The Impact of Heat Stress on the Physiological, Productive, and Reproductive Status of Dairy Cows
Abstract
:1. Introduction
2. Relevant Sections
3. Discussion
3.1. Body Temperature
3.2. Management of Heat Stress in Dairy Cattle
3.3. Physiological and Endocrine System Changes Induced by Heat Stress
3.4. Biomarkers of Heat Stress in Livestock
- (a)
- Physiological biomarkers: Pulse rate (PR), respiration rate (RR), rectal temperature (RT), skin temperature (ST), and sweating rate (SR) are basic elements in balancing heat stress. These physiological parameters subjected to heat stress present important, essential changes in identifying the exceeding of the thermal comfort threshold, i.e., the respiration rate (RR) value >80 breaths/minute, the increase in body temperature by at least 1 °C, and the increase in pulse rate (PR) are elements described by the literature as specific biomarkers of heat stress. Another physiological element considered important is skin temperature (ST), skin temperature increases by 0.22 °C for each additional degree felt by the animal, and sweating rate (SR) [26].
- (b)
- Hormonal biomarkers: These are represented by glucocorticoids, thyroid hormones (T3 and T4), catecholamines (epinephrine and norepinephrine), prolactin, and aldosterone. These were discussed previously. Thyroid hormones (triiodothyronine—T3 and thyroxine—T4) play an important role in metabolic adaptation and are true indicators of the effects of heat stress. Serum concentrations of T3 and T4 decrease because of the action of heat stress at the level of the hypothalamic–pituitary axis and at the thyroid level, which causes a decrease in thyrotropin-releasing hormone, thus limiting basal metabolism [24].
- (c)
