Research on Real-Time Groundwater Quality Monitoring System Using Sensors around Livestock Burial Sites
Abstract
1. Introduction
2. Materials and Methods
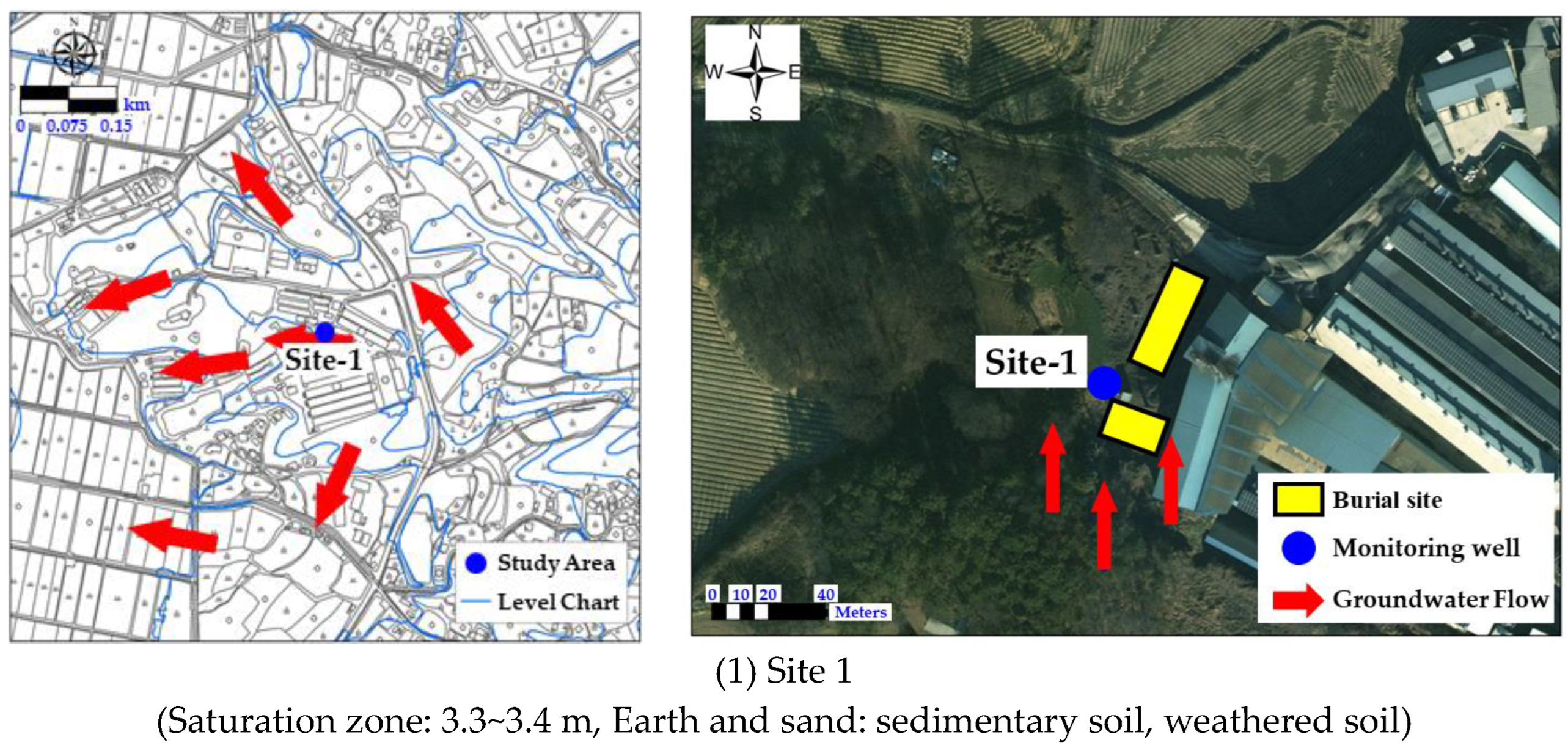
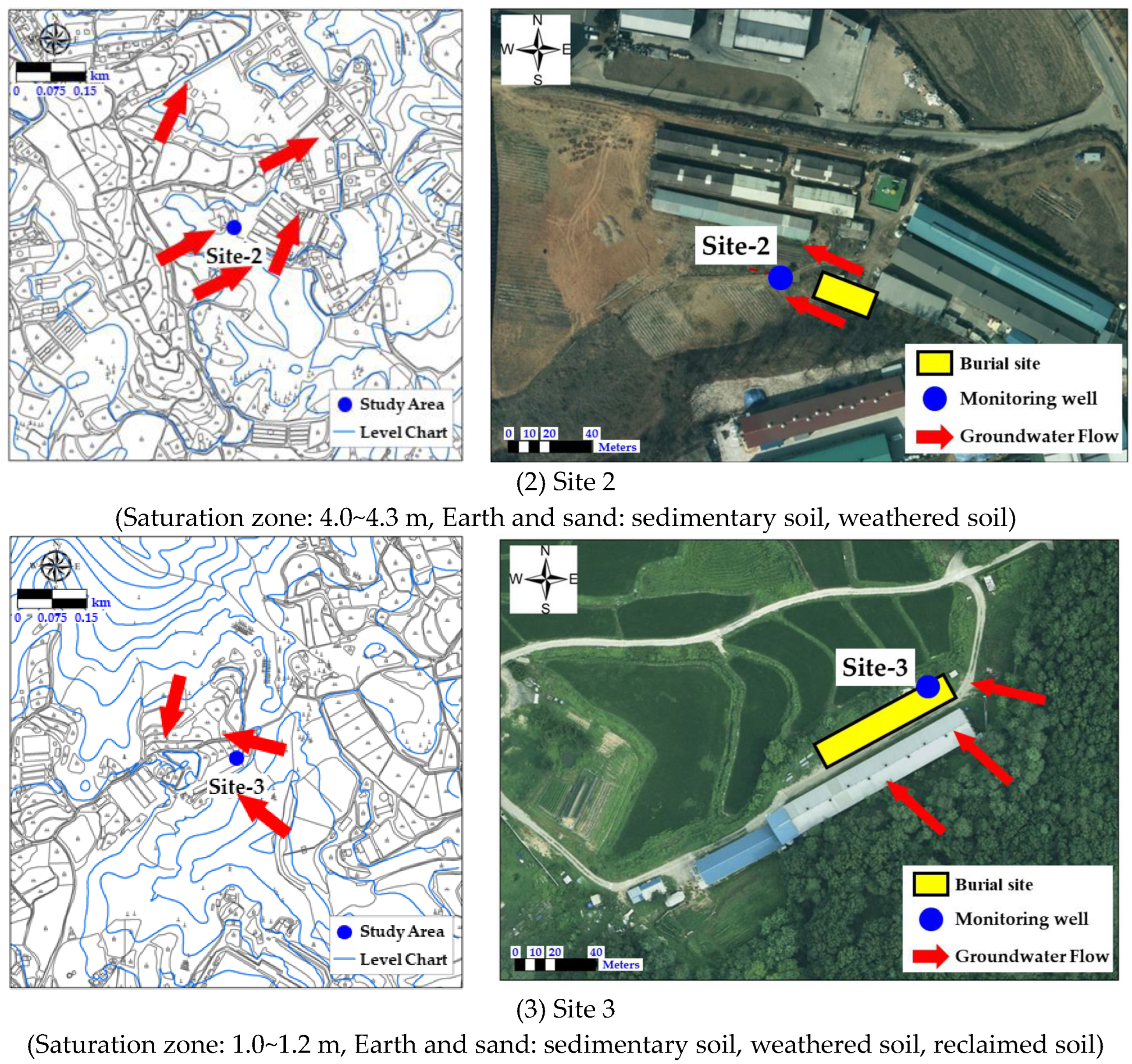
2.1. Characteristics of the Study Area
2.2. Sensor and Analytical Parameter Selection
2.3. Sensor Calibration Evaluation
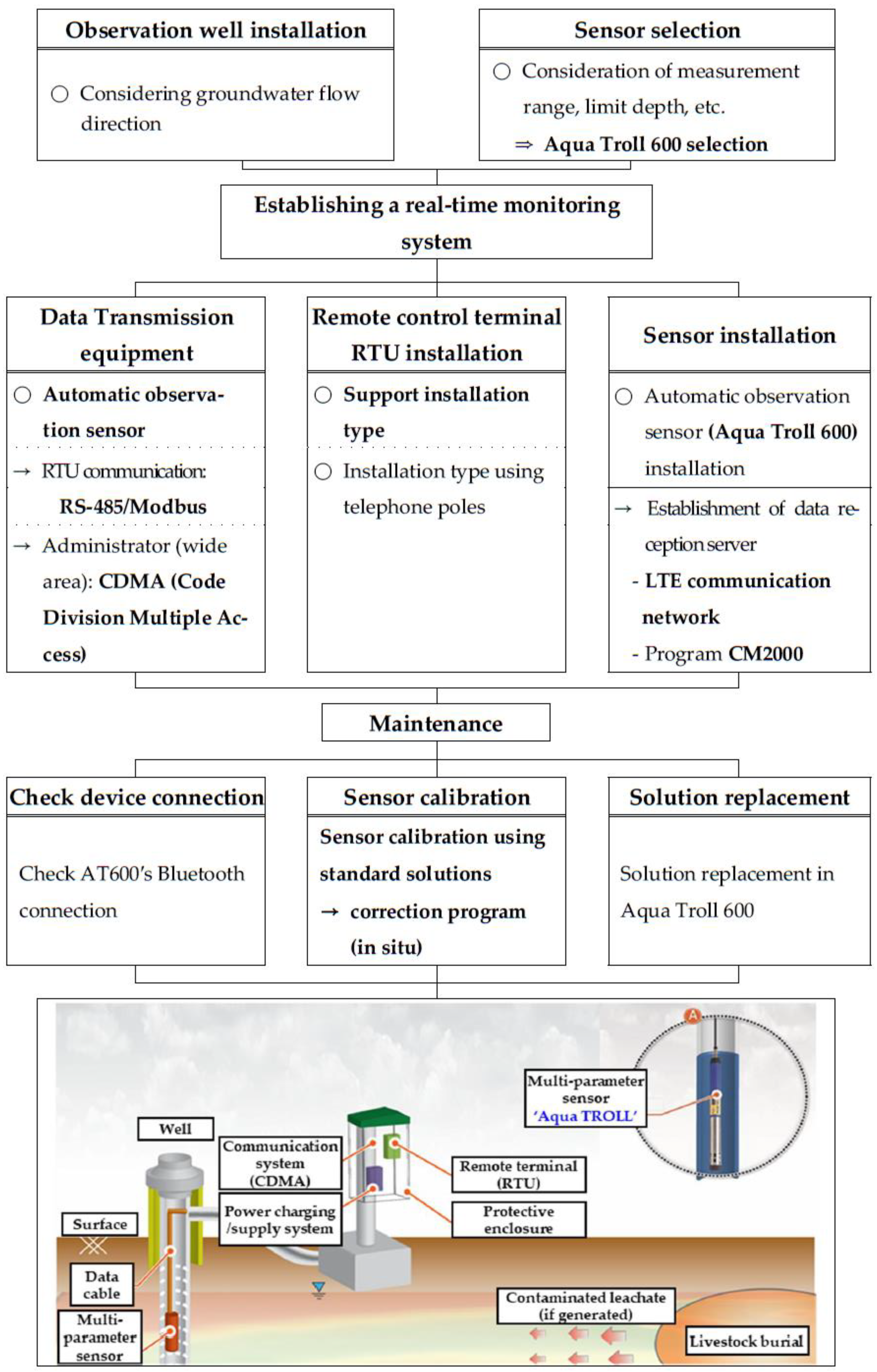
2.4. Sensor Installation and Real-Time Monitoring System Establishment
2.5. Groundwater Sample Collection
2.6. Groundwater Sample Analysis Methods
2.7. Statistical Interpretation Method
3. Results and Discussion
3.1. Sensor Quality Control and Correlation Analysis with EC through Indoor Experiments
3.1.1. Nitrate Nitrogen (NO3-N)
3.1.2. Ammonium Nitrogen (NH4-N)
3.1.3. Chloride (Cl)
3.1.4. Multiple (Two or More) Standard Substance Injections for Electrical Conductivity Change Experiment
3.1.5. Performance Verification Evaluation of Ion-Selective Electrodes (ISEs) and Maintenance Response Review
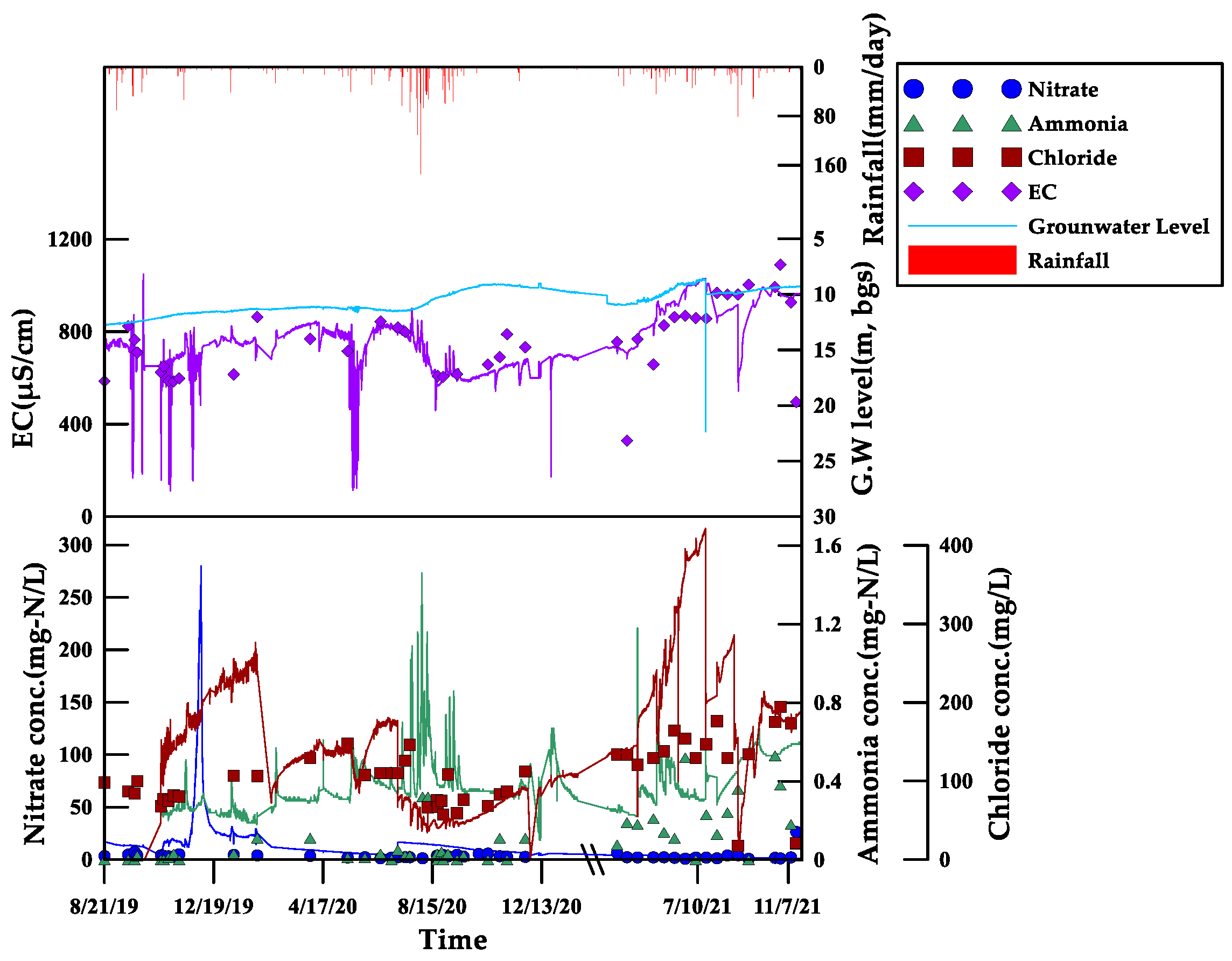
3.2. Groundwater Monitoring Results for Each Parameter Using Sensors
3.2.1. Electrical Conductivity (EC)
