Transcriptome Analysis of Ganoderma lingzhi Liquid Fermentation Process Using Corn Straw as Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Enzyme Extraction Solution and Enzymatic Activity Assay
2.3. RNA Extraction, Library Construction, and Sequencing
2.4. Bioinformatics Analysis of RNA-Seq Data
2.5. Analysis of CAZyme DEGs Related to the Decomposition of Corn Straw Lignocellulose
2.6. Analysis of Enzyme Genes Involved in Carbohydrate Metabolism and Triterpenoid Metabolism
2.7. Analysis of the Co-Regulation Mechanism of Triterpene Metabolism, Matrix Degradation, and Glucose Metabolism Genes
2.8. Real-Time Quantitative PCR Experiments
3. Results
3.1. Lignocellulose-Degrading Enzyme Activities of G. lingzhi
3.2. Functional Annotation of Novel Genes
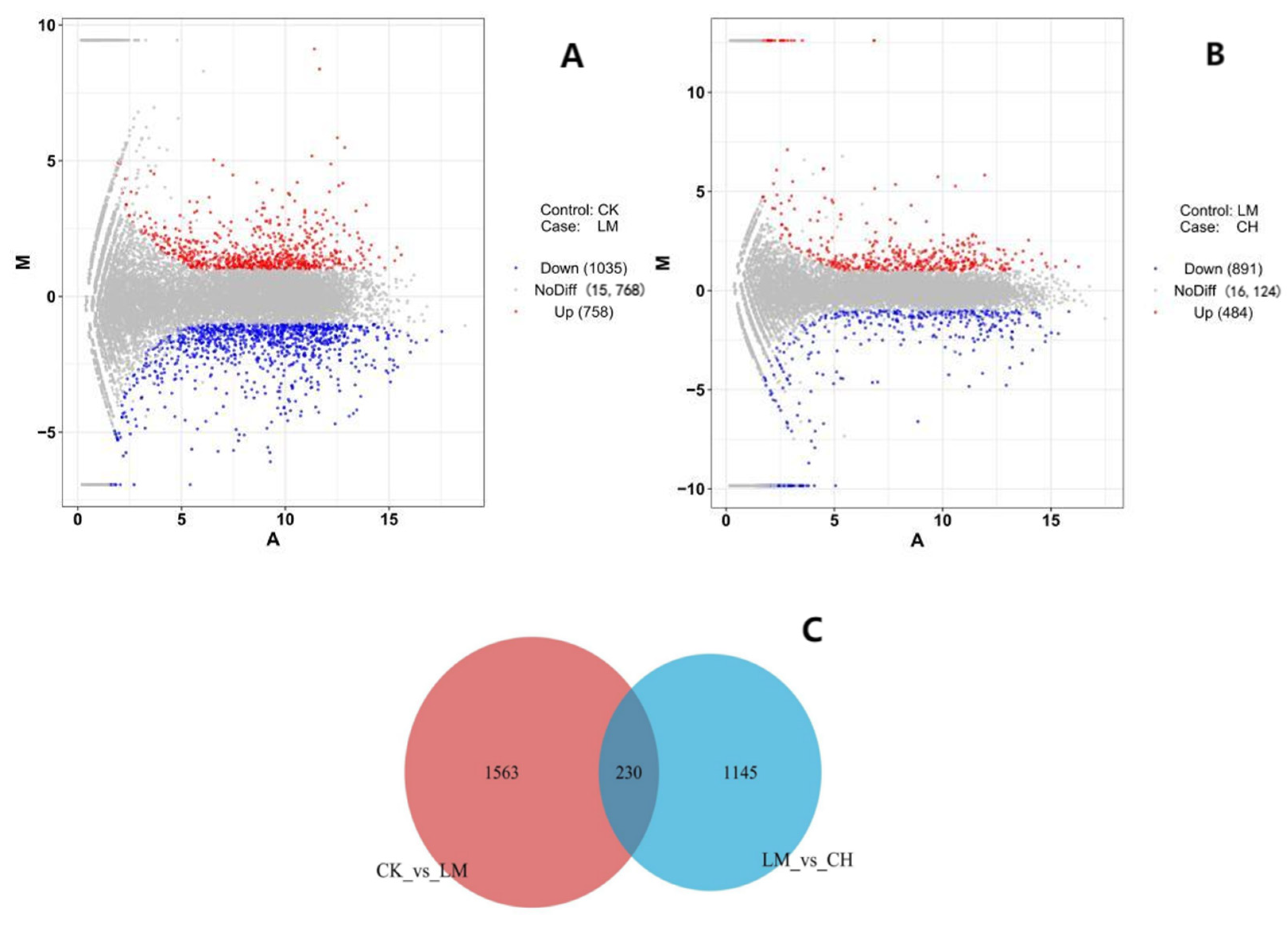
3.3. Differential Expression Analysis
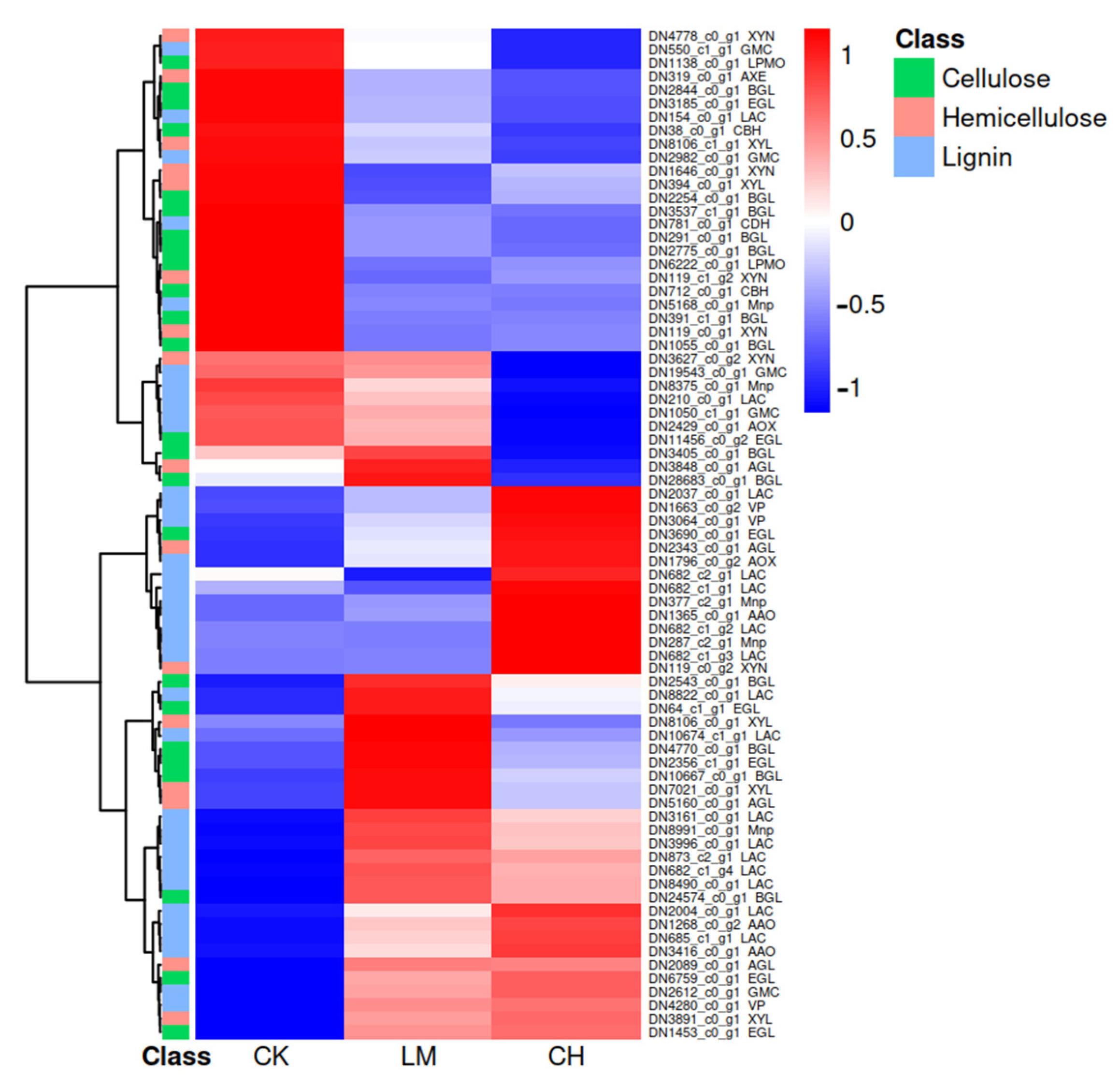
3.4. Genes Encoding Putative CAZymes Related to the Decomposition of Corn Straw Lignocellulose
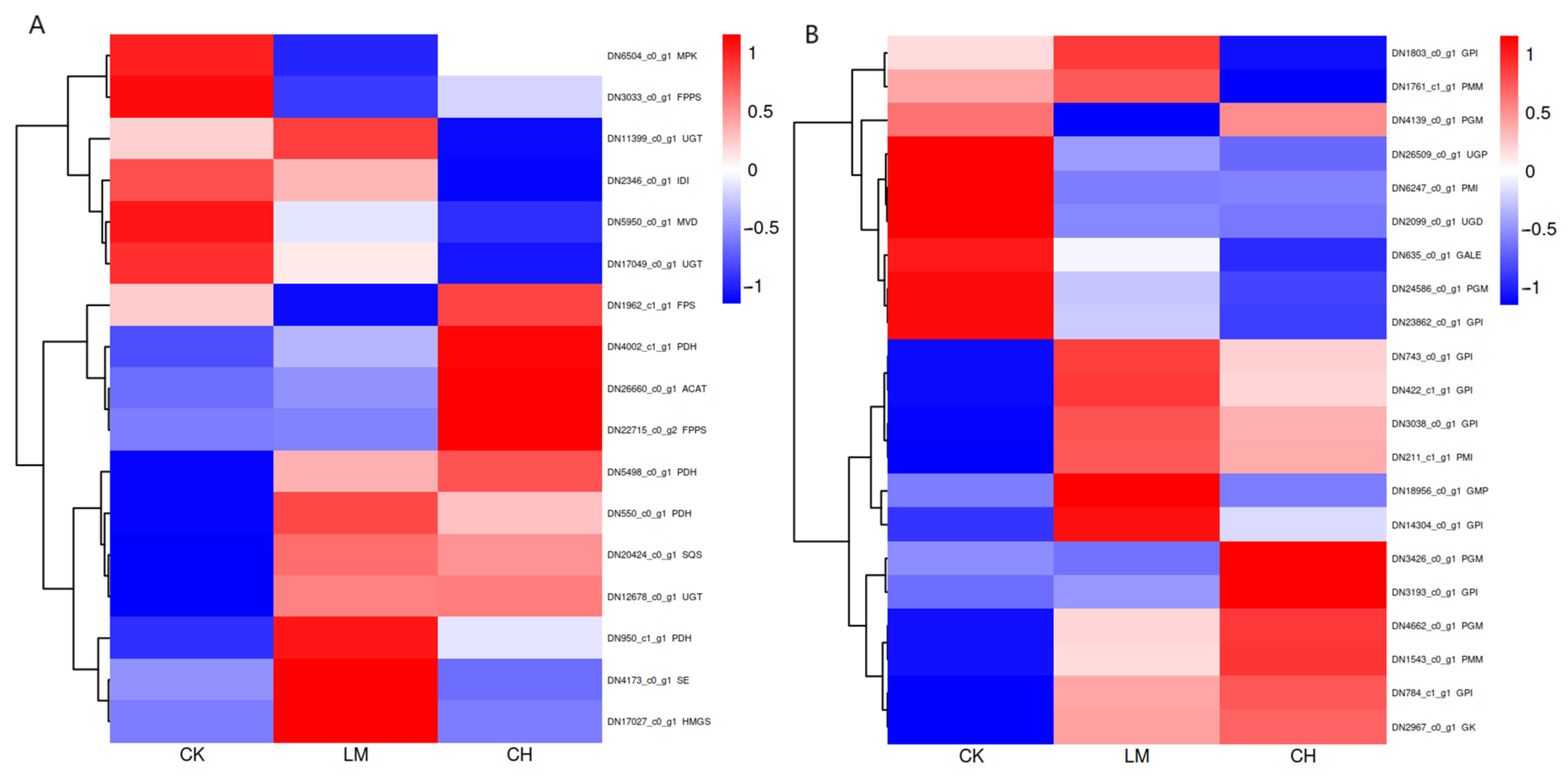
3.5. Expression Cluster Analysis of Enzyme Genes Involved in Carbohydrate and Triterpene Metabolism
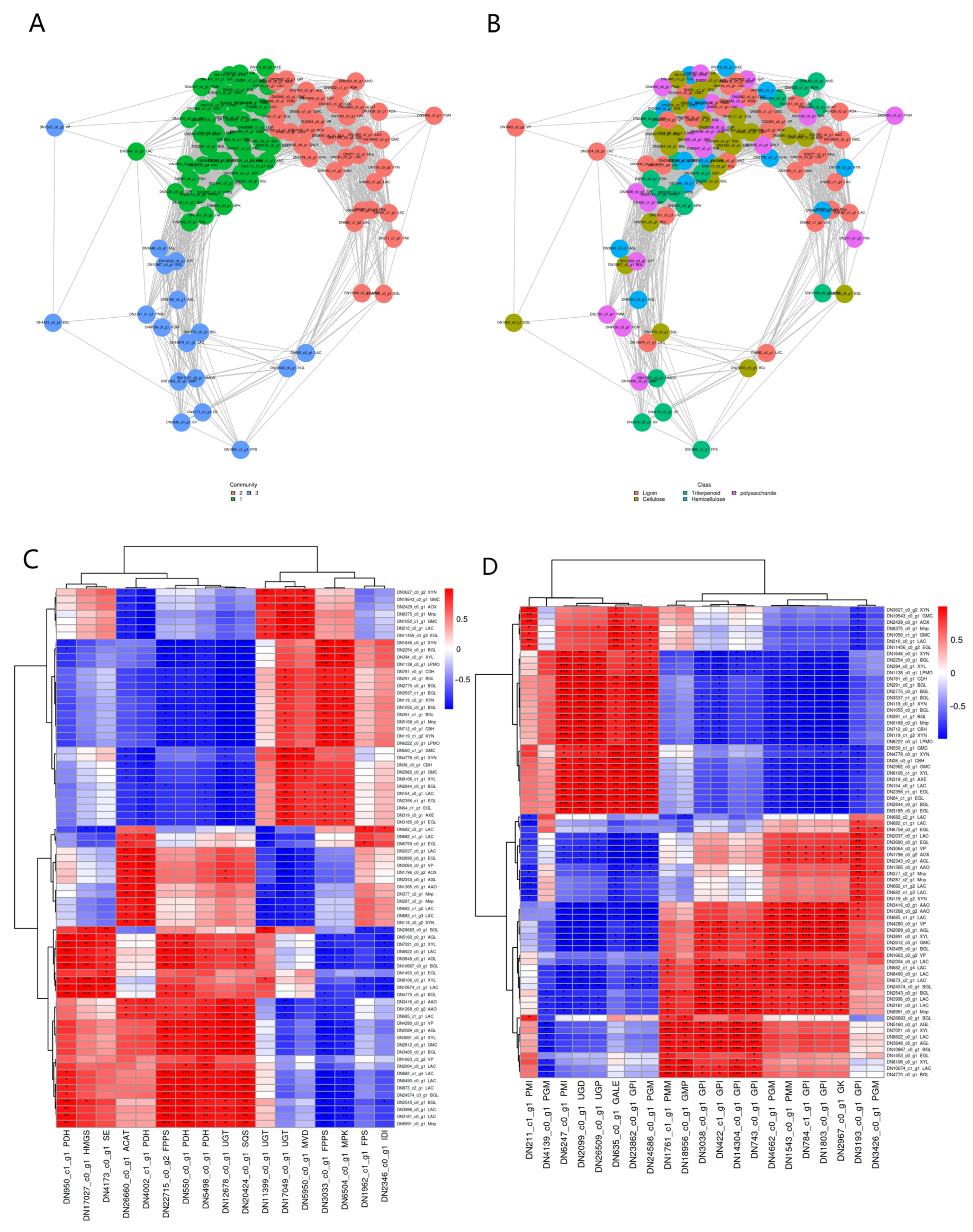
3.6. Co-Regulation of Triterpene Metabolism and Matrix Degradation and Glucose Metabolism Genes
3.7. Validating RNA-Seq Results via RT-qPCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sohretoglu, D.; Huang, S.L. Ganoderma lucidum Polysaccharides as an Anti-cancer Agent. Anti-Cancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef]
- Zhu, N.; Lv, X.C.; Wang, Y.Y.; Li, J.L.; Liu, Y.M.; Lu, W.F.; Yang, L.C.; Zhao, J.; Wang, F.J.; Zhang, L.S.W. Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int. J. Biol. Macromol. 2016, 93, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Fu, H.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, Q.P.; Xie, Y.Z.; Tan, J.B.; Ding, Y.R.; Bai, L.J. Hypoglycemic mechanisms of Ganoderma lucidum polysaccharides F31 in db/db mice via RNA-seq and iTRAQ. Food Funct. 