Culex quinquefasciatus generate cytoplasmic incompatibility. Insect Mol. Biol. 2020, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Sun, Q.; Fu, P.; Li, K.; Liang, X.; Xi, Z. Wolbachia inhibits binding of Dengue and Zika viruses to mosquito cells. Front. Microbiol. 2020, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Narmilan, A.; Gonzalez, F.; Salgadoe, A.S.A.; Powell, K. Detection of white leaf disease in sugarcane using machine learning techniques over UAV multispectral images. Drones 2020, 6, 230. [Google Scholar] [CrossRef]
- Donnua, S.; Moonjuntha, P.; Tiwari, A.K. Diversity, distribution, and status of phytoplasma diseases in Thailand. In Diversity, Distribution, and Current Status, 1st ed.; Tiwari, A.K., Caglayan, K., Al-Sadi, A.M., Azadvar, M., Abeysinghe, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 1, pp. 31–38. [Google Scholar]
- Kirdat, K.; Tiwarekar, B.; Thorat, V.; Sathe, S.; Shouche, Y.; Yadav, A. Candidatus Phytoplasma sacchari’, a novel taxon—associated with Sugarcane Grassy Shoot (SCGS) disease. Int. J. Syst. Evol. Microbiol. 2021, 71, 004591. [Google Scholar] [CrossRef] [PubMed]
- Wangkeeree, J.; Tewaruxsa, P.; Hanboonsong, Y. New bacterium symbiont in the bacteriome of the leafhopper Yamatotettix flavovittatus Matsumura. J. Asia-Pasific Entomol. 2019, 22, 889–896. [Google Scholar] [CrossRef]
- Thein, M.M.; Jamjanya, T.; Kobori, Y.; Hanboonsong, Y. Dispersal of the leafhoppers Matsumuratettix hiroglyphicus and Yamatotettix flavovittatus (Homoptera: Cicadellidae), vectors of sugarcane white leaf disease. Appl. Entomol. Zool. 2012, 47, 255–262. [Google Scholar] [CrossRef]
- Roddee, J.; Kobori, Y.; Hanboonsong, Y. Multiplication and distribution of sugarcane white leaf Phytoplasma transmitted by the leafhopper, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae), in infected sugarcane. Sugar Tech 2018, 20, 445–453. [Google Scholar] [CrossRef]
- Sukcharoenvipharat, W.; Aumarrom, T.; Usaborisut, P.; Jantra, C.; Abdullakasim, W. Design and development of a hot water treatment device for small-scale sugarcane farmers. Sugar Tech 2024, 26, 234–241. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Kobori, Y. Effects of selected insecticides on Matsumuratettix hiroglyphicus (Matsumura), a vector of sugarcane white leaf disease, and on two natural enemies of the sugarcane stem borer in sugarcane fields. Sugar Tech 2017, 19, 573–578. [Google Scholar] [CrossRef]
- Minwuyelet, A.; Petronio, G.P.; Yewhalaw, D.; Sciarretta, A.; Magnifico, I.; Nicolosi, D.; Marco, R.D.; Atenafu, G. Symbiotic Wolbachia in mosquitoes and its role in reducing the transmission of mosquito-borne diseases: Updates and prospects. Front. Microbiol. 2023, 14, 1267832. [Google Scholar] [CrossRef] [PubMed]
- Wangkeeree, J.; Suwanchaisri, K.; Roddee, J.; Hanboonsong, Y. Effect of Wolbachia infection states on the life history and reproductive traits of the leafhopper Yamatotettix flavovittatus Matsumura. J. Invertebr. Pathol. 2020, 177, 107490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rousset, F.; O’Neil, S.L. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; He, Z.; Li, S.; An, X.; Lv, N.; Ghanim, M.S.; Cuthbertson, A.G.; Ren, X.; Qiu, L. Wolbachia has two different localization patterns in whitefly Bemisia tabaci AsiaII7 species. PLoS ONE 2016, 11, e0162558. [Google Scholar] [CrossRef]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, Y.; Li, T.P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.Y.; Yang, C.; Zhang, X.; Zha, S.S.; et al. Stable introduction of plant-virus-inhibiting Wolbachia into planthoppers for rice protection. Curr. Biol. 2020, 30, 4837–4845. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Matsumoto, Y.; Gotoh, T.; Noda, H. Transinfection of Wolbachia in planthoppers: Nymphal injection of cultured Wolbachia and infection dynamics. Environ. Entomol. 2009, 38, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, B.; Zhong, Y.; Li, Z.X. Transinfected Wolbachia strains induce a complex of cytoplasmic incompatibility phenotypes: Roles of CI factor genes. Environ. Microbiol. Rep. 2023, 15, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F.; Li, Z.X. Establishment of the cytoplasmic incompatibility-inducing Wolbachia strain wMel in an important agricultural pest insect. Sci. Rep. 2016, 6, 39200. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Z.X. Bidirectional cytoplasmic incompatibility induced by cross-order transfection of Wolbachia: Implications for control of the host population. Microb. Ecol. 2014, 68, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Turelli, M.; Harshman, L.G. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 1990, 126, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Toomey, M.E.; Panaram, K.; Fast, E.M.; Beatty, C.; Frydman, H.M. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc. Natl. Acad. Sci. USA 2013, 110, 10788–10793. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Cortesero, A.M.; Poinsot, D. Influence of the symbiont Wolbachia on life history traits of the cabbage root fly (Delia radicum). J. Invertebr. Pathol. 2018, 158, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Duplouy, A.; Couchoux, C.; Hanski, I.; van Nouhuys, S. Wolbachia infection in a natural parasitoid wasp population. PLoS ONE 2015, 10, e0134843. [Google Scholar] [CrossRef] [PubMed]
