A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives
Abstract
1. Introduction
2. Methods
3. Pathogens Involved and Severity of Clinical Signs in Mastitis
4. Losses Associated with Dairy Cow Mastitis
5. Mastitis Detection
5.1. Classic on-Farm and Laboratory Methods
5.2. Physics-Based Methods
5.3. Acute-Phase Proteins
5.4. Genetic Methods
5.5. Metabolomics, Proteomics, and Other Biomarkers
6. Some Management Practices in Mastitis Prevention
7. Mastitis Immunotherapy and Nanoparticles-Based Treatments
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hagnestam, C.; Emanuelson, U.; Berglund, B. Yield Losses Associated with Clinical Mastitis Occurring in Different Weeks of Lactation. J. Dairy Sci. 2007, 90, 2260–2270. [Google Scholar] [CrossRef]
- Hussain, R.; Javed, M.T.; Khan, A. Changes in Some Biochemical Parameters and Somatic Cell Counts in the Milk of Buffalo and Cattle Suffering from Mastitis. Pak. Vet. J. 2012, 32, 418–421. [Google Scholar]
- Lipkens, Z.; Piepers, S.; De Visscher, A.; De Vliegher, S. Evaluation of Test-Day Milk Somatic Cell Count Information to Predict Intramammary Infection with Major Pathogens in Dairy Cattle at Drying Off. J. Dairy Sci. 2019, 102, 4309–4321. [Google Scholar] [CrossRef]
- Bortolami, A.; Fiore, E.; Gianesella, M.; Corrò, M.; Catania, S.; Morgante, M. Evaluation of the Udder Health Status in Subclinical Mastitis Affected Dairy Cows through Bacteriological Culture, Somatic Cell Count and Thermographic Imaging. Pol. J. Vet. Sci. 2015, 18, 104. [Google Scholar] [CrossRef]
- Senft, B.; Neudecker, J. Defense Mechanisms of the Bovine Mammary Gland. Tierärztliche Prax. 1991, 19, 357–363. [Google Scholar]
- Nickerson, S.C. Immunological Aspects of Mammary Involution. J. Dairy Sci. 1989, 72, 1665–1678. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The Role of Innate Immune Response and Microbiome in Resilience of Dairy Cattle to Disease: The Mastitis Model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef]
- Pighetti, G.M.; Sordillo, L.M. Specific Immune Responses of Dairy Cattle after Primary Inoculation with Recombinant Bovine Interferon-γ as an Adjuvant When Vaccinating against Mastitis. Am. J. Vet. Res. 1996, 57, 819–824. [Google Scholar] [CrossRef]
- Piotrowska-Tomala, K.K.; Siemieniuch, M.J.; Szóstek, A.Z.; Korzekwa, A.J.; Woclawek-Potocka, I.; Galváo, A.M.; Okuda, K.; Skarzynski, D.J. Lipopolysaccharides, Cytokines, and Nitric Oxide Affect Secretion of Prostaglandins and Leukotrienes by Bovine Mammary Gland Epithelial Cells. Domest. Anim. Endocrinol. 2012, 43, 278–288. [Google Scholar] [CrossRef]
- Mishra, S. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 8874. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Martins, R.P. Adaptive Cell-Mediated Immunity in the Mammary Gland of Dairy Ruminants. Front. Vet. Sci. 2022, 9, 4890. [Google Scholar] [CrossRef]
- Pegolo, S.; Tessari, R.; Bisutti, V.; Vanzin, A.; Giannuzzi, D.; Gianesella, M.; Lisuzzo, A.; Fiore, E.; Barberio, A.; Schiavon, E.; et al. Quarter-Level Analyses of the Associations among Subclinical Intramammary Infection and Milk Quality, Udder Health, and Cheesemaking Traits in Holstein Cows. J. Dairy Sci. 2022, 105, 3490–3507. [Google Scholar] [CrossRef]
- De Vliegher, S.; Ohnstad, I.; Piepers, S. Management and Prevention of Mastitis: A Multifactorial Approach with a Focus on Milking, Bedding and Data-Management. J. Integr. Agric. 2018, 17, 1214–1233. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, Types, Etiological Agents, Prevalence, Diagnosis, Treatment, Prevention, Effects on Human Health and Future Aspects of Bovine Mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.; Tantary, H. A Treatise on Bovine Mastitis: Disease and Disease Economics, Etiological Basis, Risk Factors, Impact on Human Health, Therapeutic Management, Prevention and Control Strategy. Adv. Dairy Res. 2015, 4, 150. [Google Scholar] [CrossRef]
- Martins, S.A.M.; Martins, V.C.; Cardoso, F.A.; Germano, J.; Rodrigues, M.; Duarte, C.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Biosensors for On-Farm Diagnosis of Mastitis. Front. Bioeng. Biotechnol. 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S. Indicators of Inflammation in the Diagnosis of Mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan — a Web and Mobile App for Systematic Reviews. Available online: https://rayyan.ai/ (accessed on 1 August 2024).
- Peters, M.D.P.; Silveira, I.D.B.; Fischer, V. Impact of Subclinical and Clinical Mastitis on Sensitivity to Pain of Dairy Cows. Animal 2015, 9, 2024–2028. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Petzer, I.M. Injectable Organic and Inorganic Selenium in Dairy Cows—Effects on Milk, Blood and Somatic Cell Count Levels. Onderstepoort J. Vet. Res. 2019, 86, 1664. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Kibebew, K. Bovine Mastitis: A Review of Causes and Epidemiological Point of View. J. Biol. Agric. Healthc. 2017, 7, 1–14. [Google Scholar]
- Bian, Y.; Lv, Y.; Li, Q. Identification of Diagnostic Protein Markers of Subclinical Mastitis in Bovine Whey Using Comparative Proteomics. Bull. Vet. Inst. Pulawy 2014, 58, 385–392. [Google Scholar] [CrossRef]
- Griffioen, K.; Velthuis, A.G.J.; Koop, G.; Lam, T.J.G.M. Effects of a Mastitis Treatment Strategy with or without On-Farm Testing. J. Dairy Sci. 2021, 104, 4665–4681. [Google Scholar] [CrossRef] [PubMed]
- Juronen, D.; Kuusk, A.; Kivirand, K.; Rinken, A.; Rinken, T. Immunosensing System for Rapid Multiplex Detection of Mastitis-Causing Pathogens in Milk. Talanta 2018, 178, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.R.; Gonçalves, J.L.; Grenfell, R.; Leite, R.F.; Juliano, L.; Santos, M.V. Direct Identification of Bovine Mastitis Pathogens by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry in Pre-Incubated Milk. Braz. J. Microbiol. 2018, 49, 801–807. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Awad, W.; Abdou, N.E.; Castañeda Vázquez, H. Molecular Biological Tools Applied for Identification of Mastitis Causing Pathogens. Int. J. Vet. Sci. Med. 2017, 5, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Awale, M.; Dudhatra, G.B.; Avinash, K.; Chauhan, B.N.; Kamani, D.R.; Modi, C.M.; Patel, H.B.; Mody, S.K. Bovine Mastitis: A Threat to Economy. Open Access Sci. Rep. 2012, 1, 1–10. Available online: https://www.omicsonline.org/scientific-reports/srep295.php (accessed on 28 May 2024).