2018, 9, 6496–6508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Yang, Y.; Lin, L.; Xu, N.; Zhao, H.; Jia, L. Purification, characterization and hepatoprotective activities of mycelia zinc polysaccharides by Pleurotus djamor. Carbohydr. Polym. 2016, 136, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Li, M.M.; Wang, R.; Wang, B.Y.; Athari, S.S.; Wang, J.L. Ganoderma modulates allergic asthma pathologic features via anti-inflammatory effects. Respir. Physiol. Neurobiol. 2022, 299, 103843. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, X.; Diao, W.; Qi, X.; Bai, Y.; Yu, X.; Li, L.; Fang, H.; Chen, Z.; Liu, Q.; et al. Comparative transcriptome analysis revealed candidate genes involved in fruiting body development and sporulation in Ganoderma lucidum. Arch. Microbiol. 2022, 204, 514. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Peng, R.H.; Xiong, A.S.; Tian, Y.; Zhao, W.; Xu, H.; Liu, D.T.; Chen, J.M.; Yao, Q.H. Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris. Mol. Biol. Rep. 2012, 39, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Huang, Z.X.; Xing, S.H.; Wang, B.Q.; Luo, X.H.; Liu, P.H. Carbon transformation and CO2 emission in cultures during growth of Ganoderma lucidum. J. Hortic. 2019, 46, 2047–2054. [Google Scholar]
- Alfaro, M.; Castanera, R.; Lavín, J.L.; Grigoriev, I.V.; Oguiza, J.A.; Ramírez, L.; Pisabarro, A.G. Comparative and transcriptional analysis of the predicted secretome in the lignocellulose-degrading basidiomycete fungus Pleurotus ostreatus. Environ. Microbiol. 2016, 18, 4710–4726. [Google Scholar] [CrossRef]
- Liu, X. Construction of Composite Bacterial Colony for Efficient Degradation of Corn Stover and Research on Its Degradation Effect; Northeast Agricultural University: Harbin, China, 2019. [Google Scholar]
