Structure–Activity Mechanism of Sodium Ion Adsorption and Release Behaviors in Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Biochar Preparation
2.3. Biochar Characterization
2.4. Batch Adsorption Experiments
2.4.1. Adsorption Kinetics
2.4.2. Adsorption Isotherm
2.4.3. Effect of Solution Equilibrium pH on Na+ Adsorption
2.4.4. Ion Exchange of Na+ on the Surface of Pyrochar
3. Results and Discussion
3.1. Characterization of Biochar
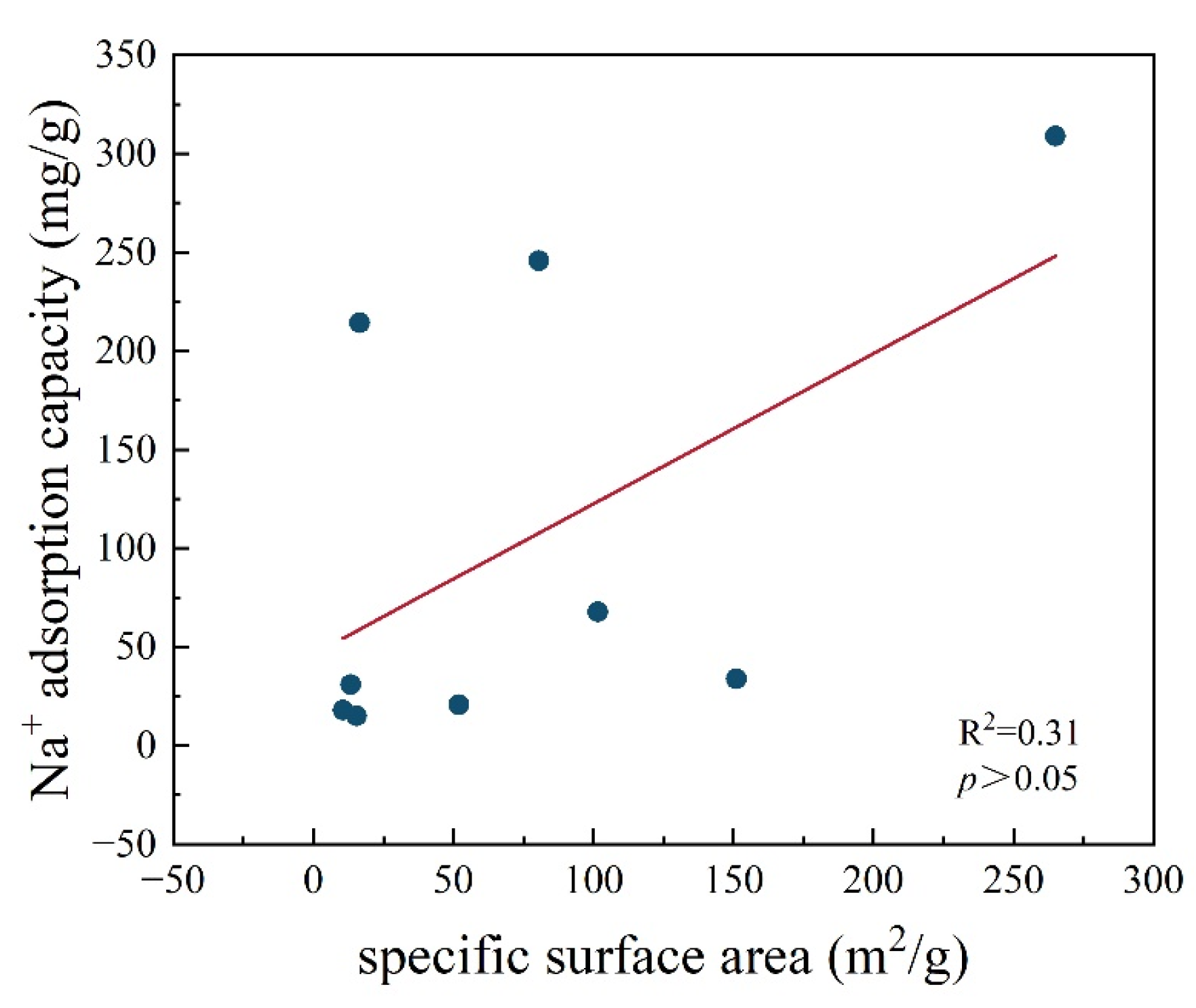
3.1.1. Surface Area and Pore Volume of Pyrochar and Hydrochar
3.1.2. Chemical Properties of Pyrochar and Hydrochar
3.2. Adsorption Kinetics of Na+ in Biochar
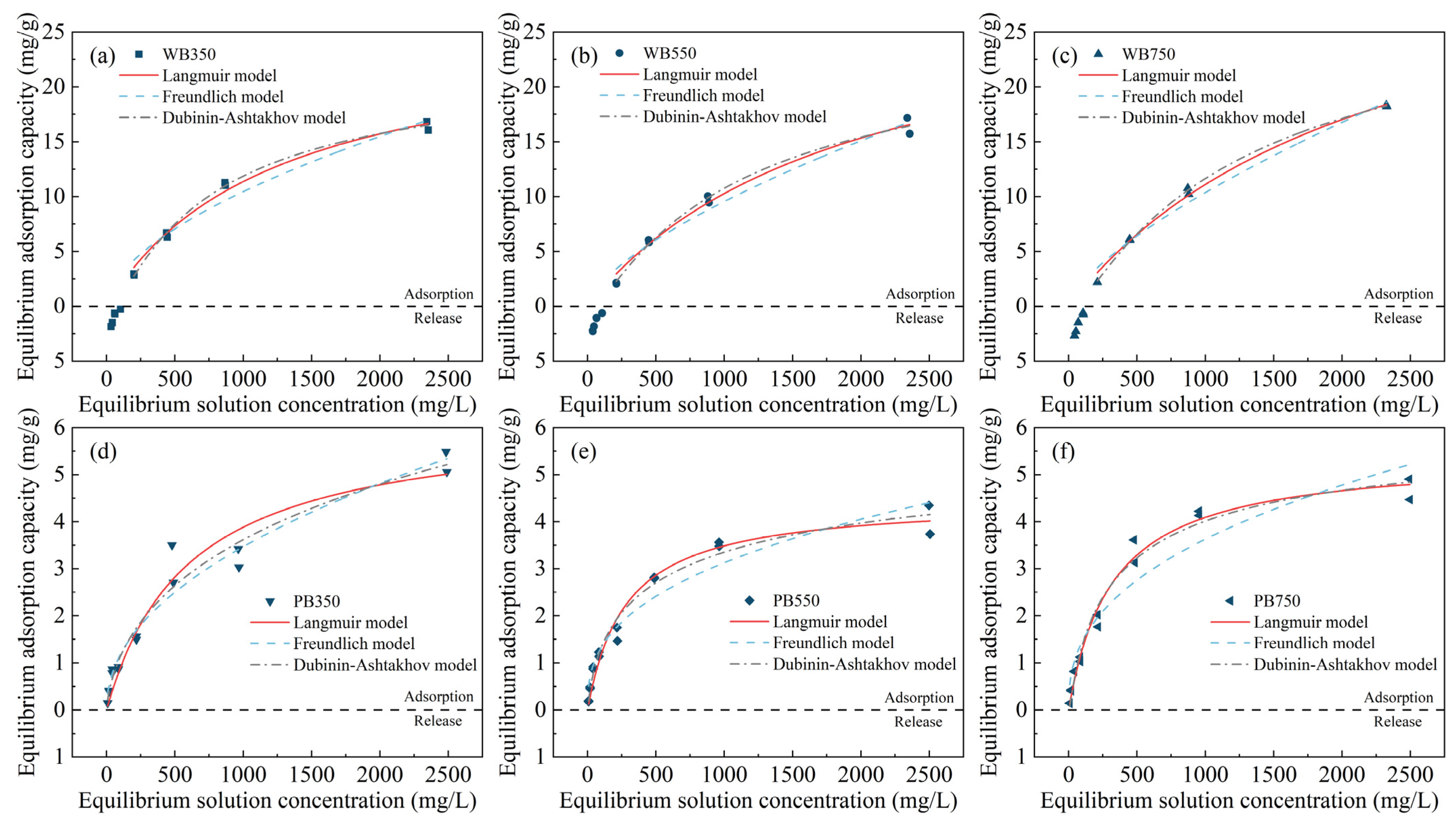
3.3. Adsorption Isotherms of Na+ in Pyrochar