- National Mastitis Council Inc. Laboratory Handbook on Bovine Mastitis, 3rd ed.; National Mastitis Council Inc.: New Prague, MN, USA, 2017. [Google Scholar]
- Kuang, Y.; Tani, K.; Synnott, A.J.; Ohshima, K.; Higuchi, H.; Nagahata, H.; Tanji, Y. Characterization of Bacterial Population of Raw Milk from Bovine Mastitis by Culture-Independent PCR-DGGE Method. Biochem. Eng. J. 2009, 45, 76–81. [Google Scholar] [CrossRef]
- Contreras, G.A.; Rodríguez, J.M. Mastitis: Comparative Etiology and Epidemiology. J. Mammary Gland Biol. Neoplasia 2011, 16, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Klaas, I.C.; Zadoks, R.N. An Update on Environmental Mastitis: Challenging Perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Saidani, M.; Messadi, L.; Soudani, A.; Daaloul-Jedidi, M.; Châtre, P.; Ben Chehida, F.; Mamlouk, A.; Mahjoub, W.; Madec, J.Y.; Haenni, M. Epidemiology, Antimicrobial Resistance, and Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Clinical Bovine Mastitis in Tunisia. Microb. Drug Resist. 2018, 24, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-Specific Production Losses in Bovine Mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Zi, C.; Zeng, D.; Ling, N.; Dai, J.; Xue, F.; Jiang, Y.; Li, B. An Improved Assay for Rapid Detection of Viable Staphylococcus Aureus Cells by Incorporating Surfactant and PMA Treatments in QPCR. BMC Microbiol. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Milanov, D.; Prunić, B.; Velhner, M.; Bojkovski, J. Diagnosis of Yeast Mastitis in Dairy Cows. Lucr. Stiint.—Univ. Stiint. Agric. A Banat. Timis. Med. Vet. 2014, 47, 56–64. [Google Scholar]
- Abou-Elmagd, S.; Kotb, H.; Sabry, K.; Refai, M. Prevalence of Candida Albicans and Cryptococcus Neoformans in Animals and Chickens in Quena Governorate, with Special Reference to RAPD-PCR Patterns of the Isolates. J. Am. Sci. 2011, 7, 20–31. [Google Scholar]
- Da Costa, G.M.; de Pereira, U.P.; Gomes Souza-Dias, M.A.; da Silva, N. Yeast Mastitis Outbreak in a Brazilian Dairy Herd. Braz. J. Vet. Res. Anim. Sci. 2012, 49, 239–243. [Google Scholar] [CrossRef][Green Version]
- Tarazona-Manrique, L.E.; Villate-Hernández, J.R.; Andrade-Becerra, R.J. Bacterial and Fungal Infectious Etiology Causing Mastitis in Dairy Cows in the Highlands of Boyacá (Colombia). Rev. Fac. Med. Vet. Zootec. 2019, 66, 208–218. [Google Scholar] [CrossRef]
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakuła, Z.; Wlazło, Ł.; Woch, P. Prevalence of Prototheca Spp. on Dairy Farms in Poland—A Cross-Country Study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef]
- Shkromada, O.; Palii, A.; Palii, A.; Skliar, O.; Dudchenko, Y.; Necherya, T. Improvement of Milk Quality for Micro-Climate Formation on Cattle Farms. Bull. Sumy Natl. Agrar. Univ. Ser. Vet. Med. 2019, 4, 43–49. [Google Scholar] [CrossRef]
- Bezman, D.; Lemberskiy-Kuzin, L.; Katz, G.; Merin, U.; Leitner, G. Influence of Intramammary Infection of a Single Gland in Dairy Cows on the Cow’s Milk Quality. J. Dairy Res. 2015, 82, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Leitner, G.; Lavon, Y. The Disruptive Effects of Mastitis on Reproduction and Fertility in Dairy Cows. Ital. J. Anim. Sci. 2015, 14, 650–654. [Google Scholar] [CrossRef]
- Van Soest, F.J.S.; Santman-Berends, I.M.G.A.; Lam, T.J.G.M.; Hogeveen, H. Failure and Preventive Costs of Mastitis on Dutch Dairy Farms. J. Dairy Sci. 2016, 99, 8365–8374. [Google Scholar] [CrossRef] [PubMed]
- Huijps, K.; Lam, T.J.G.M.; Hogeveen, H. Costs of Mastitis: Facts and Perception. J. Dairy Res. 2008, 75, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and Countrywide Prevalence of Subclinical and Clinical Mastitis in Dairy Cattle and Buffaloes by Systematic Review and Meta-Analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Piepers, S.; De Vliegher, S. Mastitis Prevention and Control Practices and Mastitis Treatment Strategies Associated with the Consumption of (Critically Important) Antimicrobials on Dairy Herds in Flanders, Belgium. J. Dairy Sci. 2016, 99, 2896–2903. [Google Scholar] [CrossRef]
- Cha, E.; Bar, D.; Hertl, J.A.; Tauer, L.W.; Bennett, G.; González, R.N.; Schukken, Y.H.; Welcome, F.L.; Gröhn, Y.T. The Cost and Management of Different Types of Clinical Mastitis in Dairy Cows Estimated by Dynamic Programming. J. Dairy Sci. 2011, 94, 4476–4487. [Google Scholar] [CrossRef]
- Liang, D.; Arnold, L.M.; Stowe, C.J.; Harmon, R.J.; Bewley, J.M. Estimating US Dairy Clinical Disease Costs with a Stochastic Simulation Model. J. Dairy Sci. 2017, 100, 1472–1486. [Google Scholar] [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The Cost of Clinical Mastitis in the First 30 Days of Lactation: An Economic Modeling Tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Tomazi, T.; Barreiro, J.R.; Beuron, D.C.; Arcari, M.A.; Lee, S.H.I.; de Martins, C.M.M.R.; Araújo Junior, J.P.; Santos, M.V. dos Effects of Bovine Subclinical Mastitis Caused by Corynebacterium Spp. on Somatic Cell Count, Milk Yield and Composition by Comparing Contralateral Quarters. Vet. J. 2016, 209, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Kamphuis, C.; Martins, C.M.M.R.; Barreiro, J.R.; Tomazi, T.; Gameiro, A.H.; Hogeveen, H.; dos Santos, M.V. Bovine Subclinical Mastitis Reduces Milk Yield and Economic Return. Livest. Sci. 2018, 210, 25–32. [Google Scholar] [CrossRef]
- Bobbo, T.; Ruegg, P.L.; Stocco, G.; Fiore, E.; Gianesella, M.; Morgante, M.; Pasotto, D.; Bittante, G.; Cecchinato, A. Associations between Pathogen-Specific Cases of Subclinical Mastitis and Milk Yield, Quality, Protein Composition, and Cheese-Making Traits in Dairy Cows. J. Dairy Sci. 2017, 100, 4868–4883. [Google Scholar] [CrossRef] [PubMed]
- Malek dos Reis, C.B.; Barreiro, J.R.; Mestieri, L.; de Porcionato, M.A.F.; dos Santos, M.V. Effect of Somatic Cell Count and Mastitis Pathogens on Milk Composition in Gyr Cows. BMC Vet. Res. 2013, 9, 67. [Google Scholar] [CrossRef]
- Batavani, R.A.; Asri, S.; Naebzadeh, H. The Effect of Subclinical Mastitis on Milk Composition in Dairy Cows. Iran. J. Vet. Res. 2007, 8, 205–211. [Google Scholar]
- Power, M.; Fell, G.; Wright, M. Principles for High-Quality, High-Value Testing. Evid. Based. Med. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential Somatic Cell Count—A Novel Method for Routine Mastitis Screening in the Frame of Dairy Herd Improvement Testing Programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef]
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium Review: Novel Strategies to Genetically Improve Mastitis Resistance in Dairy Cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Tedde, V.; Puggioni, G.M.G.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of Milk Cathelicidin for Detection of Bovine Mastitis. J. Dairy Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis Detection: Current Trends and Future Perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Adkins, P.R.F.; Middleton, J.R. Methods for Diagnosing Mastitis. Vet. Clin. North Am.—Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Rudy, C.K.; Etter, E.M.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging Flow Cytometry Elucidates Limitations of Microparticle Analysis by Conventional Flow Cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319148922. [Google Scholar]
- Langerhuus, S.N.; Ingvartsen, K.L.; Bennedsgaard, T.W.; Røntved, C.M. Gram-Typing of Mastitis Bacteria in Milk Samples Using Flow Cytometry. J. Dairy Sci. 2013, 96, 267–277. [Google Scholar] [CrossRef]