3.2.2. Nitrate Nitrogen (NO3-N)
3.2.3. Ammonia Nitrogen (NH4-N)
3.2.4. Chloride (Cl)
3.3. Comparative Evaluation of Sensor Accuracy in Measuring Field Concentrations
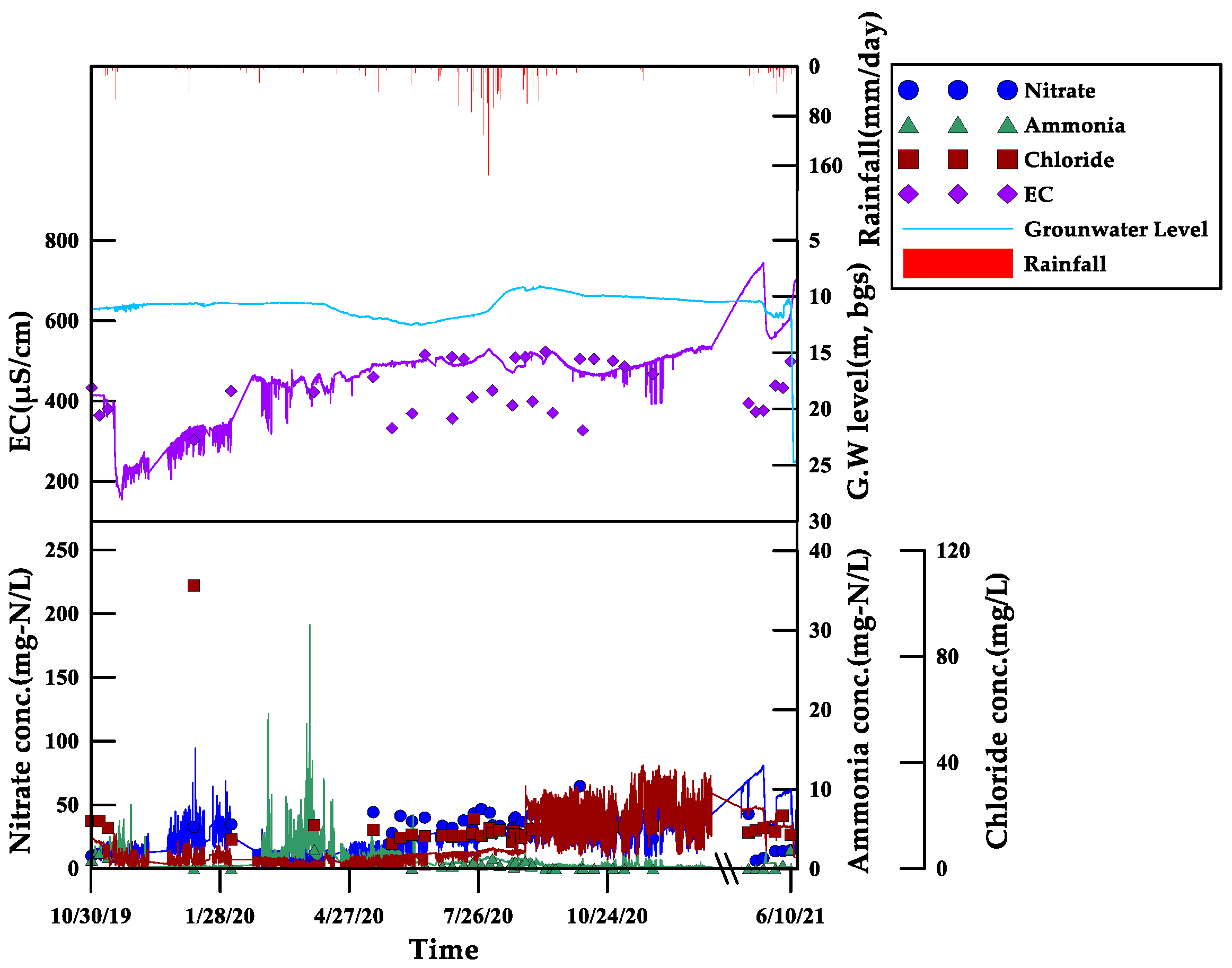
3.3.1. Monitoring Results for Site 1
3.3.2. Monitoring Results for Site 2
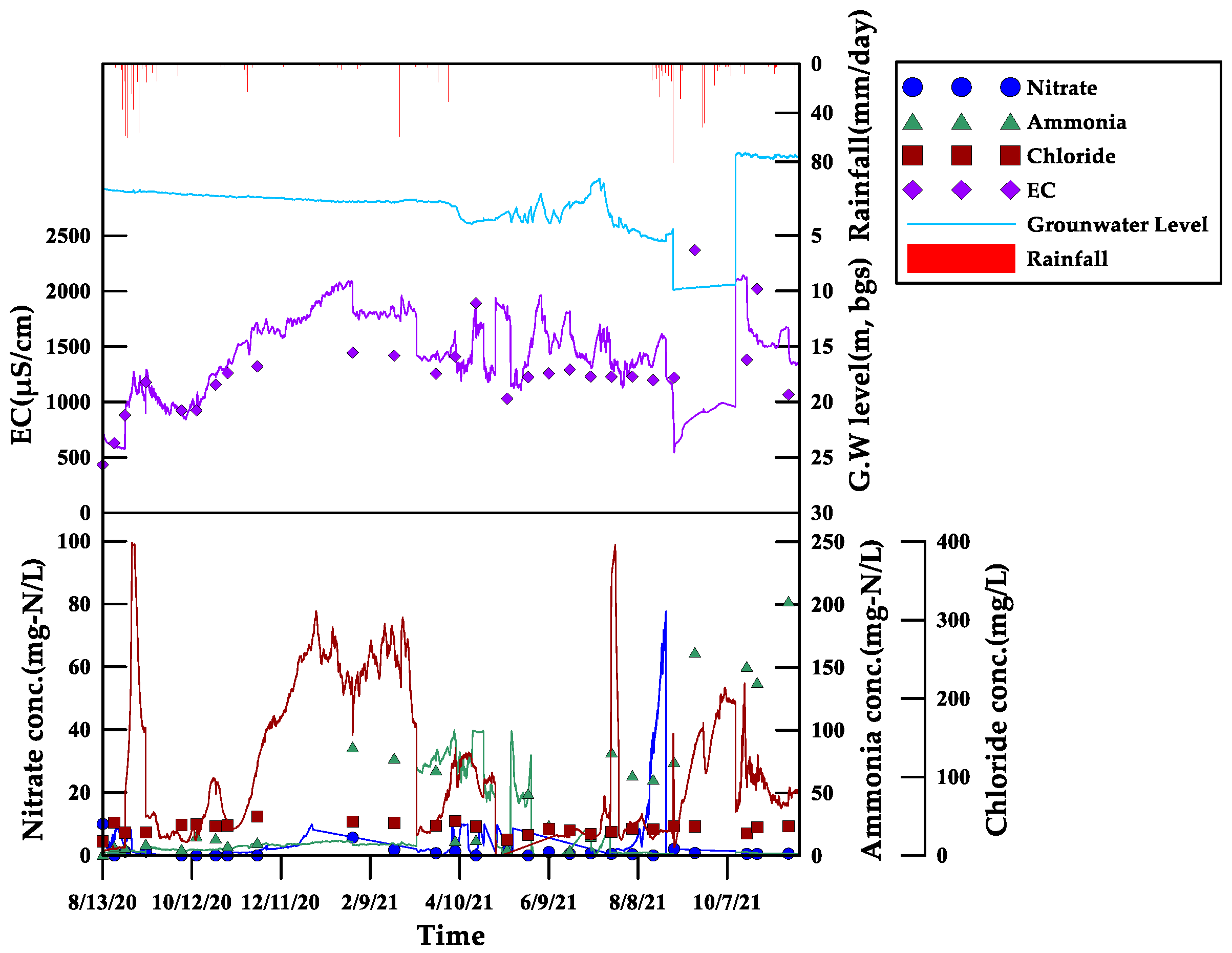
3.3.3. Monitoring Results for Site 3
3.4. Statistical Correlation Analysis using Analysis Parameters
3.5. Electrical Conductivity Characteristics in Groundwater
4. Conclusions
- Four parameters (EC, Cl, NO3-N, NH4-N) that can be used to assess the possibility of groundwater leachate discharge were selected. A sensor (Aqua Troll 600) was chosen for this study by considering factors such as the simultaneous analysis of the target parameters, the measurement range, the measurement limits, etc. The results of the quality control for sensor measurement reliability showed the following ranges for accuracy and precision: Cl: [accuracy] 99.3~100.0% and [precision] 0.1~4.0%; NO3-N: [accuracy] 93.3~104.1% and [precision] 0.5~5.0%; and NH4-N: [accuracy] 101.3~101.6% and [precision] 1.1~1.6%. These results