3.4. Effect of Equilibrium pH on Na+ Adsorption in Pyrochar
3.5. Ion Exchange of Na+ in Pyrochar
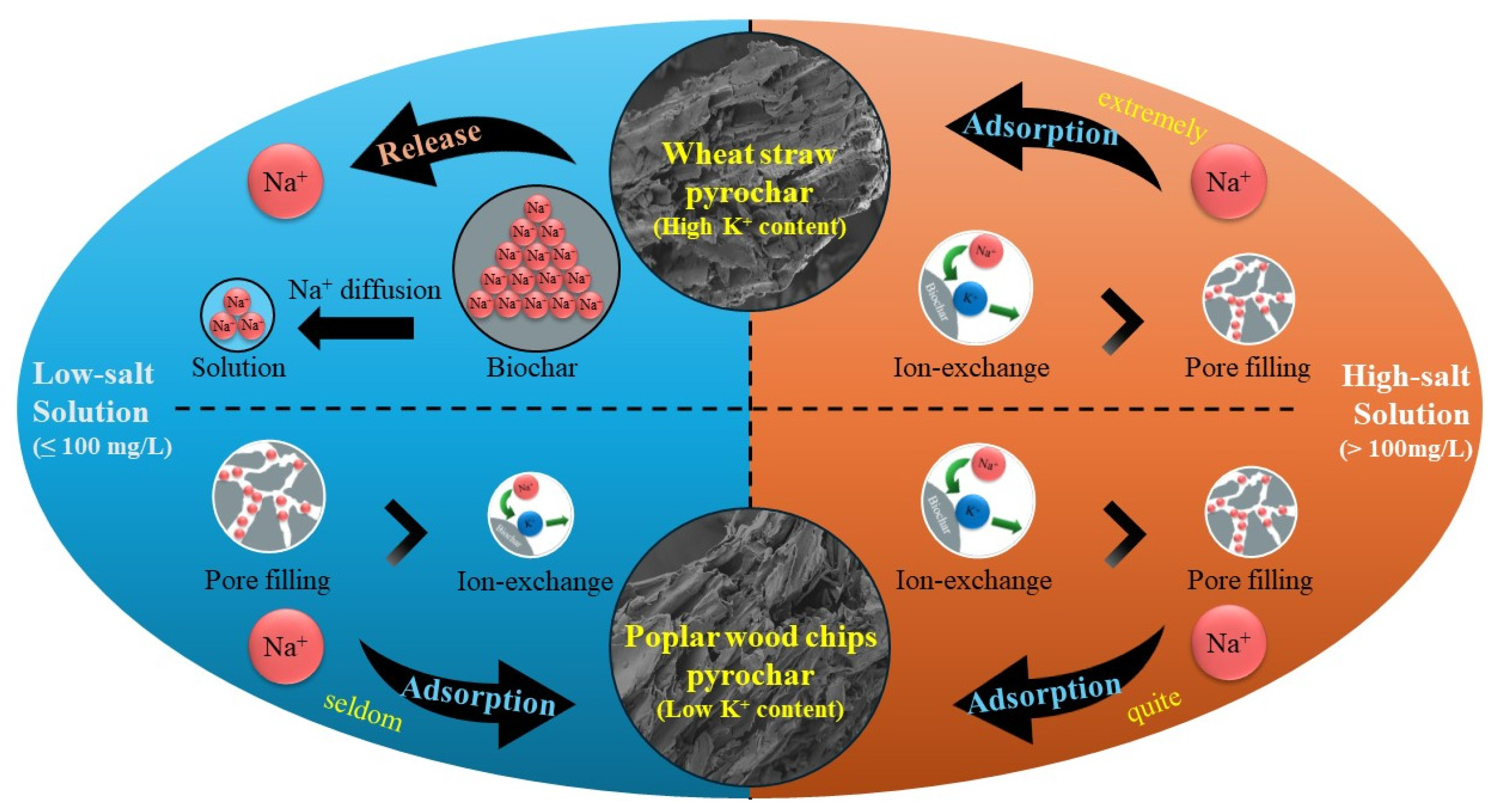
3.6. Mechanism of Na+ Adsorption in Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO: Global Map of Salt Affected Soils Version 1.0. 2021. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/ (accessed on 5 May 2024).