- Zhang, X.Q.; Wang, Z.F.; Sen, M.Y.; Bai, H.H.; Ta, N. Analysis of crop straw production and comprehensive utilisation in China. J. China Agric. Univ. 2021, 26, 30–41. [Google Scholar]
- Liu, J.M.; Ju, W.; Wu, B.; Liu, L.; Zhan, M.; Wu, P.; Wang, Y.; Liu, S.T. Lignocellulolytic Enzyme Production in Solid-State Fermentation of Corn Stalk with Ammoniation Pretreatment by Lentinus edodes L-8. Bioresources 2014, 9, 1430–1444. [Google Scholar] [CrossRef][Green Version]
- Adebayo, E.A.; Martinez-Carrera, D. Oyster mushrooms (Pleurotus) are useful for utilizing lignocellulosic biomass. Afr. J. Biotechnol. 2015, 14, 52–67. [Google Scholar]
- Hamelinck, C.N.; Hooijdonk, G.V.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-middle-and long-term. Biomass Bioeng. 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Marinovic, M.M.V.; Aguilar-Pontes, M.; Zhou, O.; Miettinen, R.P.V.; Makela, M.R.; Hilden, K. Temporal transcriptome analysis of the white-rot fungus Obba rivulosa shows expression of a constitutive set of plant cell wall degradation targeted genes during growth on solid spruce wood. Fungal Genet. Biol. 2018, 112, 47–54. [Google Scholar] [CrossRef]
- Rytioja, J.; Hildén, K.; Hatakka, A.; Mäkelä, M.R. Transcriptional analysis of selected cellulose-acting enzymes encoding genes of the white-rot fungus Dichomitus squalens on spruce wood and microcrystalline cellulose. Fungal Genet. Biol. 2014, 72, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Valaskova, V. Degradation of cellulose by basidiomycetous fungi. Fems Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Knop, D.; Yarden, O.; Hadar, Y. The ligninolytic peroxidases in the genus Pleurotus: Divergence in activities, expression, and potential applications. Appl. Microbiol. Biotechnol. 2015, 99, 1025–1038. [Google Scholar] [CrossRef]
- Xie, C.L.; Yan, S.W.; Zhang, Z.M.; Gong, W.B.; Zhu, Z.H.; Zhou, Y.J.; Yan, L.; Hu, Z.X.; Ai, L.Z.; Peng, Y.D. Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidum by untargeted metabolomics. Lwt-Food Sci. Technol. 2020, 129, 109494. [Google Scholar] [CrossRef]