meet the criteria set by domestic water quality testing standards, which require accuracy to be within 75~125% and precision to be within ±25%. As a result, the reliability of establishing a real-time monitoring system using the sensor was ensured.
- Three areas with livestock burial sites were selected as pilot areas, and a real-time monitoring system was established. The feasibility of the on-site application was evaluated. When compared to the laboratory measurement value, the field measurement value by the sensors was 1.1 times higher for EC, 1.6 times higher for Cl, and 2.5 times higher for NO3-N. Among the four parameters, the EC showed the closest similarity to the patterns of internal and external environmental changes caused by rainfall and agricultural activities. In addition, the results for EC were more reliable than for other parameters, with a relatively small error rate during the monitoring period.
- The correlation analysis between the laboratory analysis measurements and the sensor measurement results showed that the EC had the highest correlation coefficient, at 0.3834. In addition, the factor extraction results showed that the EC showed a relatively significant correlation compared to the other three parameters. This confirms that the EC is a key indicator, as supported by previous research, for indicating leachate discharge from livestock burial sites. These results suggest that the data can be used as a valuable foundation for establishing an immediate response system to incidents of leachate discharge as livestock burial sites expand in the future.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kim, H.K.; Park, S.H.; Kim, M.S.; Kim, H.J.; Lee, M.K.; Lee, G.-M.; Kim, S.-H.; Yang, J.-H.; Kim, T.S. Contamination characteristics of agricultural groundwater around Livestock burial areas in Korea. J. Eng. Geol. 2014, 24, 237–246. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; An, S.-W.; Min, J.-E.; Park, J.-W. Selection of coagulant and optimum condition for carcasses landfill leachate. In Proceedings of the Korean Society of Geotechnical and Environmental Engineering Conference, Rhodes, Greece, 17–23 June 2012; pp. 91–94. [Google Scholar]