- Biochemical biomarkers: Blood glucose, urea, amino acid, protein, free fatty acids, creatinine, haptoglobin, hemoglobin, PCV, and cholesterol are the parameters that are subject to changes under the action of heat stress. In recent years, several adaptation responses have been cited at the blood level, both biochemically and hematologically, such as an increase in the concentration of total hemoglobin (Hb) (increased oxygen requirements), an increase in plasma haptoglobin (this is an acute phase protein used to evaluate the inflammatory response), or an increase in compact cell volume (PCV) [24].
- (d)
- Enzyme biomarkers: Malondialdehyde, glutathione peroxidase (GPx), superoxide dismutase (SOD), acid phosphatase (AP), aspartate amino transferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT) are the major enzyme biomarkers involved in heat stressed livestock. Alkaline phosphatase is an important indicator of metabolic activity; its level is low in animals subjected to heat stress. Another metabolic parameter is the plasma concentration of non-esterified fatty acids (NEFA), which is low in the case of dairy cattle subjected to excessive heat [24].
- (e)
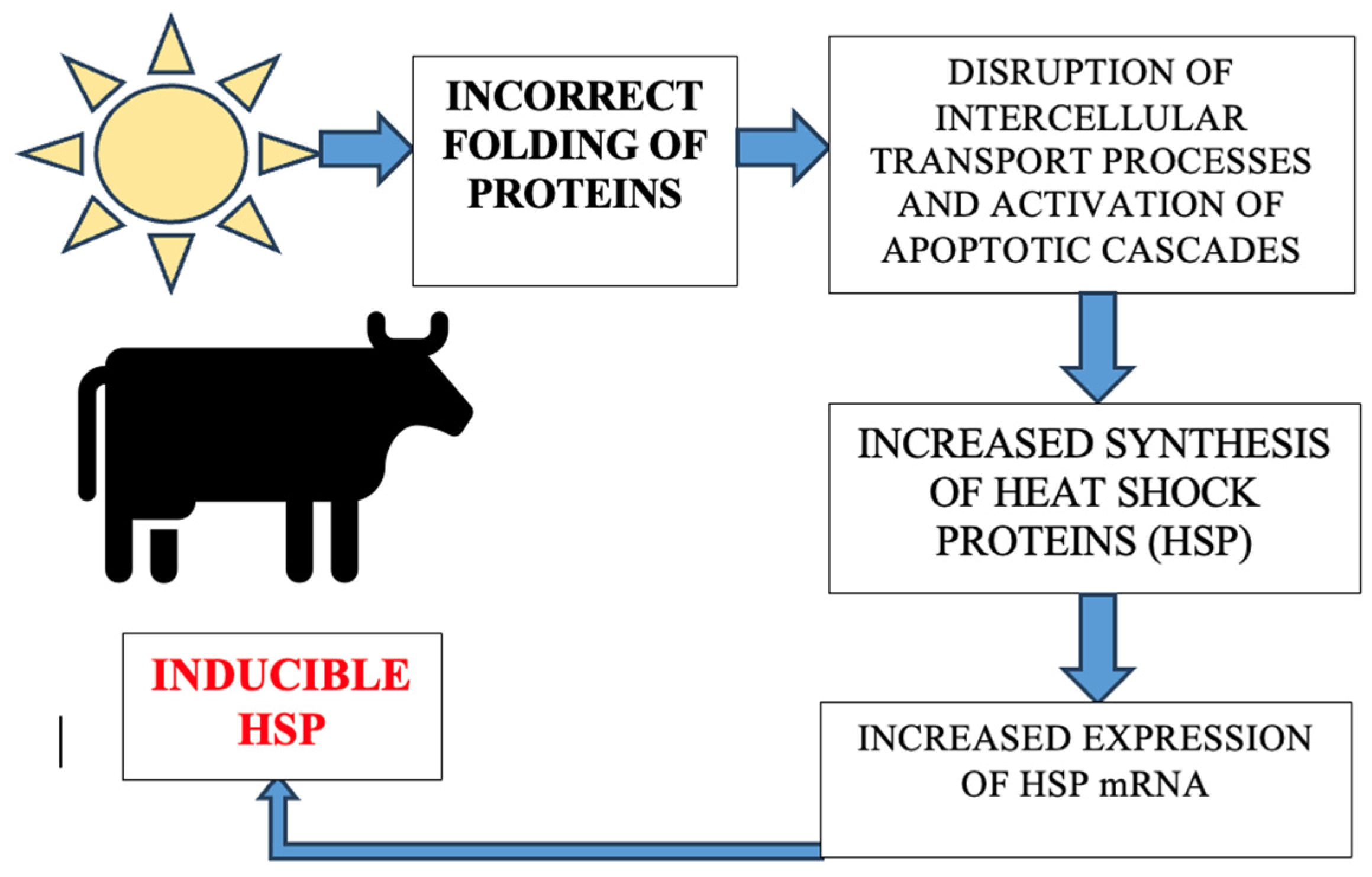
- Molecular biomarkers: The classical heat shock protein (HSP) gene, cytokines, toll-like receptors (TLRs), apoptotic gene, PMEL (premelanosome protein), MC1R (melanocortin 1 receptor), inflammatory gene (NF-κ/nuclear factor kappa B and tissue TNF-α/tumor necrotic factor), microRNAs (miRNAs), genes-associated with thermo-tolerance in ruminant livestock such as superoxide dismutase, nitric oxide synthase, thyroid hormone receptor, and prolactin receptor genes were found to be associated with thermo-tolerance in ruminant livestock. Heat shock protein (HSP) genes HSP70 and HSP90 are increased during exposure to heat stress in ruminants. Inflammatory genes (NF-κB and tissue TNF-α) show an increase directly proportional to the amplification of proinflammatory mediators and systemic inflammatory responses. Skin color genes (PMEL/premelanosome protein and melanocortin 1 MC1R receptor) are modified, and the gene expression of PME and MC1R grows in animals that are raised in environments subject to heat stress.