- Ma, Z.B.; Ye, C.; Deng, W.W.; Xu, M.M.; Wang, Q.; Liu, G.Q.; Wang, F.; Liu, L.M.; Xu, Z.H.; Shi, G.Y.; et al. Reconstruction and Analysis of a Genome-Scale Metabolic Model of Ganoderma lucidum for Improved Extracellular Polysaccharide Production. Front. Microbiol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.S. Triterpenoid natural-products in the fungus Ganderma lucidum. J. Chin. Chem. Soc. 1992, 39, 669–674. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Jian, X.X.; Lv, Y.B.; Nian, K.Q.; Gao, Q.; Chen, J.; Wei, L.J.; Hua, Q. Enhanced squalene biosynthesis in Yarrowia lipolytica based on metabolically engineered acetyl-CoA metabolism. J. Biotechnol. 2018, 281, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Kwak, S.; Liu, J.J.; Lane, S.; Hua, Q.; Kweon, D.H.; Jin, Y.S. Improved squalene production through increasing lipid contents in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2018, 115, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, Z.H.; Ren, A.; Shi, L.; Shi, D.K.; Li, X.B.; Zhao, M.W. The mitogen-activated protein kinase GlSlt2 regulates fungal growth, fruiting body development, cell wall integrity, oxidative stress and ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2017, 104, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Shi, L.; Hu, Y.R.; Liu, R.; Ren, A.; Zhao, M.W. Roles of the Skn7 response regulator in stress resistance, cell wall integrity and GA biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2018, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Z.; Storms, R.; Tsang, A. Microplate-based filter paper assay to measure total cellulase activity. Biotechnol. Bioeng. 2004, 88, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Coconi-Linares, N.; Magana-Ortiz, D.; Guzman-Ortiz, D.A.; Fernandez, F.; Loske, A.M.; Gomez-Lim, M.A. High-yield production of manganese peroxidase, lignin peroxidase, and versatile peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef]