- Koh, E.-H.; Kaown, D.; Kim, H.J.; Leea, K.-K.; Kimb, H.; Parkb, S. Nationwide groundwater monitoring around infectious-disease-caused livestock mortality burials in Korea: Superimposed influence of animal leachate on pre-existing anthropogenic pollution. Environ. Int. 2019, 129, 376–388. [Google Scholar][Green Version]
- Anderson, I. Foot and Mouth Disease 2007: A Review and Lessons Learned; Report to the Prime Minister and the Secretary of State for Environment Food and Rural Affairs: London, UK, 2008. Available online: https://www.gov.uk/government/publications/foot-and-mouth-disease-2007-a-review-and-lessons-learned (accessed on 10 June 2024).
- National Audit Office. The 2001 Outbreak of Foot and Mouth Disease; 2002. Available online: https://www.nao.org.uk/wp-content/uploads/2002/06/0102939.pdf (accessed on 2 July 2024).
- Muroga, N.; Hayama, Y.; Yamamoto, T.; Kurogi, A.; Tsuda, T.; Tsutsui, T. The 2010 foot-and-mouth disease epidemic in Japan. J. Vet. Med. Sci. 2012, 74, 399–404. [Google Scholar] [PubMed][Green Version]
- MAF BioSecurity Authority. Construction Specifications for Carcass Burial Facilities; Ministry of Agriculture and Forestry (MAF): New Zealand, 2005.