- Rowe, S.; Godden, S.; Nydam, D.V.; Gorden, P.; Lago, A.; Vasquez, A.; Royster, E.; Timmerman, J.; Thomas, M. Evaluation of Rapid Culture, a Predictive Algorithm, Esterase Somatic Cell Count and Lactate Dehydrogenase to Detect Intramammary Infection in Quarters of Dairy Cows at Dry-Off. Prev. Vet. Med. 2020, 179, 104982. [Google Scholar] [CrossRef]
- Pantoja, J.C.F.; Hulland, C.; Ruegg, P.L. Dynamics of Somatic Cell Counts and Intramammary Infections across the Dry Period. Prev. Vet. Med. 2009, 90, 43–54. [Google Scholar] [CrossRef]
- Ferronatto, J.A.; Ferronatto, T.C.; Schneider, M.; Pessoa, L.F.; Blagitz, M.G.; Heinemann, M.B.; Della Libera, A.M.M.P.; Souza, F.N. Diagnosing Mastitis in Early Lactation: Use of Somaticell®, California Mastitis Test and Somatic Cell Count. Ital. J. Anim. Sci. 2018, 17, 723–729. [Google Scholar] [CrossRef]
- Gohary, K.; McDougall, S. Predicting Intramammary Infection Status at Drying off Using Indirect Testing of Milk Samples. N. Z. Vet. J. 2018, 66, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Santschi, D.E.; Durocher, J.; Lefebvre, D.M. Evaluation of the New Differential Somatic Cell Count Parameter as a Rapid and Inexpensive Supplementary Tool for Udder Health Management through Regular Milk Recording. Prev. Vet. Med. 2020, 181, 105079. [Google Scholar] [CrossRef]
- Schwarz, D.; Lipkens, Z.; Piepers, S.; De Vliegher, S. Investigation of Differential Somatic Cell Count as a Potential New Supplementary Indicator to Somatic Cell Count for Identification of Intramammary Infection in Dairy Cows at the End of the Lactation Period. Prev. Vet. Med. 2019, 172, 104803. [Google Scholar] [CrossRef]
- Khatun, M.; Thomson, P.C.; García, S.C.; Bruckmaier, R.M. Suitability of Milk Lactate Dehydrogenase and Serum Albumin for Pathogen-Specific Mastitis Detection in Automatic Milking Systems. J. Dairy Sci. 2022, 105, 2558–2571. [Google Scholar] [CrossRef]
- Bludau, M.J.; Maeschli, A.; Leiber, F.; Steiner, A.; Klocke, P. Mastitis in Dairy Heifers: Prevalence and Risk Factors. Vet. J. 2014, 202, 566–572. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Olde Riekerink, R.G.M.; Sampimon, O.C.; van Schaik, G.; Lam, T.J.G.M. Incidence of Subclinical Mastitis in Dutch Dairy Heifers in the First 100 Days in Lactation and Associated Risk Factors. J. Dairy Sci. 2012, 95, 2476–2484. [Google Scholar] [CrossRef]
- Kandeel, S.A.; Megahed, A.A.; Ebeid, M.H.; Constable, P.D. Evaluation of 3 Esterase Tests for the Diagnosis of Subclinical Mastitis at Dry-off and Freshening in Dairy Cattle. J. Dairy Sci. 2019, 102, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.L.; Murray, R.D.; Woldehiwet, Z. California Mastitis Test Scores as Indicators of Subclinical Intra-Mammary Infections at the End of Lactation in Dairy Cows. Res. Vet. Sci. 2012, 92, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Fosgate, G.T.; Petzer, I.M.; Karzis, J. Sensitivity and Specificity of a Hand-Held Milk Electrical Conductivity Meter Compared to the California Mastitis Test for Mastitis in Dairy Cattle. Vet. J. 2013, 196, 98–102. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L. Evaluation of the California Mastitis Test to Detect an Intramammary Infection with a Major Pathogen in Early Lactation Dairy Cows. Can. Vet. J. Rev. Vet. Can. 2003, 44, 413–415. [Google Scholar]
- Sears, P.M.; Wilson, D.J.; Gonzalez, R.N.; Hancock, D.D. Microbiological Results from Milk Samples Obtained Premilking and Postmilking for the Diagnosis of Bovine Lntramammary Infections. J. Dairy Sci. 1991, 74, 4183–4188. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Jansen, J.T.; Leslie, K.E.; Smart, N.L.; Kelton, D.F. The Effect of Sampling Time and Sample Handling on the Detection of Staphylococcus Aureus in Milk from Quarters with Subclinical Mastitis. Can. Vet. J. 2002, 43, 38. [Google Scholar]
- Souza, F.N.; Cunha, A.F.; Rosa, D.L.S.O.; Brito, M.A.V.; Guimarães, A.S.; Mendonça, L.C.; Souza, G.N.; Lage, A.P.; Blagitz, M.G.; Della Libera, A.M.M.P.; et al. Somatic Cell Count and Mastitis Pathogen Detection in Composite and Single or Duplicate Quarter Milk Samples. Pesqui. Vet. Bras. 2016, 36, 811–818. [Google Scholar] [CrossRef]
- Reyher, K.K.; Dohoo, I.R. Diagnosing Intramammary Infections: Evaluation of Composite Milk Samples to Detect Intramammary Infections. J. Dairy Sci. 2011, 94, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Kleinhans, S.; Reimann, G.; Stückler, P.; Reith, F.; Ilves, K.; Pedastsaar, K.; Yan, L.; Zhang, Z.; Valdivieso, M.; et al. Investigation of Dairy Cow Performance in Different Udder Health Groups Defined Based on a Combination of Somatic Cell Count and Differential Somatic Cell Count. Prev. Vet. Med. 2020, 183, 105123. [Google Scholar] [CrossRef]
- Pilla, R.; Malvisi, M.; Snel, G.G.M.; Schwarz, D.; König, S.; Czerny, C.P.; Piccinini, R. Differential Cell Count as an Alternative Method to Diagnose Dairy Cow Mastitis. J. Dairy Sci. 2013, 96, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Robles, I.; Nolan, D.T.; Fendley, C.A.; Stokley, H.L.; France, T.L.; Ferrell, J.L.; Costa, J.H.C. Technical Note: Evaluation of a Commercial on-Farm Milk Leukocyte Differential Tester to Identify Subclinical Mastitis Cases in Dairy Cows. J. Dairy Sci. 2021, 104, 4942–4949. [Google Scholar] [CrossRef] [PubMed]
- Scherpenzeel, C.G.M.; den Uijl, I.E.M.; van Schaik, G.; Riekerink, R.G.M.O.; Hogeveen, H.; Lam, T.J.G.M. Effect of Different Scenarios for Selective Dry-Cow Therapy on Udder Health, Antimicrobial Usage, and Economics. J. Dairy Sci. 2016, 99, 3753–3764. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, A.; van Hees-Huijps, K.; van Knegsel, A.T.M.; Sampimon, O.C.; Vernooij, J.C.M.; Nielen, M.; van Werven, T. Effects of Reduced Intramammary Antimicrobial Use during the Dry Period on Udder Health in Dutch Dairy Herds. J. Dairy Sci. 2018, 101, 3248–3260. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.J.G.M.; Olde Riekerink, R.G.M.; Sampimon, O.C.; Smith, H. Mastitis Diagnostics and Performance Monitoring: A Practical Approach. Ir. Vet. J. 2009, 62, 34–39. [Google Scholar] [CrossRef]
- Maćešić, N.; Bačić, G.; Božičević, K.; Benić, M.; Karadjole, T.; Babić, N.P.; Lojkić, M.; Efendić, M.; Bačić, I.; Pavlak, M. Assessment of the Zagreb Mastitis Test in Diagnosis of Subclinical Mastitis in Dairy Cattle. Vet. Arh. 2016, 86, 475–485. [Google Scholar]
- Sargeant, J.M.; Leslie, K.E.; Shirley, J.E.; Pulkrabek, B.J.; Lim, G.H. Sensitivity and Specificity of Somatic Cell Count and California Mastitis Test for Identifying Intramammary Infection in Early Lactation. J. Dairy Sci. 2001, 84, 2018–2024. [Google Scholar] [CrossRef]
- Halasa, T.; Kirkeby, C. Differential Somatic Cell Count: Value for Udder Health Management. Front. Vet. Sci. 2020, 7, 9055. [Google Scholar] [CrossRef]
- Zecconi, A.; Vairani, D.; Cipolla, M.; Rizzi, N.; Zanini, L. Assessment of Subclinical Mastitis Diagnostic Accuracy by Differential Cell Count in Individual Cow Milk. Ital. J. Anim. Sci. 2019, 18, 460–465. [Google Scholar] [CrossRef]
- Pilla, R.; Schwarz, D.; König, S.; Piccinini, R. Microscopic Differential Cell Counting to Identify Inflammatory Reactions in Dairy Cow Quarter Milk Samples. J. Dairy Sci. 2012, 95, 4410–4420. [Google Scholar] [CrossRef]