- Fang, S.B.; Tu, W.R.; Mu, L.; Sun, Z.L.; Hu, Q.Y.; Yang, Y. Saline alkali water desalination project in Southern Xinjiang of China: A review of desalination planning, desalination schemes and economic analysis. Renew. Sustain. Energy Rev. 2019, 113, 109268. [Google Scholar] [CrossRef]
- Heng, T.; He, X.L.; Yang, L.L.; Xu, X.; Feng, Y. Mechanism of Saline-Alkali land improvement using subsurface pipe and vertical well drainage measures and its response to agricultural soil ecosystem. Environ. Pollut. 2022, 293, 118583. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Liu, X.L.; Lu, Y.C.; Wang, Y.F. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Xia, J.B.; Ren, J.Y.; Zhang, S.Y.; Wang, Y.H.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.H.; Wang, X.Y.; Ge, J.P.; Cai, B.Y. Elaeagnus angustifolia can improve salt-alkali soil and the health level of soil: Emphasizing the driving role of core microbial communities. J. Environ. Manag. 2022, 305, 114401. [Google Scholar] [CrossRef]
- Pan, X.Q.; Gu, Z.P.; Chen, W.M.; Li, Q.B. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2021, 102, 3488–3497. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.X.; Wang, H.T.; Lu, W.J.; Zhou, Z.Y.; Zhang, Y.C.; Ren, L.L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- He, L.Z.; Fan, S.L.; Müller, K.; Hu, G.T.; Huang, H.G.; Zhang, X.K.; Lin, X.M.; Che, L.; Wang, H.L. Biochar reduces the bioavailability of di-(2-ethylhexyl) phthalate in soil. Chemosphere 2016, 142, 24–27. [Google Scholar] [CrossRef]
- Guo, Z.J.; Chen, X.; Hang, J.C.; Li, Z.Z.; Zhong, C.H.; Sun, A.H.; Li, J.H.; Xu, S.Y. Oxidative magnetization of biochar at relatively low pyrolysis temperature for efficient removal of different types of pollutants. Bioresour. Technol. 2023, 387, 129572. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Wang, C.; Holm, P.E.; Hansen, H.C.B.; Brandt, K.K. Impacts of biochar materials on copper speciation, bioavailability, and toxicity in chromated copper arsenate polluted soil. J. Hazard. Mater. 2023, 459, 132067. [Google Scholar] [CrossRef]
- Ma, R.; Nie, D.Y.; Sang, M.; Wang, W.W.; Nie, G.Z. Adsorption of Rhodamine B and Pb(II) from aqueous solution by MoS2 nanosheet modified biochar: Fabrication, performance, and mechanisms. Bioresour. Technol. 2023, 386, 129548. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.M.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Fan, S.S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X.D. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Lu, H.L.; Zhang, W.H.; Yang, Y.X.; Huang, X.F.; Wang, S.Z.; Qiu, R.L. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, G.C.; Chen, L.G.; Chen, Y.X.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.Z.; Cui, Y.T.; Liang, Y.; Wang, H.Y. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Li, Z.Y.; Zhang, Z.Q.; You, L.R.; Xu, L.F.; Huang, H.Y.; Wang, X.P.; Gao, Y.; Cui, X.J. Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western Songnen Plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef]