3.5. Metabolic Changes and Immune Response
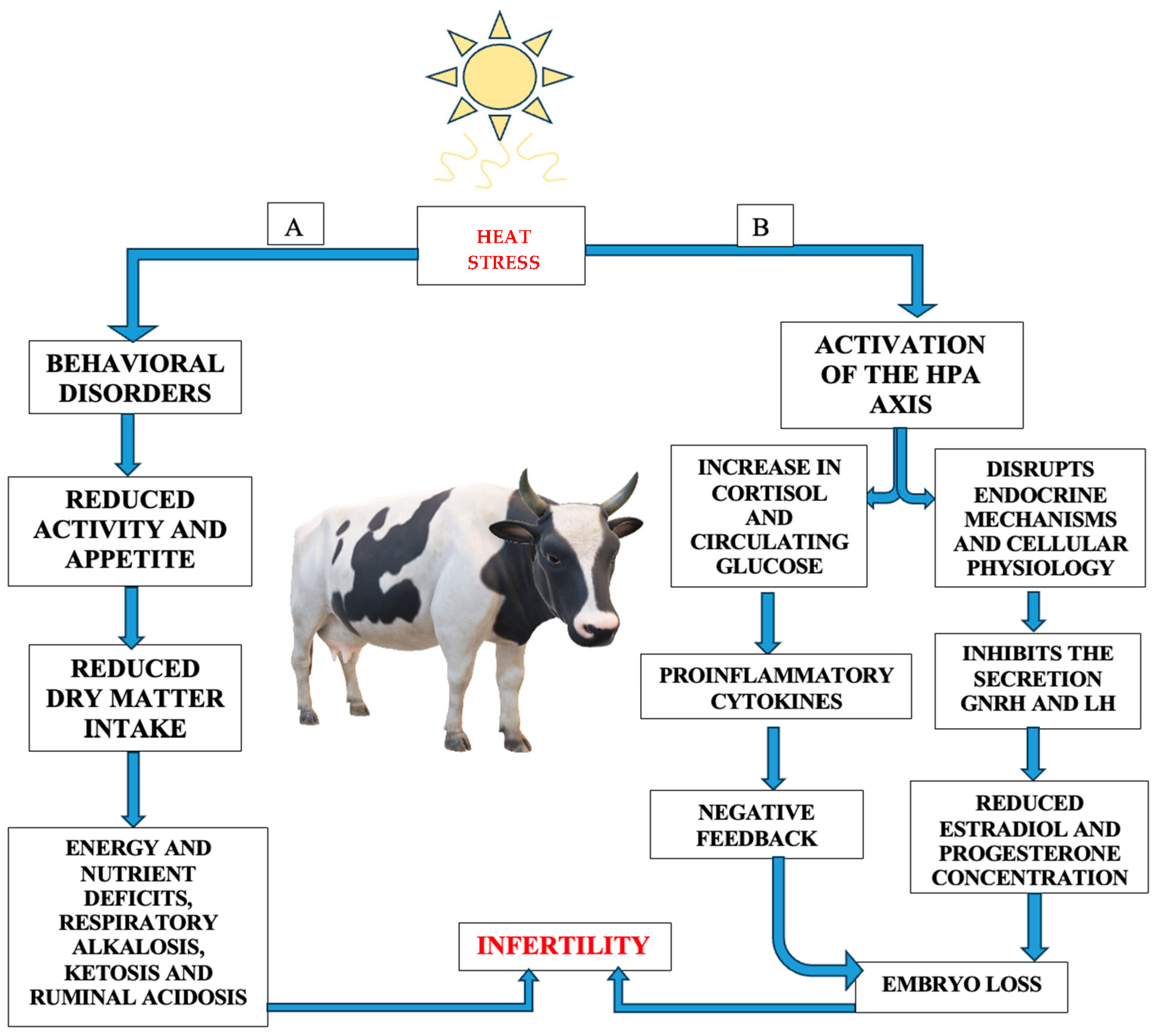
3.6. Reproduction and Fertility
3.7. The Effects of Thermal Stress on the Mammary Gland
3.8. Production Decline Analysis
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pravalie, R.; Sîrodoev, I.; Peptenatu, D. Detecting climate change effects on forest ecosystems in Southwestern Romania using Landsat TM NDVI data. J. Geogr. Sci. 2014, 24, 815–832. [Google Scholar] [CrossRef]
- Nica, M.; Petre, I.L. Nutritional Security in Romania. In Proceedings of the 1st International Conference on Economics and Social Sciences Challenges and Trends in Economic and Social Sciences Research, Kuta Selatan, Indonesia, 16–17 April 2018; The Bucharest University of Economic Studies—Romania. pp. 61–67. [Google Scholar]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milk production, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Brown-Brand, T.M.; Eigenberg, R.A.; Nienaber, J.A.; Hahn, G.L. Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle, part 1: Analyses of indicators. Biosyst. Eng. 2005, 90, 451–462. [Google Scholar] [CrossRef]
- West, J.W.; Mullinix, B.G.; Bernard, J.K. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J. Dairy. Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, C.; Sanker, C.; Gauly, M. Climatic effects on milk production traits and somatic cell score in lactating Holstein-Friesian cows in different housing systems. J. Dairy Sci. 2014, 97, 319–329. [Google Scholar] [CrossRef]
- Lozano Domínguez, R.R.; Vásquez Peláez, C.G.; Padilla, E.G. Effect of heat stress and its interaction with other management and productive variables on pregnancy rate in dairy cows in Aguascalientes, Mexico. Vet. Max 2005, 36, 245–260. [Google Scholar]
- Yang, Y.L.; Ye, B.K.; Liu, H.Y. Occurrence, danger, prevention and treatment of heat stress in dairy cattle. China Cattle Sci. 2010, 36, 63–66. [Google Scholar]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Televičius, M.; Malašauskienė, D. Evaluation of Biomarkers of Heat Stress by Using Automatic Health Monitoring System in Dairy Cows. Pol. J. Vet. Sci. 2020, 24, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Shashank, C.G.; Silpa, M.V.; Madhusoodan, A.P.; Devaraj, C.; Koenig, S. Non-Invasive Methods of Quantifying Heat Stress Response in Farm Animals with Special Reference to Dairy Cattle. Atmosphere 2022, 13, 1642. [Google Scholar] [CrossRef]
- Riaz, U.; Idris, M.; Ahmed, M.; Ali, F.; Yang, L. Infrared Thermography as a Potential Non-Invasive Tool for Estrus Detection in Cattle and Buffaloes. Animals 2023, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Godyń, D.; Hoffmann, G. The physiological and productivity effects of heat stress in cattle—A review. Anim. Sci. 2019, 19, 579–594. [Google Scholar] [CrossRef]
- Cartwright, S.L.; Schmied, J.; Karrow, N.; Mallard, B.A. Impact of heat stress on dairy cattle and selection strategies for thermotolerance: A review. Front. Vet. Sci. 2023, 10, 1198697. [Google Scholar] [CrossRef] [PubMed]
- Carabaño, M.J.; Ramón, M.; Díaz, C.; Molina, A.; Pérez-Guzmán, M.D.; Serradilla, J.M. Breeding and genetics symposium: Breeding for resilience to heat stress effects in dairy ruminants. A comprehensive review. J. Anim. Sci. 2017, 95, 1813. [Google Scholar] [CrossRef]