- Græsbøll, K.; Kirkeby, C.; Nielsen, S.S.; Halasa, T.; Toft, N.; Christiansen, L.E. Models to Estimate Lactation Curves of Milk Yield and Somatic Cell Count in Dairy Cows at the Herd Level for the Use in Simulations and Predictive Models. Front. Vet. Sci. 2016, 3, 115. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, C.; Toft, N.; Schwarz, D.; Farre, M.; Nielsen, S.S.; Zervens, L.; Hechinger, S.; Halasa, T. Differential Somatic Cell Count as an Additional Indicator for Intramammary Infections in Dairy Cows. J. Dairy Sci. 2020, 103, 1759–1775. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Zanini, L.; Cipolla, M.; Stefanon, B. Factors Affecting the Patterns of Total Amount and Proportions of Leukocytes in Bovine Milk. Animals 2020, 10, 992. [Google Scholar] [CrossRef]
- Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M.; Schwarz, D. Differential Somatic Cell Count in Milk before, during, and after Lipopolysaccharide- and Lipoteichoic-Acid-Induced Mastitis in Dairy Cows. J. Dairy Sci. 2018, 101, 5362–5373. [Google Scholar] [CrossRef]
- Thyarla, A.; Agrawal, A.K.; Sandey, K.K.; Suresh, V.; Prasanth, B.; Umapathy, K.S.; Sinha, G. Study of Physical and Electrical Characteristics of Skim Milk. Asian J. Dairy Food Res. 2018, 37, 18–21. [Google Scholar] [CrossRef][Green Version]
- Hovinen, M.; Pyörälä, S. Invited Review: Udder Health of Dairy Cows in Automatic Milking. J. Dairy Sci. 2011, 94, 547–562. [Google Scholar] [CrossRef]
- Khatun, M.; Thomson, P.C.; Kerrisk, K.L.; Lyons, N.A.; Clark, C.E.F.; Molfino, J.; García, S.C. Development of a New Clinical Mastitis Detection Method for Automatic Milking Systems. J. Dairy Sci. 2018, 101, 9385–9395. [Google Scholar] [CrossRef]
- Wethal, K.B.; Svendsen, M.; Heringstad, B. A Genetic Study of New Udder Health Indicator Traits with Data from Automatic Milking Systems. J. Dairy Sci. 2020, 103, 7188–7198. [Google Scholar] [CrossRef]
- Youssif, N.H.; Hafiz, N.M.; Halawa, M.A.; Saad, M.F. Association of Selected Risk Factors with Bovine Subclinical Mastitis. Acta Vet. Bras. 2021, 15, 153–160. [Google Scholar] [CrossRef]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.C.; Sloth, K.H.M.N.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.; Page, K.L.; Walton, A.W.; Hillerton, J.E. Detection of Clinical Mastitis by Changes in Electrical Conductivity of Foremilk before Visible Changes in Milk. J. Dairy Sci. 1996, 79, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.M.; Reneau, J.K.; Wallace, R.; Hawkins, D.; Munoz-Zanzi, C. A Novel Method of Analyzing Daily Milk Production and Electrical Conductivity to Predict Disease Onset. J. Dairy Sci. 2009, 92, 5964–5976. [Google Scholar] [CrossRef] [PubMed]
- Kunes, R.; Bartos, P.; Iwasaka, G.K.; Lang, A.; Hankovec, T.; Smutny, L.; Cerny, P.; Poborska, A.; Smetana, P.; Kriz, P.; et al. In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review. Agric. 2021, 11, 239. [Google Scholar] [CrossRef]
- Naqvi, S.A.; King, M.T.M.; Matson, R.D.; DeVries, T.J.; Deardon, R.; Barkema, H.W. Mastitis Detection with Recurrent Neural Networks in Farms Using Automated Milking Systems. Comput. Electron. Agric. 2022, 192, 106618. [Google Scholar] [CrossRef]
- Juozaitienė, V.; Juozaitis, A.; Brazauskas, A.; Žymantienė, J.; Žilaitis, V.; Antanaitis, R.; Stankevičius, R.; Bobinienė, R. Investigation of Electrical Conductivity of Milk in Robotic Milking System and Its Relationship with Milk Somatic Cell Count and Other Quality Traits. J. Meas. Eng. 2015, 3, 63–70. [Google Scholar]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Bolaños, J.; Ceballes-Serrano, C.C.; Velásquez-Mejía, D.; Riaño-Rojas, J.C.; Giraldo, C.E.; Carmona, J.U.; Ceballos-Márquez, A. Application of Udder Surface Temperature by Infrared Thermography for Diagnosis of Subclinical Mastitis in Holstein Cows Located in Tropical Highlands. J. Dairy Sci. 2021, 104, 10310–10323. [Google Scholar] [CrossRef]
- Grodkowski, G.; Szwaczkowski, T.; Koszela, K.; Mueller, W.; Tomaszyk, K.; Baars, T.; Sakowski, T. Early Detection of Mastitis in Cows Using the System Based on 3D Motions Detectors. Sci. Rep. 2022, 12, 21215. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Das, D.N.; Kataktalware, M.A.; Jayaprakash, G.; Patbandha, T.K. Investigation of Body and Udder Skin Surface Temperature Differentials as an Early Indicator of Mastitis in Holstein Friesian Crossbred Cows Using Digital Infrared Thermography Technique. Vet. World 2016, 9, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Stordeur, P.; Wallemacq, H.; Schynts, F.; Stevens, M.; Boutet, P.; Peelman, L.J.; De Spiegeleer, B.; Duchateau, L.; Bureau, F.; et al. Variation of Inflammatory Dynamics and Mediators in Primiparous Cows after Intramammary Challenge with Escherichia Coli. Vet. Res. 2011, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using Infrared Thermography to Detect Subclinical Mastitis in Dairy Cows in Compost Barn Systems. J. Therm. Biol. 2021, 97, 102881. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, M.; Siivonen, J.; Taponen, S.; Hänninen, L.; Pastell, M.; Aisla, A.M.; Pyörälä, S. Detection of Clinical Mastitis with the Help of a Thermal Camera. J. Dairy Sci. 2008, 91, 4592–4598. [Google Scholar] [CrossRef]
- Sepúlveda-Varas, P.; Proudfoot, K.L.; Weary, D.M.; von Keyserlingk, M.A.G. Changes in Behaviour of Dairy Cows with Clinical Mastitis. Appl. Anim. Behav. Sci. 2016, 175, 8–13. [Google Scholar] [CrossRef]
- Hallowell, G.D.; Palgrave, K. Ultrasound of the Bovine Mammary Gland and Its Role in Mastitis Control. Int. Dairy Top. 2012, 11, 43. [Google Scholar]
- Suzuki, N.; Kurose, T.; Kaneko, S.; Haraguchi, A.; Isobe, N. Outcome Prediction from the First Examination in Clinical Mastitis Using Ultrasonography in Dairy Cows. Anim. Sci. J. 2020, 91, 13452. [Google Scholar] [CrossRef]
- Flöck, M.; Winter, P. Diagnostic Ultrasonography in Cattle with Diseases of the Mammary Gland. Vet. J. 2006, 171, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Patil, D.; Kelawala, D.; Parikh, P.; Mer, D.; Gameti, K.; Gohil, K. Ultrasonography of Udder and Teat in Dairy Animals. Rumin. Sci. 2017, 6, 171–175. [Google Scholar]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Turk, R.; Piras, C.; Kovačić, M.; Samardžija, M.; Ahmed, H.; De Canio, M.; Urbani, A.; Meštrić, Z.F.; Soggiu, A.; Bonizzi, L.; et al. Proteomics of Inflammatory and Oxidative Stress Response in Cows with Subclinical and Clinical Mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.C.; Waterston, M.; Hastie, P.; Parkin, T.; Haining, H.; Eckersall, P.D. The Major Acute Phase Proteins of Bovine Milk in a Commercial Dairy Herd. BMC Vet. Res. 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic-Filipovic, M.; Ilić, V.; Vujčić, Z.; Dojnov, B.; Stevanov-Pavlović, M.; Mijačević, Z.; Božić, T. Serum Amyloid A Isoforms in Serum and Milk from Cows with Staphylococcus Aureus Subclinical Mastitis. Vet. Immunol. Immunopathol. 2012, 145, 120–128. [Google Scholar] [CrossRef]
- Kleczkowski, M.; Kluciński, W.; Czerski, M.; Kudyba, E. Association between Acute Phase Response, Oxidative Status and Mastitis in Cows. Vet. Stanica 2017, 48, 177–186. [Google Scholar]
- Eckersall, P.D.; Young, F.J.; Nolan, A.M.; Knight, C.H.; McComb, C.; Waterston, M.M.; Hogarth, C.J.; Scott, E.M.; Fitzpatrick, J.L. Acute Phase Proteins in Bovine Milk in an Experimental Model of Staphylococcus Aureus Subclinical Mastitis. J. Dairy Sci. 2006, 89, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Sugiyama, M.; Ito, T.; Tsukano, K.; Oikawa, S.; Suzuki, K. Diagnostic Utility of Measuring Serum Amyloid a with a Latex Agglutination Turbidimetric Immunoassay in Bovine Mastitis: Comparison with Haptoglobin and Alpha 1 Acid Glycoprotein. J. Vet. Med. Sci. 2021, 83, 329–332. [Google Scholar] [CrossRef]