- Ruiz-Duenas, F.J.; Guillén, F.; Camarero, S.; Pérez-Boada, M.; Martínez, M.J.; Martínez, A.T. Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl. Environ. Microbiol. 1999, 65, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Yang, Y.D.; Jian, Q.L.I.; Song, F.W.U.; Yun, P.Z.H.U.; Yao, W.C.; Fu, H.E. Integrated nr database in Protein Annotation System and Its Localization. Comput. Eng. 2006, 32, 71–73,76. [Google Scholar]
- Renaux, A.; UniProt, C. UniProt: The universal protein knowledgebase (vol 45, pg D158, 2017). Nucleic Acids Res. 2018, 46, 2699. [Google Scholar]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.S.; Liu, J.H.; Li, Y.T.; Yan, L.Y.; Pang, G.C. Establishment of multi-strain co-fermentation system and bioconversion of corn stover. Guangzhou Chem. Ind. 2000, 4, 69–73+27. [Google Scholar]
- Monclaro, A.V.; Ferreira Filho, E.X. Fungal lytic polysaccharide monooxygenases from family AA9: Recent developments and application in lignocelullose breakdown. Int. J. Biol. Macromol. 2017, 102, 771–778. [Google Scholar] [CrossRef]
- Lei, T.C.; Long, J.X.; Tian, C.E. Screening of laccase-producing straw mushroom strains. Ind. Microbiol. 2008, 3, 51–55. [Google Scholar]
- Chen, Q.H.; Zhou, Y.P.; Zhou, Y.P.; Bi, F.S.; Cheng, H.Z.; Tian, C.E. Screening of high yielding strains of fungal laccase. J. Guangzhou Univ. (Nat. Sci. Ed.) 2009, 8, 53–57. [Google Scholar]
- Liu, X.D.; Liu, X.D.; Wang, J.L.; Wang, X. Selection of laccase high-yielding strains by compound mutagenesis of tree tongue Ganoderma lucidum. North. Hortic. 2019, 14, 124–129. [Google Scholar]