- Choi, N.-C.; Choi, E.-J.; Kim, B.-J.; Park, J.-A.; Kim, S.-B.; Park, C.-Y. Characterization of Water Quality and Bacteria of Leachate from Animal Carcass Disposal on the Disposal Lapse Time. Econ. Environ. Geol. 2013, 23, 37–46. [Google Scholar][Green Version]
- Chang, H.; Moon, B.; Yoon, S.; Jin, T. Development and performance evaluation of multiple sensor for Groundwater Quality Monitoring and Remote Control System using IoT. J. Korea Inst. Inf. Commun. Eng. 2017, 21, 1957–1963. [Google Scholar][Green Version]
- Parra, L.; Sendra, S.; Lloret, J.; Bosch, I. Development of a conductivity sensor for monitoring groundwater resources to optimize water management in smart city environments. Sensors 2015, 15, 20990–21015. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Partheeban, P.; Baskaran, A.; Krishnan, D.; Sridhar, M. Wireless sensor network technology and geospatial technology for groundwater quality monitoring. J. Ind. Inf. Integr. 2024, 38, 100569. [Google Scholar]
- Burbery, L.; Abraham, P.; Wood, D.; de Lima, S. Applications of a UV optical nitrate sensor in a surface water/groundwater quality field study. Environ. Monit. Assess. 2021, 193, 303. [Google Scholar] [PubMed]
- Lim, W.-s.; Hyun, M.-h.; Gyubeom, K. New development and application of a remote controlled groundwater monitoring equipment. J. Geol. 2014, 50, 689–696. [Google Scholar]
- Calderwood, A.J.; Pauloo, R.A.; Yoder, A.M.; Fogg, G.E. Low-cost, open source wireless sensor network for real-time, scalable groundwater monitoring. Water 2020, 12, 1066. [Google Scholar] [CrossRef]
- Kim, G.-J.; Se-Hoon, J.; Shim, C.-b. Implementation of user interface and geoSensor based traveling Type Sub-observation prototype system for monitoring of groundwater. J. Korea Comput. Inf. Soc. 2012, 17, 183–192. [Google Scholar]
- Chi-hyeong, L.; Kim, G.-S.; Choi, J.; Lee, H.; Park, R.; Yong-soon, P. Development of a remote integrated management system for groundwater near livestock burial sites. J. Korean Soc. Geotech. Eng. 2014, 2014, 213–214. [Google Scholar]
- Kim, H.K.; Kim, K.H.; Yun, S.T.; Oh, J.; Kim, H.R.; Park, S.H.; Kim, M.S.; Kim, T.S. Probabilistic assessment of potential leachate leakage from livestock mortality burial pits: A supervised classification approach using a Gaussian mixture model (GMM) fitted to a groundwater quality monitoring dataset. Process Saf. Environ. Prot. 2019, 129, 326–338. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Wei, G.H. The design and application of the groundwater monitoring system based on the internet of things in the HeiHe river basin. Appl. Mech. Mater. 2014, 511, 319–325. [Google Scholar] [CrossRef]
- Lee, I.-j. Development of a Real-Time Monitoring System to Prevent Pre-Contamination in Areas Concerned about Groundwater Contamination; GAIA Project technical data book; Korea Environmental Industry and Technology Institute: Seoul, Republic of Korea, 2011.
- USGS. National Field Manual for the Collection of Water-Quality Data, 6.0 Guidelines for Field-Measured Water-Quality Properties. 2010. Available online: https://pubs.usgs.gov/publication/tm9A6.0 (accessed on 2 July 2024).
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Lee, H.-S. SPSS 18.0 Manual; Jhjbook Publishing: Seoul, Republic of Korea, 2011; pp. 362–389. [Google Scholar]
- Bjerg, P.L.; Ruegge, K.; Pedersen, J.K.; Christensen, T.H. Distribution of redox-sensitive groundwater quality parameters downgradient of a landfill (Grindsted, Denmark). Environ. Sci. Technol. 1995, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.J.; Lee, J.Y. A study on the characteristics of landfill gas and leachate in Nanjido after constructed final cover system. J. Korea Soc. Waste Manag. 2007, 24, 744–751. [Google Scholar]
- Huh, I.-R.; Kim, K.-W.; Choi, G.-J.; Lee, T.-S. Monitoring water quality of groundwater observation wells near foot-and-mouth disease livestock burial sites. J. Korean Soc. Environ. Health 2014, 40, 47–54. [Google Scholar] [CrossRef]
- Kaown, D.; Hyun, Y.; Bae, G.O.; Lee, K.K. Factors affecting the spatial pattern of nitrate contamination in shallow groundwater. J. Environ. Qual. 2007, 36, 1479–1487. [Google Scholar] [CrossRef]
- Son, J.K.; Kwon, T.K.; Kong, M.J.; Kang, T.G.; Lim, R.G.; Kim, N.C.; Park, M.J. Measurement of groundwater penetration and Water Balance Analysis according to Land Use Materials. J. Korea Acad. -Ind. Coop. Soc. 2021, 22, 613–622. [Google Scholar]