- El-Deeb, W.M. Clinicobiochemical Investigations of Gangrenous Mastitis in Does: Immunological Responses and Oxidative Stress Biomarkers. J. Zhejiang Univ. Sci. B 2013, 14, 33–39. [Google Scholar] [CrossRef]
- Tothova, C.; Nagy, O.; Kovac, G. Acute Phase Proteins and Their Use in the Diagnosis of Diseases in Ruminants: A Review. Vet. Med. 2014, 59, 163–180. [Google Scholar] [CrossRef]
- Sadek, K.; Saleh, E.; Ayoub, M. Selective, Reliable Blood and Milk Bio-Markers for Diagnosing Clinical and Subclinical Bovine Mastitis. Trop. Anim. Health Prod. 2017, 49, 431–437. [Google Scholar] [CrossRef]
- Dalanezi, F.M.; Schmidt, E.M.S.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Cerri, R.L.A.; Chadwick, C.; Langoni, H. Concentrations of Acute-Phase Proteins in Milk from Cows with Clinical Mastitis Caused by Different Pathogens. Pathogens 2020, 9, 706. [Google Scholar] [CrossRef]
- Wollowski, L.; Heuwieser, W.; Kossatz, A.; Addis, M.F.; Puggioni, G.M.G.; Meriaux, L.; Bertulat, S. The Value of the Biomarkers Cathelicidin, Milk Amyloid A, and Haptoglobin to Diagnose and Classify Clinical and Subclinical Mastitis. J. Dairy Sci. 2021, 104, 2106–2122. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, M.C.; Healy, A.M.; Harte, D.; Walshe, K.G.; Torgerson, P.R.; Doherty, M.L. Milk Amyloid A: Correlation with Cellular Indices of Mammary Inflammation in Cows with Normal and Raised Serum Amyloid A. Res. Vet. Sci. 2006, 80, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hiss, S.; Mueller, U.; Neu-Zahren, A.; Sauerwein, H. Haptoglobin and Lactate Dehydrogenase Measurements in Milk for the Identification of Subclinically Diseased Udder Quarters. Vet. Med. 2007, 52, 245–252. [Google Scholar] [CrossRef]
- Safi, S.; Khoshvaghti, A.; Jafarzadeh, S.R.; Bolourchi, M.; Nowrouzian, I. Acute Phase Proteins in the Diagnosis of Bovine Subclinical Mastitis. Vet. Clin. Pathol. 2009, 38, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, G.A.; Wieliczko, R.J.; Pryor, S.M.; Broadhurst, M.K.; Wheeler, T.T.; Haigh, B.J. The Abundance of Milk Cathelicidin Proteins during Bovine Mastitis. Vet. Immunol. Immunopathol. 2011, 143, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, T.; Cacciotto, C.; Pisanu, S.; Tedde, V.; Alberti, A.; Pittau, M.; Dore, S.; Cannas, A.; Uzzau, S.; Rocca, S.; et al. Cathelicidin Production and Release by Mammary Epithelial Cells during Infectious Mastitis. Vet. Immunol. Immunopathol. 2017, 189, 66–70. [Google Scholar] [CrossRef]
- Ali, A.; Rehman, M.U.; Mushtaq, S.; Ahmad, S.B.; Khan, A.; Karan, A.; Bashir Wani, A.; Ganie, S.A.; Mir, M.U.R. Biochemical and Computational Assessment of Acute Phase Proteins in Dairy Cows Affected with Subclinical Mastitis. Curr. Issues Mol. Biol. 2023, 45, 338. [Google Scholar] [CrossRef]
- O’Reilly, E.L.; Viora, L.; Malcata, F.; Pepler, P.T.; Zadoks, R.; Brady, N.; Hanh, H.Q.; McLaughlin, M.; Horvatic, A.; Gelemanovic, A.; et al. Biomarker and Proteome Analysis of Milk from Dairy Cows with Clinical Mastitis: Determining the Effect of Different Bacterial Pathogens on the Response to Infection. Res. Vet. Sci. 2024, 172, 105240. [Google Scholar] [CrossRef] [PubMed]
- Spittel, S.; Hoedemaker, M. Mastitis Diagnosis in Dairy Cows Using PathoProof Real-Time Polymerase Chain Reaction Assay in Comparison with Conventional Bacterial Culture in a Northern German Field Study. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 494–502. [Google Scholar]
- Loy, J.D.; Clawson, M.L.; Adkins, P.R.F.; Middleton, J.R. Current and Emerging Diagnostic Approaches to Bacterial Diseases of Ruminants. Vet. Clin. North Am. Food Anim. Pract. 2023, 39, 93–114. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Cole, J.B.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Cattle Quantitative Trait Locus Database. Available online: https://www.animalgenome.org/cgi-bin/QTLdb/BT/nscape?isID=1439 (accessed on 20 June 2024).
- Mai, M.D.; Rychtářová, J.; Zink, V.; Lassen, J.; Guldbrandtsen, B. Quantitative Trait Loci for Milk Production and Functional Traits in Two Danish Cattle Breeds. J. Anim. Breed. Genet. 2010, 127, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, G.; Nicolazzi, E.L.; Strozzi, F.; Stella, A.; Negrini, R.; Ajmone-Marsan, P.; Williams, J.L. Genome Wide Scan for Somatic Cell Counts in Holstein Bulls. In Proceedings of the International Symposium on Animal Genomics for Animal Health (AGAH 2010), Paris, France, 31 May–2 June 2010; Volume 5. [Google Scholar]
- Sodeland, M.; Kent, M.P.; Olsen, H.G.; Opsal, M.A.; Svendsen, M.; Sehested, E.; Hayes, B.J.; Lien, S. Quantitative Trait Loci for Clinical Mastitis on Chromosomes 2, 6, 14 and 20 in Norwegian Red Cattle. Anim. Genet. 2011, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Meredith, B.K.; Kearney, F.J.; Finlay, E.K.; Bradley, D.G.; Fahey, A.G.; Berry, D.P.; Lynn, D.J. Genome-Wide Associations for Milk Production and Somatic Cell Score in Holstein-Friesian Cattle in Ireland. BMC Genet. 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wijga, S.; Bastiaansen, J.W.M.; Wall, E.; Strandberg, E.; de Haas, Y.; Giblin, L.; Bovenhuis, H. Genomic Associations with Somatic Cell Score in First-Lactation Holstein Cows. J. Dairy Sci. 2012, 95, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sahana, G.; Ma, P.; Su, G.; Yu, Y.; Zhang, S.; Lund, M.S.; Sørensen, P. Exploring the Genetic Architecture and Improving Genomic Prediction Accuracy for Mastitis and Milk Production Traits in Dairy Cattle by Mapping Variants to Hepatic Transcriptomic Regions Responsive to Intra-Mammary Infection. Genet. Sel. Evol. 2017, 49, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kurz, J.P.; Yang, Z.; Weiss, R.B.; Wilson, D.J.; Rood, K.A.; Liu, G.E.; Wang, Z. A Genome-Wide Association Study for Mastitis Resistance in Phenotypically Well-Characterized Holstein Dairy Cattle Using a Selective Genotyping Approach. Immunogenetics 2019, 71, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Welderufael, B.G.; Løvendahl, P.; de Koning, D.J.; Janss, L.L.G.; Fikse, W.F. Genome-Wide Association Study for Susceptibility to and Recoverability from Mastitis in Danish Holstein Cows. Front. Genet. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.; Bortfeldt, R.H.; Reißmann, M.; Brockmann, G.A. P5000 Confirmation of Genome-Wide Associations for Clinical Mastitis in German Holstein Cattle. J. Anim. Sci. 2016, 94, 115–116. [Google Scholar] [CrossRef]
- Mustafa, H. Performance of Bovine High Density SNPs Genotyping Array in Indigenous Pakistani Cattle Breeds. Pure Appl. Biol. 2018, 7, 221–226. [Google Scholar] [CrossRef]
- Jamrozik, J.; Koeck, A.; Miglior, F.; Kistemaker, G.J.; Schenkel, F.S.; Kelton, D.F.; Doormaal, B.J. Van Genetic and Genomic Evaluation of Mastitis Resistance in Canada. Interbull Bull. 2013, 47, 43–51. [Google Scholar]
- Koeck, A.; Miglior, F.; Kelton, D.F.; Schenkel, F.S. Alternative Somatic Cell Count Traits to Improve Mastitis Resistance in Canadian Holsteins. J. Dairy Sci. 2012, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Canadian Dairy Network Heritability Estimates Used for Genetic Evaluation in Canada. Available online: https://lactanet.ca/en/heritability-estimates-used-for-genetic-evaluation-in-canada/ (accessed on 20 July 2024).