- Osei-Amponsah, R.; Chauhan, S.S.; Leury, B.J.; Cheng, L.; Cullen, B.; Clarke, I.J.; Dunshea, F.R. Genetic selection for thermotolerance in ruminants. Animals 2019, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.; Lee, S.S. Heat stress: Effects on rumen microbes and host physiology and strategies to alleviate the negative impacts on lactating dairy cows. Front. Microbiol. 2022, 13, 804562. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.L.; McKechnie, M.; Schmied, J.; Livernois, A.M.; Mallard, B.A. Effect of in vitro heat stress challenge on the function blood mononuclear cells from dairy cattle ranked as high, average and low immune responders. BMC Vet. Res. 2021, 17, 233. [Google Scholar] [CrossRef]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the Heat Stress-Induced Responses in Dairy Cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef]
- Napolitano, F.; Rosa, G.D.; Chay-Canul, A.; Álvarez-Macías, A.; Pereira, A.M.; Bragaglio, A.; Mora-Medina, P.; Rodríguez-González, D.; García-Herrera, R.; Hernández-Ávalos, I.; et al. The Challenge of Global Warming in Water Buffalo Farming: Physiological and Behavioral Aspects and Strategies to Face Heat Stress. Animals 2023, 13, 3103. [Google Scholar] [CrossRef] [PubMed]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat Stress: A Serious Disruptor of the Reproductive Physiology of Dairy Cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12 (Suppl. S2), s431–s444. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Roshna, K.; Anandu, S.; Tanuj, G.N. Biomarkers of heat stress in livestock. Indian Farmers 2023, 10, 335–338. [Google Scholar]
- Gantner, V.; Mijić, P.; Kuterovac, K.; Solić, D.; Gantner, R. Temperature-humidity index values and their significance on the daily production of dairy cattle. Mljekarstvo 2011, 61, 56–63. [Google Scholar]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef]
- Salak-Johnson, J.L.; McGlone, J.J. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 2007, 85, E81–E88. [Google Scholar] [CrossRef]
- Ghosh, C.P.; Kesh, S.S.; Tudu, N.K.; Datta, S. Heat stress in dairy animals-Its impact and remedies: A review. Int. J. Pure Appl. Biosci. 2017, 5, 953–965. [Google Scholar] [CrossRef]
- Min, L.; Zhao, S.; Tian, H. Metabolic responses and “omics” technologies for elucidating the effects of heat stress in dairy cows. Int. J. Biometeorol. 2017, 61, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; Von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy. Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Schüller, L.K.; Michaelis, I.; Heuwieser, W. Impact of heat stress on estrus expression and follicle size in estrus under field conditions in dairy cows. Theriogenology 2017, 102, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Orief, Y.I.; Karkor, T.A.E.; Saleh, H.A.; El Hadidy, A.S.; Badr, N. Comparative evaluation of vascular endothelial growth factor-A expression in pre-ovulatory follicular fluid in normogonadotrophic and endometriotic patients undergoing assisted reproductive techniques. Middle East Fertil. Soc. J. 2014, 19, 248–261. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2018, 9, 32–38. [Google Scholar] [CrossRef]