- Azhdarpoora, A.; Radfarda, M.; Pakdelb, M.; Abbasniac, A.; Badeenezhadd, A.; Mohammadie, A.A.; Yousefi, M. Assessing fluoride and nitrate contaminants in drinking water resources and their health risk assessment in a semiarid region of southwest Iran. Desalination Water Treat. 2019, 149, 43–51. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Muniraj, K.; Duraisamy, K.; Pande, C.; Karuppannan, S.; Thomas, M. An integrated approach to explore the suitability of nitrate-contaminated groundwater for drinking purposes in a semiarid region of India. Environ. Geochem. Health 2023, 45, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Cellone, F.; Carol, E.; Pugliese, I.; Cordoba, J.; Butler, L.; Lamarche, L. Nitrate pollution in dairy farms and its impact on groundwater quality in a sector of the Pampas plain, Argentina. Environ. Earth Sci. 2020, 79, 1–7. [Google Scholar] [CrossRef]
- Zghibi, A.; Tarhouni, J.; Zouhri, L. Assessment of seawater intrusion and nitrate contamination on the groundwater quality in the Korba coastal plain of Cap-Bon (North-east of Tunisia). J. Afr. Earth Sci. 2013, 87, 1–12. [Google Scholar]


| Division | Time of Burial | Livestock Burial (ea) | Well Depth (m) | Average Water Level (m) | Observation Period | Rainfall (mm/month) |
|---|---|---|---|---|---|---|
| Site 1 | 2016 | Laying hen 292,950 | 20.0 | 10.5 | Observation Period of Site-1: August 2019–November 2021 (27 month) | 107.1 |
| Site 2 | 2016 | Laying hen 54,029 | 20.0 | 10.3 | Observation Period of Site-2: October 2019–November 2021 (26 month) | 94.6 |
| Site 3 | 2014 | Laying hen 109,502 | 15.0 | 1.2 | Observation Period of Site-3: August 2019–November 2021 (27 month) | 61.8 |
| Division | Aqua TROLL 600 | EXO1 | DS 5 | |
|---|---|---|---|---|
| Sensor source | Manufacturer | In situ | YSI | HYDROLAB |
| Standard | Diameter: 47 mm Length: 59.16 cm | Diameter: 4 mm Length: 64.77 cm | Diameter 44 mm Length 74.9 cm | |
| Weight | 1.45 kg | 1.42 kg | 1.3 kg | |
| Temp Range | −5~50 °C | −5~45 °C | −5~50 °C | |
| Maximum installation depth | 0.21 MPa (NH4-N, NO3-N) | |||
| Communication method | RS485/MODBUS, SDI-12, Bluetooth | RS232/SDI-12/Bluetooth | RS-232, SDI-12, RS-485 | |
| Simultaneous measurable item | Temp., pressure, water level, pH, ORP, EC, turbidity, TDS, salinity, NH4-N, NO3-N, Cl− | |||
| Measurement limit depth | 25 M | 15 M | 15 M | |
| External material | PVC·Titanium | PVC | PVC | |
| Application range for each sensor measurement item | EC | - Range: 0~350 μS/cm - Error: ± 0.5% (of reading + 0.001 μS/cm) | - Range: 0~200 μS/cm - Error: ± 0.5% (of reading + 0.001 μS/cm) | - Range: 0~100 μS/cm - Error: ± 0.5% (of reading + 0.001 μS/cm) |
| Cl− | - Range: 0~150,000 mg/L - Error: ±10% (of reading or 2 mg/L) | - Range: 0.5~18,000 mg/L - Error: ±15% (of reading or 5 mg/L) | - Range: 0.5~18,000 mg/L - Error: ±5% (of reading or 2 mg/L) | |
| NO3-N | - Range: 0~40,000 mg-N/L - Error: ±10% (of reading or 2 mg/L) | - Range: 0~200 mg-N/L - Error: ±10% (of reading or 2 mg/L) | - Range: 0~100 mg/L - Error: ±5% (of reading or 2 mg/L) | |
| NH4-N | - Range: 0~10,000 mg/L - Error: ±10% (of reading or 2 mg/L) | - Range: 10~200 mg/L - Error: ±10% (of reading or 2 mg/L) | - Range: 0~100 mg/L - Error: ±5% (of reading or 2 mg/L) | |
| Reference | https://in-situ.com/en/aqua-troll-600-multiparameter-sonde (2 November 2020) | https://www.ysi.com/exo1 (2 November 2020) | https://www.ott.com/en-uk/products/water-quality-106/hydrolab-ds5-multioarameter-data-sonde-2348/ (2 November 2020) | |
| Standard Direct Field Measurement | Stabilization Criteria for Measurements (Variability Should Be within the Value Shown) | |
|---|---|---|
| Temperature | Thermistor thermometer | ±0.2 °C |
| Conductivity | when ≤100 μS/cm | ±5% |
| when >100 μS/cm | ±3% | |
| pH | Meter displays to 0.01 | ±0.1 unit |
| Dissolved oxygen | Amperometric method | ±0.3 mg/L |
| Concentration (mg/L) | Sensor Measurement Parameters | 1st | 2nd | 3rd | Average | Deviation | Accuracy (%) | Precision (%) |
|---|---|---|---|---|---|---|---|---|
| 0 (D·I water) | NO3-N (mg/L) | 0 | - | - | - | |||
| EC (μS/cm) | 0.01 | - | - | - | ||||
| 1 | NO3-N (mg/L) | 1.0 | 1.1 | 1.0 | 1.0 | 0.1 | 104.1 | 5.0 |
| EC (μS/cm) | 0.012 | 0.013 | 0.013 | 0.013 | - | - | - | |
| 50 | NO3-N (mg/L) | 47.0 | 46.6 | 46.4 | 46.6 | 0.3 | 93.3 | 0.5 |
| EC (μS/cm) | 0.460 | 0.460 | 0.460 | 0.460 | - | - | - | |
| 100 | NO3-N (mg/L) | 100.3 | 99.0 | 99.5 | 99.5 | 0.7 | 99.2 | 0.7 |
| EC (μS/cm) | 0.903 | 0.903 | 0.903 | 0.903 | - | - | - | |