- Lisuzzo, A.; Laghi, L.; Fiore, E.; Cecchinato, A.; Bisutti, V.; Pegolo, S.; Giannuzzi, D.; Tessari, R.; Barberio, A.; Schiavon, E.; et al. Serum Metabolome Differences Associated with Subclinical Intramammary Infection Caused by Streptococcus Agalactiae and Prototheca Spp. in Multiparous Dairy Cows. J. Dairy Sci. 2024, 107, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Dunn, S.M.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. GC-MS Metabolomics Identifies Metabolite Alterations That Precede Subclinical Mastitis in the Blood of Transition Dairy Cows. J. Proteome Res. 2017, 16, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, M.; Simojoki, H.; Pösö, R.; Suolaniemi, J.; Kalmus, P.; Suojala, L.; Pyörälä, S. N-Acetyl-β-D-Glucosaminidase Activity in Cow Milk as an Indicator of Mastitis. J. Dairy Res. 2016, 83, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Nyman, A.K.; Emanuelson, U.; Waller, K.P. Diagnostic Test Performance of Somatic Cell Count, Lactate Dehydrogenase, and N-Acetyl-β-d-Glucosaminidase for Detecting Dairy Cows with Intramammary Infection. J. Dairy Sci. 2016, 99, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Shtenberg, G. N-Acetyl-β-D-Glucosaminidase Biomarker Quantification in Milk Using Ag-Porous Si SERS Platform for Mastitis Severity Evaluation. Appl. Surf. Sci. 2021, 566, 150700. [Google Scholar] [CrossRef]
- Nirala, N.R.; Shtenberg, G. Gold Nanoparticle Size-Dependent Enhanced Chemiluminescence for Ultra-Sensitive Haptoglobin Biomarker Detection. Biomolecules 2019, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Harel, Y.; Lellouche, J.P.; Shtenberg, G. Ultrasensitive Haptoglobin Biomarker Detection Based on Amplified Chemiluminescence of Magnetite Nanoparticles. J. Nanobiotechnol. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Frundzhyan, V.G.; Parkhomenko, I.M.; Brovko, L.Y.; Ugarova, N.N. Improved Bioluminescent Assay of Somatic Cell Counts in Raw Milk. J. Dairy Res. 2008, 75, 3282. [Google Scholar] [CrossRef]
- Bobbo, T.; Ruegg, P.L.; Fiore, E.; Gianesella, M.; Morgante, M.; Pasotto, D.; Gallo, L.; Bittante, G.; Cecchinato, A. Short Communication: Association between Udder Health Status and Blood Serum Proteins in Dairy Cows. J. Dairy Sci. 2017, 100, 9775–9780. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, T.; Fiore, E.; Gianesella, M.; Morgante, M.; Gallo, L.; Ruegg, P.L.; Bittante, G.; Cecchinato, A. Variation in Blood Serum Proteins and Association with Somatic Cell Count in Dairy Cattle from Multi-Breed Herds. Animal 2017, 11, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Decter, D.H.; Bicalho, R.C. Evaluation of an On-Farm Culture System (Accumast) for Fast Identification of Milk Pathogens Associated with Clinical Mastitis in Dairy Cows. PLoS ONE 2016, 11, e0155314. [Google Scholar] [CrossRef] [PubMed]
- Besier, J.; Lind, O.; Bruckmaier, R.M. Dynamics of Teat-End Vacuum during Machine Milking: Types, Causes and Impacts on Teat Condition and Udder Health—A Literature Review. J. Appl. Anim. Res. 2016, 44, 263–272. [Google Scholar] [CrossRef]
- Cerqueira, J.L.; Araújo, J.P.; Cantalapiedra, J.; Blanco-Penedo, I. How Is the Association of Teat-End Severe Hyperkeratosis on Udder Health and Dairy Cow Behavior? Rev. Med. Vet. 2018, 169, 30–37. [Google Scholar]
- Cardozo, L.L.; Thaler Neto, A.; Souza, G.N.; Picinin, L.C.A.; Felipus, N.C.; Reche, N.L.M.; Schmidt, F.A.; Werncke, D.; Simon, E.E. Risk Factors for the Occurrence of New and Chronic Cases of Subclinical Mastitis in Dairy Herds in Southern Brazil. J. Dairy Sci. 2015, 98, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Guarín, J.F.; Ruegg, P.L. Short Communication: Pre- and Postmilking Anatomical Characteristics of Teats and Their Associations with Risk of Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2016, 99, 8323–8329. [Google Scholar] [CrossRef] [PubMed]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine Mastitis: Prevalence, Risk Factors and Isolation of Staphylococcus Aureus in Dairy Herds at Hawassa Milk Shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef]
- Guarín, J.F.; Paixão, M.G.; Ruegg, P.L. Association of Anatomical Characteristics of Teats with Quarter-Level Somatic Cell Count. J. Dairy Sci. 2017, 100, 643–652. [Google Scholar] [CrossRef]
- Neijenhuis, F.; Barkema, H.W.; Hogeveen, H.; Noordhuizen, J.P.T.M. Relationship between Teat-End Callosity and Occurrence of Clinical Mastitis. J. Dairy Sci. 2001, 84, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, J.C.F.; Correia, L.B.N.; Rossi, R.S.; Latosinski, G.S. Association between Teat-End Hyperkeratosis and Mastitis in Dairy Cows: A Systematic Review. J. Dairy Sci. 2020, 103, 1843–1855. [Google Scholar] [CrossRef]
- Mein, G.A. The Role of the Milking Machine in Mastitis Control. Vet. Clin. North Am.—Food Anim. Pract. 2012, 28, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Vlkova, H.; Babak, V.; Vrtkova, I.; Cervinkova, D.; Marosevic, D.; Moravkova, M.; Jaglic, Z. Epidemiology of Intramammary Infections with Staphylococcus Aureus and Mastitis Streptococci in a Dairy Cattle Herd with a History of Recurrent Clinical Mastitis. Pol. J. Vet. Sci. 2017, 20, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shittu, A.; Abdullahi, J.; Jibril, A.; Mohammed, A.A.; Fasina, F.O. Sub-Clinical Mastitis and Associated Risk Factors on Lactating Cows in the Savannah Region of Nigeria. BMC Vet. Res. 2012, 8, 134. [Google Scholar] [CrossRef]
- Abrahmsén, M.; Persson, Y.; Kanyima, B.M.; Båge, R. Prevalence of Subclinical Mastitis in Dairy Farms in Urban and Peri-Urban Areas of Kampala, Uganda. Trop. Anim. Health Prod. 2014, 46, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Tranter, W.; Laven, R. Longitudinal Study of Herd Udder Hygiene and Its Association with Clinical Mastitis in Pasture-Based Dairy Cows. J. Dairy Sci. 2021, 104, 6051–6060. [Google Scholar] [CrossRef]
- Schreiner, D.A.; Ruegg, P.L. Relationship between Udder and Leg Hygiene Scores and Subclinical Mastitis. J. Dairy Sci. 2003, 86, 3460–3465. [Google Scholar] [CrossRef]
- Köster, G.; Tenhagen, B.A.; Scheibe, N.; Heuwieser, W. Factors Associated with High Milk Test Day Somatic Cell Counts in Large Dairy Herds in Brandenburg. II. Milking Practices. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2006, 53, 209–214. [Google Scholar] [CrossRef]
- Zucali, M.; Bava, L.; Tamburini, A.; Brasca, M.; Vanoni, L.; Sandrucci, A. Effects of Season, Milking Routine and Cow Cleanliness on Bacterial and Somatic Cell Counts of Bulk Tank Milk. J. Dairy Res. 2011, 78, 436–441. [Google Scholar] [CrossRef]
- Sandrucci, A.; Bava, L.; Zucali, M.; Tamburini, A. Management Factors and Cow Traits Influencing Milk Somatic Cell Counts and Teat Hyperkeratosis during Different Seasons. Rev. Bras. Zootec. 2014, 43, 505–511. [Google Scholar] [CrossRef]