- Cui, M.L.; Yang, H.Y.; He, G.Q. Submerged fermentation production and characterization of intracellular triterpenoids from Ganoderma lucidum using HPLC-ESI-MS. J. Zhejiang Univ. Sci. B 2015, 16, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M. Effects of Maize Straw Cultivation of Pleurotus ostreatus and Its Residue on the Growth of Maize Seedling; Tianjin Agricultural College: Tianjin, China, 2018. [Google Scholar]
- Li, Y.L.; Liu, J.H.; Wang, G.; Yang, M.Y.; Yang, X.; Li, T.B.; Chen, G. De novo transcriptome analysis of Pleurotus djamor to identify genes encoding CAZymes related to the decomposition of corn stalk lignocellulose. J. Biosci. Bioeng. 2019, 128, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Aguilar-Pontes, M.V.; Hainaut, M.; Henrissat, B.; Hilden, K.; Makela, M.R.; de Vries, R.P. Comparative analysis of basidiomycete transcriptomes reveals a core set of expressed genes encoding plant biomass degrading enzymes. Fungal Genet. Biol. 2017, 112, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, G.R.; Davies, G.J.; Walton, P.H. Recent insights into copper-containing lytic polysaccharide mono-oxygenases. Curr. Opin. Struct. Biol. 2013, 23, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, G.R.; Johnston, E.M.; Davies, G.J.; Walton, P.H. Lytic Polysaccharide Monooxygenases in Biomass Conversion. Trends Biotechnol. 2015, 33, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Salame, T.M.; Knop, D.; Levinson, D.; Mabjeesh, S.J.; Yarden, O.; Hadar, Y. Release of Pleurotus ostreatus Versatile-Peroxidase from Mn2+ Repression Enhances Anthropogenic and Natural Substrate Degradation. PLoS ONE 2012, 7, e52446. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Boada, M.; Ruiz-Dueñas, F.J.; Pogni, R.; Basosi, R.; Choinowski, T.; Martínez, M.J.; Piontek, K.; Martínez, A.T. Versatile peroxidase oxidation of high redox potential aromatic compounds: Site-directed mutagenesis, spectroscopic and crystallographic investigation of three long-range electron transfer pathways. J. Mol. Biol. 2005, 354, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Li, N.; Yu, X.Y.; Zhao, P.; Li, T.; Xu, J.W. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynthesis in Ganoderma lingzhi. Phytochemistry 2017, 134, 46–53. [Google Scholar] [CrossRef]