- Paes, V.M.; Vieira, L.A.; Correia, H.H.V.; Sa, N.A.R.; Moura, A.A.A.; Sales, A.D.; Rodrigues, A.P.R.; Magalhães-Padilha, D.M.; Santos, F.W.; Apgar, G.A.; et al. Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus-oocyte complex. Theriogenology 2016, 86, 994–1003. [Google Scholar] [CrossRef]
- Roth, Z.; Meidan, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Trout, J.P.; McDowell, L.R.; Hansen, P.J. Characteristics of the estrous cycle and antioxidant status of lactating Holstein cows exposed to heat stress. J. Dairy Sci. 1998, 81, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Marion, R.S.; Spain, J.N.; Spiers, D.E.; Keisler, D.H.; Lucy, M.C. Effects of controlled heat stress on ovarian function of dairy cattle. 1. Lactating cows. J. Dairy Sci. 1998, 81, 2124–2131. [Google Scholar] [CrossRef]
- Howell, J.L.; Fuquay, J.W.; Smith, A.E. Corpus luteum growth and function in lactating Holstein cows during spring and summer. J. Dairy Sci. 1994, 77, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Hansen, P.J. Disruption of nuclear maturation and rearrangement of cytoskeletal elements in bovine oocytes exposed to heat shock during maturation. Reproduction 2005, 129, 235–244. [Google Scholar] [CrossRef]
- Payton, R.R.; Romar, R.; Coy, P.; Saxton, A.M.; Lawrence, J.L.; Edwards, J.L. Susceptibility of Bovine Germinal Vesicle-Stage Oocytes from Antral Follicles to Direct Effects of Heat Stress in Vitro. Biol. Reprod. 2004, 71, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Soto, P.; Smith, L.C. BH4 peptide derived from Bcl-xL and Bax-inhibitor peptide suppresses apoptotic mitochondrial changes in heat stressed bovine oocytes. Mol. Reprod. Dev. 2009, 76, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Schatten, H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006, 131, 193–205. [Google Scholar] [CrossRef]
- Ju, J.C.; Jiang, S.; Tseng, J.K.; Parks, J.E.; Yang, X. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology 2005, 64, 1677–1689. [Google Scholar] [CrossRef]
- Nabenishi, H.; Ohta, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012, 20, 249–259. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature-humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pavlok, A.; Kopecný, V.; Lucas-Hahn, A.; Niemann, H. Transcriptional activity and nuclear ultrastructure of 8-cell bovine embryos developed by in vitro maturation and fertilization of oocytes from different growth categories of antral follicles. Mol. Reprod. Dev. 1993, 35, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Nasr-Esfahani, M.H. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994, 16, 31–38. [Google Scholar] [CrossRef]
- Cartmill, J.A.; El-Zarkouny, S.Z.; Hensley, B.A.; Rozell, T.G.; Smith, J.F.; Stevenson, J.S. An alternative AI breeding protocol for dairy cows exposed to elevated ambient temperatures before or after calving or both. J. Dairy Sci. 2001, 84, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Monteiro, A.P.A. Effects of late-gestation heat stress on immunity and performance of calves. J. Dairy Sci. 2016, 99, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.; Dahl, G.E. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 2014, 97, 6426–6439. [Google Scholar] [CrossRef]