| Concentration (mg/L) | Sensor Measurement Parameters | 1st | 2nd | 3rd | Average | Deviation | Accuracy (%) | Precision (%) |
|---|---|---|---|---|---|---|---|---|
| 0 (D·I water) | NH4-N (mg/L) | 0 | - | - | - | |||
| EC (μS/cm) | 0.01 | - | - | - | ||||
| 1 | NH4-N (mg/L) | 0.98 | 1.00 | 1.00 | 0.99 | 0.0 | 101.3 | 1.2 |
| EC (μS/cm) | 0.013 | 0.013 | 0.013 | 0.013 | - | - | - | |
| 50 | NH4-N(mg/L) | 49.9 | 50.7 | 51.5 | 50.7 | 0.8 | 101.6 | 1.6 |
| EC (μS/cm) | 0.484 | 0.484 | 0.484 | 0.484 | - | - | - | |
| 100 | NH4-N (mg/L) | 100.0 | 101.3 | 102.2 | 101.6 | 1.1 | 101.6 | 1.1 |
| EC (μS/cm) | 0.942 | 0.942 | 0.942 | 0.942 | - | - | - | |
| Concentration (mg/L) | Sensor Measurement Parameters | 1st | 2nd | 3rd | Average | Deviation | Accuracy (%) | Precision (%) |
|---|---|---|---|---|---|---|---|---|
| 0 (D·I water) | Cl (mg/L) | 0 | - | - | - | |||
| EC (μS/cm) | 0.01 | - | - | - | ||||
| 5 | Cl (mg/L) | 5 | 5 | 5 | 5 | 0.2 | 100.0 | 4.0 |
| EC (μS/cm) | 0.019 | 0.019 | 0.019 | 0.019 | - | - | - | |
| 150 | Cl (mg/L) | 158 | 157 | 156 | 157 | 1.1 | 99.3 | 0.7 |
| EC (μS/cm) | 0.499 | 0.499 | 0.499 | 0.499 | - | - | - | |
| 250 | Cl (mg/L) | 246 | 244 | 243 | 244 | 1.3 | 99.4 | 0.5 |
| EC (μS/cm) | 0.734 | 0.734 | 0.734 | 0.734 | - | - | - | |
| 500 | Cl (mg/L) | 500 | 499 | 499 | 499 | 0.7 | 99.9 | 0.1 |
| EC (μS/cm) | 1.451 | 1.451 | 1.451 | 1.451 | - | - | - | |
| Concentration (mg/L) | EC (μS/cm) | Average (μS/cm) | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| D·I water | 0.01 | - | ||||
| ① | NO3-N NH4-N | 2 mg/L 2 mg/L | 0.046 | 0.046 | 0.046 | 0.046 |
| ② | NO3-N Cl | 2 mg/L 10 mg/L | 0.054 | 0.054 | 0.054 | 0.054 |
| ③ | NH4-N Cl | 2 mg/L 10 mg/L | 0.054 | 0.054 | 0.054 | 0.054 |
| ④ | NO3-N NH4-N Cl | 2 mg/L 2 mg/L 10 mg/L | 0.071 | 0.071 | 0.071 | 0.071 |
| Parameter | Period | Sensor Monitoring Result (Average, Min~Max) | ||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | ||
| EC (μS/cm) | 2019 | 693.6 (112.0~1048.0) | 290.4 (154.0~415.0) | - |
| 2020 | 715.3 (114.0~901.0) | 470.0 (255.0~538.0) | 1243.8 (573.0~1841.0) | |
| 2021 | 875.7 (543.0~1030.0) | 628.8 (526.0~745.0) | 1497.2 (543.0~2142.0) | |
| Total | 775.5 (112.0~1048.0) | 515.8 (154.0~745.0) | 1420.7 (543.0~2142.0) | |
| NO3-N (mg/L) | 2019 | 31.0 (3.8~279.7) | 13.8 (0.3~44.8) | - |
| 2020 | 11.0 (4.0~31.5) | 21.8 (0.1~94.5) | 1.7 (0.1~9.9) | |
| 2021 | 3.2 (1.0~5.6) | 39.5 (3.5~89.8) | 4.9 (0.1~77.6) | |
| Total | 10.6 (1.0~279.7) | 27.6 (0.1~94.5) | 3.5 (0.1~77.6) | |
| NH4-N (mg/L) | 2019 | 0.262 (0.200~0.510) | 1.224 (0.150~8.070) | - |
| 2020 | 0.378 (0.100~1.460) | 1.057 (0.160~30.670) | 6.040 (2.060~10.470) | |
| 2021 | 0.419 (0.220~1.180) | 0.475 (0.000~2.110) | 16.735 (0.000~99.940) | |
| Total | 0.386 (0.100~1.460) | 0.854 (0.000~30.670) | 13.451 (0.000~99.940) | |
| Cl (mg/L) | 2019 | 101.3 (0.2~243.7) | 4.8 (0.4~15.8) | - |
| 2020 | 111.9 (0.2~276.5) | 9.6 (0.1~39.0) | 96.4 (6.0~398.6) | |
| 2021 | 205.8 (10.4~421.6) | 19.7 (2.9~51.3) | 120.1 (0.0~396.1) | |
| Total | 147.2 (0.2~421.6) | 13.0 (0.1~51.3) | 112.3 (0.0~398.6) | |
| Variable | Site 1 | Site 2 | Site 3 | |||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | Factor 1 | Factor 2 | |
| Cl | 0.945 | −0.201 | 0.781 | 0.210 | 0.851 | −0.282 |
| EC | 0.884 | 0.341 | 0.839 | 0.142 | 0.695 | 0.551 |
| NH4-N | −0.104 | 0.845 | −0.112 | −0.940 | 0.070 | 0.601 |
| NO3-N | −0.151 | −0.679 | 0.524 | 0.632 | −0.208 | 0.618 |
| Eigenvalue | 1.709 | 1.333 | 1.601 | 1.347 | 1.255 | 1.127 |
| Variance explained (%) | 42.736 | 33.316 | 40.023 | 33.671 | 31.371 | 28.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Park, S.; Han, K. Research on Real-Time Groundwater Quality Monitoring System Using Sensors around Livestock Burial Sites. Agriculture 2024, 14, 1278. https://doi.org/10.3390/agriculture14081278
Yoon J, Park S, Han K. Research on Real-Time Groundwater Quality Monitoring System Using Sensors around Livestock Burial Sites. Agriculture. 2024; 14(8):1278. https://doi.org/10.3390/agriculture14081278
Chicago/Turabian StyleYoon, Jonghyun, Sunhwa Park, and Kyungjin Han. 2024. "Research on Real-Time Groundwater Quality Monitoring System Using Sensors around Livestock Burial Sites" Agriculture 14, no. 8: 1278. https://doi.org/10.3390/agriculture14081278
APA StyleYoon, J., Park, S., & Han, K. (2024). Research on Real-Time Groundwater Quality Monitoring System Using Sensors around Livestock Burial Sites. Agriculture, 14(8), 1278. https://doi.org/10.3390/agriculture14081278