- Feng, Z.R.; Feng, Z.R.; Li, H.J.; Xu, J.W. Ganoderic Acid Accumulation and Biosynthetic Gene Expression during Fruiting Body Development in Ganoderma lucidum. In Proceedings of the 2015 Asia-Pacific Energy Equipment Engineering Research Conference (ap3er 2015), Zhuhai, China, 13–14 June 2015; pp. 354–358. [Google Scholar]
- Zhou, J.S.; Ji, S.L.; Ren, M.F.; He, Y.L.; Jing, X.R.; Xu, J.W. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem. Eng. J. 2014, 90, 178–183. [Google Scholar] [CrossRef]
- Ma, L.; Chen, L.; Zhang, L.; Zou, G.; Liu, R.; Jiang, Y.P.; Zhou, Z.H. RNA Sequencing Reveals Xyr1 as a Transcription Factor Regulating Gene Expression beyond Carbohydrate Metabolism. Biomed Res. Int. 2016, 2016, 4841756. [Google Scholar] [CrossRef] [PubMed]





| Gene ID | Description | Forward Primer Sequences (5′-3′) | Reverse Primer Sequences (5′-3′) |
|---|---|---|---|
| DN4778_c0_g1 | Endo-1,4-beta-xylanase 3 | TGAAGAACCTTGCTGCACTC | TCTCAAAGACACAAGTCGAGC |
| DN1646_c0_g1 | Endo-1,4-beta-xylanase A | GGGACTTCACCGACAAGTATTC | TGCCACGATACCGTTGTAC |
| DN5168_c0_g1 | Manganese dependent endoglucanase Eg5A | TGGTTCAGCAACTTCTACGAC | CAGACGCAAACTGGTCATTG |
| DN38_c0_g1 | Exoglucanase 1 | CTGGATGGTGCTGACTACG | AATATCTCGTACTTGGCGTCG |
| DN377_c2_g1 | Manganese transporter pdt1 | TCATCTTCGCACTCGCAC | GCCGATGAGTCTGGTGATAAT |
| DN3627_c0_g2 | Beta-xylanase | TTAACCAGCTCAACGGTCC | CACCAAAAGCGATGCAAGAG |
| DN682_c2_g1 | Laccase 1 | TCGTGGTCAATGGTGTCTTC | GTTCGTGCCCTTTTGGAAG |
| DN2037_c0_g1 | Laccase C | TCACTGCCACATTGACTGG | TGGGATCGTTTGCTATGGAC |
| Sample | Read No. | Clean Read No. | Clean Read % | N (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| CK_1 | 49,716,670 | 47,483,608 | 95.5 | 0.001485 | 98.32 | 95.15 |