- Wang, X.L.; Riaz, M.; Xia, X.Y.; Babar, S.; El-Desouki, Z.; Li, Y.X.; Wang, J.Y.; Jiang, C.C. Alleviation of cotton growth suppression caused by salinity through biochar is strongly linked to the microbial metabolic potential in saline-alkali soil. Sci. Total Environ. 2024, 922, 171407. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.P.; Zhang, H.P.; Xu, Y.; Wang, S.M.; Kong, Y.Z.; Zhou, G.K.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Tang, J.W.; Zhang, S.D.; Zhang, X.T.; Chen, J.H.; He, X.Y.; Zhang, Q.Z. Effects of pyrolysis temperature on soil -plant -microbe responses to Solidago canadensis L.-derived biochar in coastal saline-alkali soil. Sci. Total Environ. 2020, 731, 138938. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Dinh, G.D.; Dong, H.P.; Le, L.B. Sodium adsorption isotherm and characterization of biochars produced from various agricultural biomass wastes. J. Clean. Prod. 2022, 346, 131250. [Google Scholar] [CrossRef]
- Yu, J.; Chang, J.S.; Guo, H.L.; Han, S.; Lee, D.J. Sodium ions removal by sulfuric acid-modified biochars. Environ. Res. 2023, 235, 116592. [Google Scholar] [CrossRef]
- Awan, S.; Ippolito, J.A.; Ullman, J.L.; Ansari, K.; Cui, L.Q.; Siyal, A.A. Biochars reduce irrigation water sodium adsorption ratio. Biochar 2021, 3, 77–87. [Google Scholar] [CrossRef]
- Zhang, K.; Mao, J.F.; Chen, B.L. Reconsideration of heterostructures of biochars: Morphology, particle size, elemental composition, reactivity and toxicity. Environ. Pollut. 2019, 254, 113017. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.W.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X.D. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Xue, Y.W.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200, 673–680. [Google Scholar] [CrossRef]
- Mao, J.F.; Zhang, K.; Chen, B.L. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef]
- Guo, S.Q.; Dong, X.Y.; Wu, T.T.; Shi, F.J.; Zhu, C.X. Characteristic evolution of hydrochar from hydrothermal carbonization of corn stalk. J. Anal. Appl. Pyrolysis 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.D.; Lin, Y.C.; Ho, S.H.; Zhou, Y.; Ren, N.Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, Y.; Zong, Y.M.; Yu, J.; Ding, H.; Kong, Y.L.; Ma, J.Y.; Ding, L. Efficient removal of cadmium by salts modified-biochar: Performance assessment, theoretical calculation, and quantitative mechanism analysis. Bioresour. Technol. 2022, 361, 127717. [Google Scholar] [CrossRef]
- Chen, T.; Wei, Y.F.; Yang, W.J.; Liu, C.B. Highly efficient As(III) removal in water using millimeter-sized porous granular MgO-biochar with high adsorption capacity. J. Hazard. Mater. 2021, 416, 125822. [Google Scholar] [CrossRef]
- Yang, K.; Yan, X.X.; Xu, J.L.; Jiang, L.; Wu, W.H. Sorption of organic compounds by pyrolyzed humic acids. Sci. Total Environ. 2021, 781, 146646. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B.S. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef]
- Qi, G.D.; Pan, Z.F.; Zhang, X.Y.; Miao, X.D.; Xiang, W.; Gao, B. Effect of ball milling with hydrogen peroxide or ammonia hydroxide on sorption performance of volatile organic compounds by biochar from different pyrolysis temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, B.; Zheng, Y.L.; Hu, X.; Creamer, A.E.; Annable, M.D.; Li, Y.C. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017, 245, 606–614. [Google Scholar] [CrossRef]