- Brown, D.E.; Harrison, P.C.; Hinds, F.C.; Lewis, J.A.; Wallace, M.H. Heat stress effects on fetal development during late gestation in the ewe. J. Anim. Sci. 1977, 44, 442–446. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Guo, J.; Weng, X.; Ahmed, B.M.; Hayen, M.J.; Dahl, G.E.; Bernard, J.K.; Tao, S. Effect of maternal heat stress during the dry period on growth and metabolism of calves. J. Dairy Sci. 2016, 99, 3896–3907. [Google Scholar] [CrossRef] [PubMed]
- Laporta, J.; Ferreira, F.C.; Ouellet, V.; Dado-Senn, B.; Almeida, A.K.; De Vries, A.; Dahl, G.E. Late-gestation heat stress impairs daughter and granddaughter lifetime performance. J. Dairy Sci. 2020, 103, 7555–7568. [Google Scholar] [CrossRef] [PubMed]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Gao, S.T.; Ma, L.; Zhou, Z.; Zhou, Z.K.; Baumgard, L.H.; Jiang, D.; Bionaz, M.; Bu, D.P. Heat stress negatively affects the transcriptome related to overall metabolism and milk protein synthesis in mammary tissue of midlactating dairy cows. Physiol. Genom. 2019, 51, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef]
- Gantner, V.; Bobic, T.; Gantner, R.; Gregic, M.; Kuterovac, K.; Novakovic, J.; Potocnik, K. Differences in response to heat stress due to production level and breed of dairy cows. Int. J. Biometeorol. 2017, 61, 1675–1685. [Google Scholar] [CrossRef]
- Garner, J.B.; Douglas, M.; Williams, S.R.O.; Wales, W.J.; Marett, L.C.; DiGiacomo, K.; Leury, B.J.; Hayes, B.J. Responses of dairy cows to short-term heat stress in controlled-climate chambers. Anim. Prod. Sci. 2017, 57, 1233–1241. [Google Scholar] [CrossRef]
- Popescu, A.; Tindeche, C.; Marcuta, A.; Marcuta, L.; Hontus, A.; Stanciu, M. Concentration trends in milk production and number of dairy cows in Romania 2013–2022. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2023, 23, 677–688. [Google Scholar]
- Kelemen, A.; Mărginean, G.E.; Vidu, L. Practical and theoretical aspects regarding the precision dairy farming concept in Romania. Sci. Papers. Ser. D Anim. Sci. 2016, 59, 210. [Google Scholar]
- Broucek, J.; Ryba, S.; Mihina, S.; Uhrincat, M.; Kisac, P. Impact of thermal-humidity index on milk yield under conditions of different dairy management. J. Anim. Feed. Sci. 2007, 16, 329. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țogoe, D.; Mincă, N.A. The Impact of Heat Stress on the Physiological, Productive, and Reproductive Status of Dairy Cows. Agriculture 2024, 14, 1241. https://doi.org/10.3390/agriculture14081241
Țogoe D, Mincă NA. The Impact of Heat Stress on the Physiological, Productive, and Reproductive Status of Dairy Cows. Agriculture. 2024; 14(8):1241. https://doi.org/10.3390/agriculture14081241
Chicago/Turabian StyleȚogoe, Dorin, and Nicoleta Andreea Mincă. 2024. "The Impact of Heat Stress on the Physiological, Productive, and Reproductive Status of Dairy Cows" Agriculture 14, no. 8: 1241. https://doi.org/10.3390/agriculture14081241
APA StyleȚogoe, D., & Mincă, N. A. (2024). The Impact of Heat Stress on the Physiological, Productive, and Reproductive Status of Dairy Cows. Agriculture, 14(8), 1241. https://doi.org/10.3390/agriculture14081241