| CK_2 | 44,655,274 | 42,407,348 | 94.96 | 0.001512 | 98.32 | 95.23 |
| CK_3 | 51,196,280 | 48,724,776 | 95.17 | 0.001471 | 98.33 | 95.25 |
| LM_1 | 48,361,614 | 46,107,672 | 95.33 | 0.001457 | 98.3 | 95.15 |
| LM_2 | 44,591,096 | 42,515,008 | 95.34 | 0.001436 | 98.28 | 95.08 |
| LM_3 | 54,795,248 | 52,157,262 | 95.18 | 0.00147 | 98.32 | 95.17 |
| CH_1 | 43,475,624 | 41,499,254 | 95.45 | 0.00157 | 98.09 | 94.67 |
| CH_2 | 44,928,998 | 42,852,550 | 95.37 | 0.001836 | 97.85 | 94.05 |
| CH_3 | 43,479,448 | 41,455,396 | 95.34 | 0.001553 | 98.06 | 94.53 |
| Total Length (bp) | Sequence Number | Max. Length (bp) | Mean Length (bp) | N50 (bp) | N90 (bp) | GC% | |
|---|---|---|---|---|---|---|---|
| Transcript | 167,022,854 | 81,677 | 15,429 | 2044.92 | 2800 | 1089 | 57.07 |
| Unigene | 29,277,836 | 18,157 | 15,429 | 1612.48 | 2661 | 654 | 56.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, J.; Fan, Q.; Wang, S.; Sun, C.; Yan, M. Transcriptome Analysis of Ganoderma lingzhi Liquid Fermentation Process Using Corn Straw as Matrix. Agriculture 2024, 14, 1271. https://doi.org/10.3390/agriculture14081271
Wang S, Li J, Fan Q, Wang S, Sun C, Yan M. Transcriptome Analysis of Ganoderma lingzhi Liquid Fermentation Process Using Corn Straw as Matrix. Agriculture. 2024; 14(8):1271. https://doi.org/10.3390/agriculture14081271
Chicago/Turabian StyleWang, Sheng, Jintao Li, Qi Fan, Shufang Wang, Changwei Sun, and Meixia Yan. 2024. "Transcriptome Analysis of Ganoderma lingzhi Liquid Fermentation Process Using Corn Straw as Matrix" Agriculture 14, no. 8: 1271. https://doi.org/10.3390/agriculture14081271
APA StyleWang, S., Li, J., Fan, Q., Wang, S., Sun, C., & Yan, M. (2024). Transcriptome Analysis of Ganoderma lingzhi Liquid Fermentation Process Using Corn Straw as Matrix. Agriculture, 14(8), 1271. https://doi.org/10.3390/agriculture14081271