- Huijps, K.; Hogeveen, H.; Lam, T.J.G.M.; Oude Lansink, A.G.J.M. Costs and Efficacy of Management Measures to Improve Udder Health on Dutch Dairy Farms. J. Dairy Sci. 2010, 93, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vissio, C.; Bouman, M.; Larriestra, A.J. Milking Machine and Udder Health Management Factors Associated with Bulk Milk Somatic Cell Count in Uruguayan Herds. Prev. Vet. Med. 2018, 150, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; O’Sullivan, K.; Berry, D.P.; More, S.J.; Meaney, W.J.; O’Callaghan, E.J.; O’Brien, B. Farm Management Factors Associated with Bulk Tank Somatic Cell Count in Irish Dairy Herds. Ir. Vet. J. 2009, 62, 45–51. [Google Scholar] [CrossRef]
- Cortinhas, C.S.; Botaro, B.G.; de Macedo, S.N.; dos Santos, M.V. Herd Characteristics and Management Practices Associated with Bulk Tank Milk Quality of Dairy Herds in Southeastern Brazil. Trop. Anim. Health Prod. 2018, 50, 1605–1610. [Google Scholar] [CrossRef]
- Halasa, T.; Nielen, M.; van Werven, T.; Hogeveen, H. A Simulation Model to Calculate Costs and Benefits of Dry Period Interventions in Dairy Cattle. Livest. Sci. 2010, 129, 80–87. [Google Scholar] [CrossRef]
- Schewe, R.L.; Kayitsinga, J.; Contreras, G.A.; Odom, C.; Coats, W.A.; Durst, P.; Hovingh, E.P.; Martinez, R.O.; Mobley, R.; Moore, S.; et al. Herd Management and Social Variables Associated with Bulk Tank Somatic Cell Count in Dairy Herds in the Eastern United States. J. Dairy Sci. 2015, 98, 7650–7665. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Asthana, R.K.; Kumar, S.; Kumar, S.; Ghosh, S.; Upadhaya, B. Use of Dry Cow Therapy for Control of Mastitis in Dairy Animals. J. Krishi Vigyan 2020, 8, 303. [Google Scholar] [CrossRef]
- Stevens, M.; Piepers, S.; De Vliegher, S. The Effect of Mastitis Management Input and Implementation of Mastitis Management on Udder Health, Milk Quality, and Antimicrobial Consumption in Dairy Herds. J. Dairy Sci. 2019, 102, 2401–2415. [Google Scholar] [CrossRef]
- Ingle, H.D.; Rice, C.A.; Black, R.A.; Childers, S.Z.; Eberhart, N.L.; Prado, M.E.; Krawczel, P.D. Effect of Switch Trimming on Udder and Teat Hygiene of Dairy Cows. J. Appl. Anim. Welf. Sci. 2018, 21, 239–243. [Google Scholar] [CrossRef]
- Nickerson, S.C.; Oliver, S.P. REVIEW: How Well Have United States Dairy Producers Adopted Mastitis-Control Technologies for Reducing Herd Somatic Cell Counts Improving Milk Quality? Prof. Anim. Sci. 2014, 30, 115–124. [Google Scholar] [CrossRef]
- Salovuo, H.; Ronkainen, P.; Heino, A.; Suokannas, A.; Ryhänen, E.L. Introduction of Automatic Milking System in Finland: Effect on Milk Quality. Agric. Food Sci. 2005, 14, 346–353. [Google Scholar] [CrossRef]
- Kolenda, M.; Piwczyński, D.; Brzozowski, M.; Sitkowska, B.; Wójcik, P. Comparison of Yield, Composition and Quality of Milk of Polish Holstein-Friesian Cows in Conventional and Automatic Milking Systems. Ann. Anim. Sci. 2021, 21, 709–720. [Google Scholar] [CrossRef]
- Ivemeyer, S.; Simantke, C.; Ebinghaus, A.; Poulsen, P.H.; Sorensen, J.T.; Rousing, T.; Palme, R.; Knierim, U. Herd-Level Associations between Human–Animal Relationship, Management, Fecal Cortisol Metabolites, and Udder Health of Organic Dairy Cows. J. Dairy Sci. 2018, 101, 7361–7374. [Google Scholar] [CrossRef] [PubMed]
- Winnicki, S.; Jugowar, J.; Aerts, J.; Sobek, Z. The Effect of Milking Systems on the Auantity and Quality of Cow Milk. J. Res. Appl. Agric. 2017, 62, 193–196. [Google Scholar]
- Toušová, R.; Ducháček, J.; Stádník, L.; Ptáček, M.; Beran, J. Porovnání Produkce a Kvality Mléka v Dojírně a Systému AMS. J. Cent. Eur. Agric. 2014, 15, 100–114. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Immunotherapies against Human Bacterial and Fungal Infectious Diseases: A Review. Front. Med. 2023, 10, 1135541. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Saleem Bhat, S.; Omonijo, A.F.; Ganai, A.N.; Ibeagha-Awemu, M.E.; Mudasir Ahmad, S. Immunotherapy in Mastitis: State of Knowledge, Research Gaps and Way Forward. Vet. Q. 2024, 44, 1–23. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class i and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Rainard, P.; Gilbert, F.B.; Germon, P.; Foucras, G. Invited Review: A Critical Appraisal of Mastitis Vaccines for Dairy Cows. J. Dairy Sci. 2021, 104, 10427–10448. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Dubovi, E.J.; Gröhn, Y.T.; Brunner, M.A.; Hertl, J.A. Response to Modified Live and Killed Multivalent Viral Vaccine in Regularly Vaccinated, Fresh Dairy Cows. Vet. Ther. 2000, 1, 49–58. [Google Scholar] [PubMed]
- Herry, V.; Gitton, C.; Tabouret, G.; Répérant, M.; Forge, L.; Tasca, C.; Gilbert, F.B.; Guitton, E.; Barc, C.; Staub, C.; et al. Local Immunization Impacts the Response of Dairy Cows to Escherichia Coli Mastitis. Sci. Rep. 2017, 7, 3441. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, L.; Wang, T.; Cao, L.; Liu, F.; Song, J.; Zhang, Y. Construction of the WaaF Subunit and DNA Vaccine Against Escherichia Coli in Cow Mastitis and Preliminary Study on Their Immunogenicity. Front. Vet. Sci. 2022, 9, 877685. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, C.; Gong, R.; Chen, Y.; Ren, N.; Xiao, G.; Xie, Q.; Zhang, M.; Liu, Q.; Guo, A.; et al. Evaluation of a Novel Chimeric B Cell Epitope-Based Vaccine against Mastitis Induced by Either Streptococcus Agalactiae or Staphylococcus Aureus in Mice. Clin. Vaccine Immunol. 2011, 18, 893–900. [Google Scholar] [CrossRef][Green Version]
- Kiku, Y.; Ozawa, T.; Takahashi, H.; Kushibiki, S.; Inumaru, S.; Shingu, H.; Nagasawa, Y.; Watanabe, A.; Hata, E.; Hayashi, T. Effect of Intramammary Infusion of Recombinant Bovine GM-CSF and IL-8 on CMT Score, Somatic Cell Count, and Milk Mononuclear Cell Populations in Holstein Cows with Staphylococcus Aureus Subclinical Mastitis. Vet. Res. Commun. 2017, 41, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Kiku, Y.; Mizuno, M.; Inumaru, S.; Kushibiki, S.; Shingu, H.; Matsubara, T.; Takahashi, H.; Hayashi, T. Effect of Intramammary Infusion of RbGM-CSF on SCC and Expression of Polymorphonuclear Neutrophil Adhesion Molecules in Subclinical Mastitis Cows. Vet. Res. Commun. 2012, 36, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.B.; Albright, B.N.; Caswell, J.L. Effect of Interleukin-8 and Granulocyte Colony-Stimulating Factor on Priming and Activation of Bovine Neutrophils. Infect. Immun. 2003, 71, 1643–1649. [Google Scholar] [CrossRef]
- Daley, M.J.; Furda, G.; Dougherty, R.; Coyle, P.A.; Williams, T.J.; Johnston, P. Potentiation of Antibiotic Therapy for Bovine Mastitis by Recombinant Bovine Interleukin-2. J. Dairy Sci. 1992, 75, 3330–3338. [Google Scholar] [CrossRef]
- Daley, M.J.; Coyle, P.A.; Williams, T.J.; Furda, G.; Dougherty, R.; Hayes, P.W. Staphylococcus Aureus Mastitis: Pathogenesis and Treatment with Bovine Interleukin-1β and Interleukin-2. J. Dairy Sci. 1991, 74, 4413–4424. [Google Scholar] [CrossRef]