- López-Madrigal, S.; Duarte, E.H. Titer regulation in arthropod-Wolbachia symbioses. FEMS Microbiol. Lett. 2019, 366, fnz232. [Google Scholar] [CrossRef] [PubMed]
- Madhav, M.; Brown, G.; Morgan, J.A.T.; Asgari, S.; McGraw, E.A.; James, P. Transinfection of buffalo flies (Haematobia irritans exigua) with Wolbachia and effect on host biology. Parasit. Vectors 2020, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Li, Z.X. A novel Wolbachia strain from the rice moth Corcyra cephalonica induces reproductive incompatibility in the whitefly Bemisia tabaci: Sequence typing combined with phenotypic evidence. Environ. Microbiol. Rep. 2015, 7, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Massaki, N.; Kubo, T. Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity 2005, 95, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Jaenike, J. Spontaneous emergence of a new Wolbachia phenotype. Evolution 2007, 61, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Veneti, Z.; Zabalou, S.; Papafotiou, G.; Paraskevopoulos, C.; Pattas, S.; Livadaras, I.; Markakis, G.; Herren, J.K.; Jaenike, J.; Bourtziz, K. Loss of reproductive parasitism following transfer of male-killing Wolbachia to Drosophila melanogaster and Drosophila simulans. Heredity 2012, 109, 306–312. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Li, Y.R.; Dong, B.; Li, Z.X. The intruding Wolbachia strain from the moth fails to establish itself in the fruit fly due to immune and exclusion reactions. Curr. Microbiol. 2020, 77, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Turelli, M.; Katznelson, A.; Ginsberg, P.S. Why Wolbachia-induced cytoplasmic incompatibility is so common. Proc. Natl. Acad. Sci. USA 2022, 19, e2211637119. [Google Scholar] [CrossRef] [PubMed]
- Shropshire, J.D.; Hamant, E.; Cooper, B.S. Male age and Wolbachia dynamics: Investigating how fast and why bacterial densities and cytoplasmic incompatibility strengths vary. mBio 2021, 12, e0299821. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Werren, J.H. Bidirectional incompatibility among divergent Wolbachia and incompatibility level differences among closely related Wolbachia in Nasonia. Heredity 2007, 99, 278–287. [Google Scholar] [CrossRef] [PubMed]


| Experiment | No. of Injected Nymphs/ No. of Surviving Nymphs (%) | No. of Surviving Nymphs/ No. of Females Emerged (G0) (%) | No. of G0 Female/No. of Wolbachia-Infected (%) | No. of Injected Nymphs/ /No. of Wolbachia-Infected Female (%) |
|---|---|---|---|---|
| 1 | 50/15 (30.00%) | 15/5 (33.33%) | 5/4 (80.00%) | 50/4 (8.00%) |
| 2 | 50/21 (42.00%) | 21/8 (38.09%) | 8/4 (50.00%) | 50/4 (8.00%) |
| 3 | 50/17 (34.00%) | 17/6 (35.29%) | 6/3 (50.00%) | 50/3 (8.00%) |
| 4 | 50/22 (44.00%) | 22/7 (31.82%) | 7/5 (71.43%) | 50/5 (10.00%) |
| Total | 200/75 (37.50%) | 75/26 (34.67%) | 26/16 (61.54%) | 200/16 (8.00%) |
| Developmental Stage (G1) | Infection Frequency of Wolbachia (% ± Standard Error) * |
|---|---|
| Egg | 62.00 ± 3.74 |
| 1st nymph | 60.00 ± 4.47 |
| 2nd nymph | 70.00 ± 7.07 |
| 3rd nymph | 58.00 ± 4.90 |
| 4th nymph | 68.00 ± 3.74 |
| 5th nymph | 64.00 ± 5.10 |
| Generation | Wolbachia Infection Frequency (% ± Standard Error) * |
|---|---|
| G1 | 64.00 ± 2.44 ab |
| G2 | 72.50 ± 4.28 a |
| G3 | 80.00 ± 8.16 a |
| G4 | 67.50 ± 4.28 ab |
| G5 | 72.50 ± 5.63 a |
| G6 | 62.50 ± 2.24 ab |
| G7 | 55.00 ± 5.77 b |
| G8 | 62.50 ± 2.24 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwanchaisri, K.; Roddee, J.; Wangkeeree, J. Wolbachia Transinfection and Effect on the Biological Traits of Matsumuratettix hiroglyphicus (Matsumura), the Leafhopper Vector of Sugarcane White Leaf Disease. Agriculture 2024, 14, 1236. https://doi.org/10.3390/agriculture14081236
Suwanchaisri K, Roddee J, Wangkeeree J. Wolbachia Transinfection and Effect on the Biological Traits of Matsumuratettix hiroglyphicus (Matsumura), the Leafhopper Vector of Sugarcane White Leaf Disease. Agriculture. 2024; 14(8):1236. https://doi.org/10.3390/agriculture14081236
Chicago/Turabian StyleSuwanchaisri, Kamonrat, Jariya Roddee, and Jureemart Wangkeeree. 2024. "Wolbachia Transinfection and Effect on the Biological Traits of Matsumuratettix hiroglyphicus (Matsumura), the Leafhopper Vector of Sugarcane White Leaf Disease" Agriculture 14, no. 8: 1236. https://doi.org/10.3390/agriculture14081236
APA StyleSuwanchaisri, K., Roddee, J., & Wangkeeree, J. (2024). Wolbachia Transinfection and Effect on the Biological Traits of Matsumuratettix hiroglyphicus (Matsumura), the Leafhopper Vector of Sugarcane White Leaf Disease. Agriculture, 14(8), 1236. https://doi.org/10.3390/agriculture14081236