- Sun, Y.N.; Gao, B.; Yao, Y.; Fang, J.N.; Zhang, M.; Zhou, Y.M.; Chen, H.; Yang, L.Y. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.D.; Masek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.M.; Chen, C.L.; Lin, J.Y.; Tzeng, J.H.; Huang, C.P.; Dong, C.D.; Huang, C.P. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Liu, Y.G.; Zeng, G.M.; Wang, X.; Hu, X.J.; Gu, Y.L.; Yang, Z.Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.Q.; Zhang, X.; Huang, D.J.; Huang, G.L.; Fan, J.L.; Peng, H.; Dai, Y.; Prabaharan, K. Copper hexacyanoferrate nanoparticle-decorated biochar produced from pomelo peel for cesium removal from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 322, 791–799. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Jiang, J.; Xu, R.K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Wei, C.B.; Zhang, S.R.; Wang, Y.D.; Kuzyakov, Y.; Ding, X.D. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Cui, X.Q.; Fang, S.Y.; Yao, Y.Q.; Li, T.Q.; Ni, Q.J.; Yang, X.E.; He, Z.L. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Huang, S.; Dong, C.Q.; Meng, Z.W.; Wang, X.G. Competitive adsorption behaviour and mechanisms of cadmium, nickel and ammonium from aqueous solution by fresh and ageing rice straw biochars. Bioresour. Technol. 2020, 303, 122853. [Google Scholar] [CrossRef]
- Medynska-Juraszek, A.; Alvarez, M.L.; Bialowiec, A.; Jerzykiewicz, M. Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes. Materials 2021, 14, 4714. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Nufida, B.A.; Ismail, N.M.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Al-Dhawi, B.N.S. Modeling and Optimization of Biochar Based Adsorbent Derived from Kenaf Using Response Surface Methodology on Adsorption of Cd2+. Water 2021, 13, 999. [Google Scholar] [CrossRef]
- Chen, Z.M.; Xiao, X.; Chen, B.L.; Zhu, L.Z. Quantification of Chemical States, Dissociation Constants and Contents of Oxygen-containing Groups on the Surface of Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2015, 49, 309–317. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting Hydrogen-Bond Strengths from Acid-Base Molecular Properties. The pKa Slide Rule: Toward the Solution of a Long-Lasting Problem. Accounts Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef]
- Wang, J.M.; Teng, X.J.; Wang, H.; Ban, H. Characterizing the metal adsorption capability of a class F coal fly ash. Environ. Sci. Technol. 2004, 38, 6710–6715. [Google Scholar] [CrossRef]


| Biochar | SBET (m2/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | Ash (%) | pH | Elemental Composition (%) | Atomic Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | H/C | O/C | ||||||
| WB350 | 4.63 | 0.008 | 0.072 | 19.71 ± 0.03 | 8.6 | 56.37 ± 0.04 | 3.64 ± 0.02 | 19.47 ± 0.03 | 0.73 ± 0.01 | 0.09 ± 0.00 | 0.06 | 0.35 |
| WB550 | 194.66 | 0.012 | 0.108 | 25.67 ± 0.34 | 11.0 | 60.54 ± 0.02 | 2.14 ± 0.03 | 10.94 ± 0.01 | 0.63 ± 0.04 | 0.08 ± 0.00 | 0.04 | 0.18 |
| WB750 | 417.34 | 0.021 | 0.141 | 26.91 ± 0.10 | 10.6 | 59.96 ± 0.04 | 1.62 ± 0.00 | 10.81 ± 0.00 | 0.66 ± 0.04 | 0.05 ± 0.00 | 0.03 | 0.18 |
| PB350 | 109.05 | 0.006 | 0.114 | 0.45 ± 0.07 | 6.0 | 70.45 ± 0.01 | 3.76 ± 0.02 | 25.03 ± 0.00 | 0.26 ± 0.02 | 0.07 ± 0.00 | 0.05 | 0.36 |
| PB550 | 484.27 | 0.025 | 0.164 | 2.06 ± 0.80 | 9.0 | 82.23 ± 0.04 | 2.88 ± 0.02 | 12.41 ± 0.05 | 0.37 ± 0.01 | 0.06 ± 0.01 | 0.04 | 0.15 |
| PB750 | 601.90 | 0.125 | 0.207 | 1.95 ± 0.06 | 10.0 | 85.76 ± 0.04 | 2.26 ± 0.02 | 9.51 ± 0.07 | 0.44 ± 0.05 | 0.09 ± 0.00 | 0.03 | 0.11 |
| HWB180 | 8.46 | 0.033 | 0.019 | 9.81 ± 0.33 | 4.6 | 45.53 ± 0.02 | 4.98 ± 0.09 | 39.17 ± 0.15 | 0.48 ± 0.04 | 0.04 ± 0.00 | 0.11 | 0.86 |
| HWB220 | 12.54 | 0.055 | 0.033 | 9.96 ± 0.61 | 4.0 | 52.34 ± 0.02 | 5.85 ± 0.04 | 31.30 ± 0.00 | 0.52 ± 0.02 | 0.03 ± 0.00 | 0.11 | 0.60 |
| HPB180 | 2.15 | 0.005 | 0.011 | 1.19 ± 0.35 | 4.5 | 49.33 ± 0.04 | 6.36 ± 0.05 | 42.91 ± 0.11 | 0.16 ± 0.01 | 0.05 ± 0.01 | 0.13 | 0.87 |
| HPB220 | 3.47 | 0.011 | 0.030 | 0.15 ± 0.07 | 4.2 | 55.36 ± 0.04 | 6.64 ± 0.02 | 37.66 ± 0.08 | 0.14 ± 0.05 | 0.06 ± 0.01 | 0.12 | 0.68 |
| Biochar | Metal Element Content (mg/g) | |||
|---|---|---|---|---|
| Na | K | Ca | Mg | |
| WB350 | 3.95 | 49.80 | 4.49 | 3.24 |
| WB550 | 4.44 | 58.50 | 6.00 | 4.29 |
| WB750 | 4.94 | 60.60 | 5.61 | 4.33 |
| PB350 | 0.66 | 2.97 | 3.42 | 0.47 |
| PB550 | 0.70 | 3.95 | 4.94 | 0.93 |
| PB750 | 0.62 | 5.40 | 6.79 | 1.42 |
| Biochar | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (/h) | R2 | qe (mg/g) | k2 (g/mg/h) | R2 | |
| PB350 | 0.32 ± 0.02 | 1.36 ± 0.63 | 0.61 | 0.32 ± 0.03 | 7.79 ± 5.52 | 0.58 |
| PB550 | 0.38 ± 0.01 | 3.78 ± 0.85 | 0.88 | 0.39 ± 0.01 | 20.98 ± 8.33 | 0.88 |
| PB750 | 0.23 ± 0.01 | 5.02 ± 2.84 | 0.58 | 0.23 ± 0.02 | 63.74 ± 80.61 | 0.57 |
| Biochar | Freundlich Model | Langmuir Model | D–A Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | KF | R2 | qmax | KL | R2 | Q0 | E | b | R2 | |
| WB350 | 1.77 ± 0.19 | 0.21 ± 0.09 | 0.9564 | 25.44 ± 1.68 | 0.0008 ± 0.0001 | 0.9883 | 21.54 ± 1.42 | 18.97 ± 0.14 | 5.13 ± 0.54 | 0.9965 |
| WB550 | 1.52 ± 0.14 | 0.10 ± 0.04 | 0.9688 | 30.21 ± 3.15 | 0.0005 ± 0.0001 | 0.9859 | 24.88 ± 3.83 | 18.28 ± 0.22 | 4.47 ± 0.85 | 0.9926 |
| WB750 | 1.44 ± 0.11 | 0.09 ± 0.03 | 0.9786 | 36.45 ± 3.19 | 0.0004 ± 0.0001 | 0.9927 | 29.47 ± 2.10 | 18.02 ± 0.10 | 4.29 ± 0.34 | 0.9989 |
| PB350 | 2.11 ± 0.16 | 0.13 ± 0.03 | 0.9624 | 6.23 ± 0.53 | 0.0017 ± 0.0004 | 0.9524 | 13.72 ± 7.39 | 19.42 ± 2.43 | 1.90 ± 0.66 | 0.9674 |
| PB550 | 2.67 ± 0.23 | 0.24 ± 0.05 | 0.9452 | 4.46 ± 0.21 | 0.0036 ± 0.0005 | 0.9718 | 5.67 ± 0.79 | 23.51 ± 0.50 | 3.09 ± 0.54 | 0.9780 |
| PB750 | 2.52 ± 0.26 | 0.23 ± 0.07 | 0.9265 | 5.41 ± 0.19 | 0.0031 ± 0.0003 | 0.9862 | 5.85 ± 0.54 | 22.71 ± 0.39 | 4.09 ± 0.61 | 0.9814 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Jing, W.; Wang, J.; Zhang, K.; Li, Y.; Xia, M.; Zhang, K.; Mao, J. Structure–Activity Mechanism of Sodium Ion Adsorption and Release Behaviors in Biochar. Agriculture 2024, 14, 1246. https://doi.org/10.3390/agriculture14081246
Yang K, Jing W, Wang J, Zhang K, Li Y, Xia M, Zhang K, Mao J. Structure–Activity Mechanism of Sodium Ion Adsorption and Release Behaviors in Biochar. Agriculture. 2024; 14(8):1246. https://doi.org/10.3390/agriculture14081246
Chicago/Turabian StyleYang, Kai, Wei Jing, Jing Wang, Kaizhao Zhang, Yaoming Li, Meng Xia, Kun Zhang, and Jiefei Mao. 2024. "Structure–Activity Mechanism of Sodium Ion Adsorption and Release Behaviors in Biochar" Agriculture 14, no. 8: 1246. https://doi.org/10.3390/agriculture14081246