- Quiroga, G.H.; Sordillo, L.M.; Adkinson, R.W.; Nickerson, S.C. Cytologic Responses of Staphylococcus Aureus-Infected Mammary Glands of Heifers to Interferon Gamma and Interleukin-2 Treatment. Am. J. Vet. Res. 1993, 54, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Piccinini, R.; Fiorina, S.; Cabrini, L.; Daprà, V.; Amadori, M. Evaluation of Interleukin-2 Treatment for Prevention of Intramammary Infections in Cows after Calving. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Yagi, Y.; Shiono, H.; Yokomizo, Y.; Inumaru, S. Effects of Intramammary Infusions of Interleukin-8 on Milk Protein Composition and Induction of Acute-Phase Protein in Cows during Mammary Involution. Can. J. Vet. Res. 2008, 72, 291–296. [Google Scholar] [PubMed]
- Watanabe, A.; Hirota, J.; Shimizu, S.; Inumaru, S.; Kimura, K. Single Intramammary Infusion of Recombinant Bovine Interleukin-8 at Dry-off Induces the Prolonged Secretion of Leukocyte Elastase, Inflammatory Lactoferrin-Derived Peptides, and Interleukin-8 in Dairy Cows. Vet. Med. Int. 2012, 2012, 172072. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Yagi, Y.; Shiono, H.; Yokomizo, Y. Effect of Intramammary Infusion of Tumour Necrosis Factor-α on Milk Protein Composition and Induction of Acute-Phase Protein in the Lactating Cow. J. Vet. Med. Ser. B 2000, 47, 653–662. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Babiuk, L.A. Controlling Acute Escherichia Coli Mastitis during the Periparturient Period with Recombinant Bovine Interferon Gamma. Vet. Microbiol. 1991, 28, 189–198. [Google Scholar] [CrossRef]
- Solis-Castillo, L.A.; Garcia-Romo, G.S.; Diaz-Rodriguez, A.; Reyes-Hernandez, D.; Tellez-Rivera, E.; Rosales-Garcia, V.H.; Mendez-Cruz, A.R.; Jimenez-Flores, J.R.; Villafana-Vazquez, V.H.; Pedroza-Gonzalez, A. Tumor-Infiltrating Regulatory T Cells, CD8/Treg Ratio, and Cancer Stem Cells Are Correlated with Lymph Node Metastasis in Patients with Early Breast Cancer. Breast Cancer 2020, 27, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F. Molecular and Cellular Pathways of Immunoglobulin G Activity In Vivo. ISRN Immunol. 2014, 2014, 524081. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, X.Y.; Jin, L.J.; You, J.S.; Zhou, Y.; Li, S.Y.; Xu, Y.P. Characterization of Chicken Egg Yolk Immunoglobulins (IgYs) Specific for the Most Prevalent Capsular Serotypes of Mastitis-Causing Staphylococcus Aureus. Vet. Microbiol. 2011, 149, 415–421. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Jin, L.J.; Guo, J.; Li, X.Y.; Li, Z.; Fang, R.; Xu, Y.P. Characterization of Specific Egg Yolk Immunoglobulin (IgY) against Mastitis-Causing Staphylococcus Aureus. J. Appl. Microbiol. 2008, 105, 1529–1535. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Jin, L.J.; Li, X.Y.; Guo, J.; Li, Z.; Zhang, B.J.; Fang, R.; Xu, Y.P. Efficacy of Specific Egg Yolk Immunoglobulin (IgY) to Bovine Mastitis Caused by Staphylococcus Aureus. Vet. Microbiol. 2009, 133, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Kerro-Dego, O.; Prado, M.E.; Headrick, S.I.; Lewis, M.J.; Siebert, L.J.; Pighetti, G.M.; Oliver, S.P. Protective Effect of Anti-SUAM Antibodies on Streptococcus Uberis Mastitis. Vet. Res. 2015, 46, 133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Tian, X.; Wang, Q.; Hao, J.; Jiang, J.; Wang, H. Regulation Mechanisms and Maintenance Strategies of Stemness in Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2024, 20, 455–483. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.F.; Higashi, Y. Mesenchymal Stem/Stromal Cells for Therapeutic Angiogenesis. Cells 2023, 12, 2162. [Google Scholar] [CrossRef] [PubMed]
- Peralta, O.A.; Carrasco, C.; Vieytes, C.; Tamayo, M.J.; Muñoz, I.; Sepulveda, S.; Tadich, T.; Duchens, M.; Melendez, P.; Mella, A.; et al. Safety and Efficacy of a Mesenchymal Stem Cell Intramammary Therapy in Dairy Cows with Experimentally Induced Staphylococcus Aureus Clinical Mastitis. Sci. Rep. 2020, 10, 2843. [Google Scholar] [CrossRef] [PubMed]
- Saenz-de-Juano, M.D.; Silvestrelli, G.; Bauersachs, S.; Ulbrich, S.E. Determining Extracellular Vesicles Properties and MiRNA Cargo Variability in Bovine Milk from Healthy Cows and Cows Undergoing Subclinical Mastitis. BMC Genom. 2022, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Tamma, M.; Pathigadapa, U.; Reddanna, P.; Yenuganti, V.R. Drug Loading and Functional Efficacy of Cow, Buffalo, and Goat Milk-Derived Exosomes: A Comparative Study. Mol. Pharm. 2022, 19, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The Bioactivity of Colostrum and Milk Exosomes of High, Average, and Low Immune Responder Cows on Human Intestinal Epithelial Cells. J. Dairy Sci. 2021, 104, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of Highly Purified Bovine Milk-Derived Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; de las Hazas, M.C.L.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for Mirna-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Sun, X.; Huang, M.; Ma, Q.; Du, L.; Cui, Y. Enhanced Neuroprotective Effects of Epicatechin Gallate Encapsulated by Bovine Milk-Derived Exosomes against Parkinson’s Disease through Antiapoptosis and Antimitophagy. J. Agric. Food Chem. 2021, 69, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shang, F.; Chen, X.; Ni, J.; Yu, L.; Zhang, M.; Sun, D.; Xue, T. The Anti-Biofilm Effect of Silvernanoparticle-Decorated Quercetin Nanoparticles on a Multi-Drug Resistant Escherichia Coli Strain Isolated from a Dairy Cow with Mastitis. PeerJ 2018, 2018, 5711. [Google Scholar] [CrossRef] [PubMed]
- Castelani, L.; Arcaro, J.R.P.; Braga, J.E.P.; Bosso, A.S.; Moura, Q.; Esposito, F.; Sauter, I.P.; Cortez, M.; Lincopan, N. Short Communication: Activity of Nisin, Lipid Bilayer Fragments and Cationic Nisin-Lipid Nanoparticles against Multidrug-Resistant Staphylococcus Spp. Isolated from Bovine Mastitis. J. Dairy Sci. 2019, 102, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and Copper Nanoparticles—An Alternative in Future Mastitis Treatment and Prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro Machado, G.T.; Veleirinho, M.B.; Mazzarino, L.; Machado Filho, L.C.P.; Maraschin, M.; Cerri, R.L.A.; Kuhnen, S. Development of Propolis Nanoparticles for the Treatment of Bovine Mastitis: In Vitro Studies on Antimicrobial and Cytotoxic Activities. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar] [CrossRef]
- Orellano, M.S.; Isaac, P.; Breser, M.L.; Bohl, L.P.; Conesa, A.; Falcone, R.D.; Porporatto, C. Chitosan Nanoparticles Enhance the Antibacterial Activity of the Native Polymer against Bovine Mastitis Pathogens. Carbohydr. Polym. 2019, 213, 1–9. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, X.; Xu, Q.; Su, J.; Li, X.; Zhou, W. Evaluation of the Antibacterial Activity of Tilmicosin-SLN against Streptococcus Agalactiae: In Vitro and in Vivo Studies. Int. J. Nanomed. 2018, 13, 4747–4755. [Google Scholar] [CrossRef]
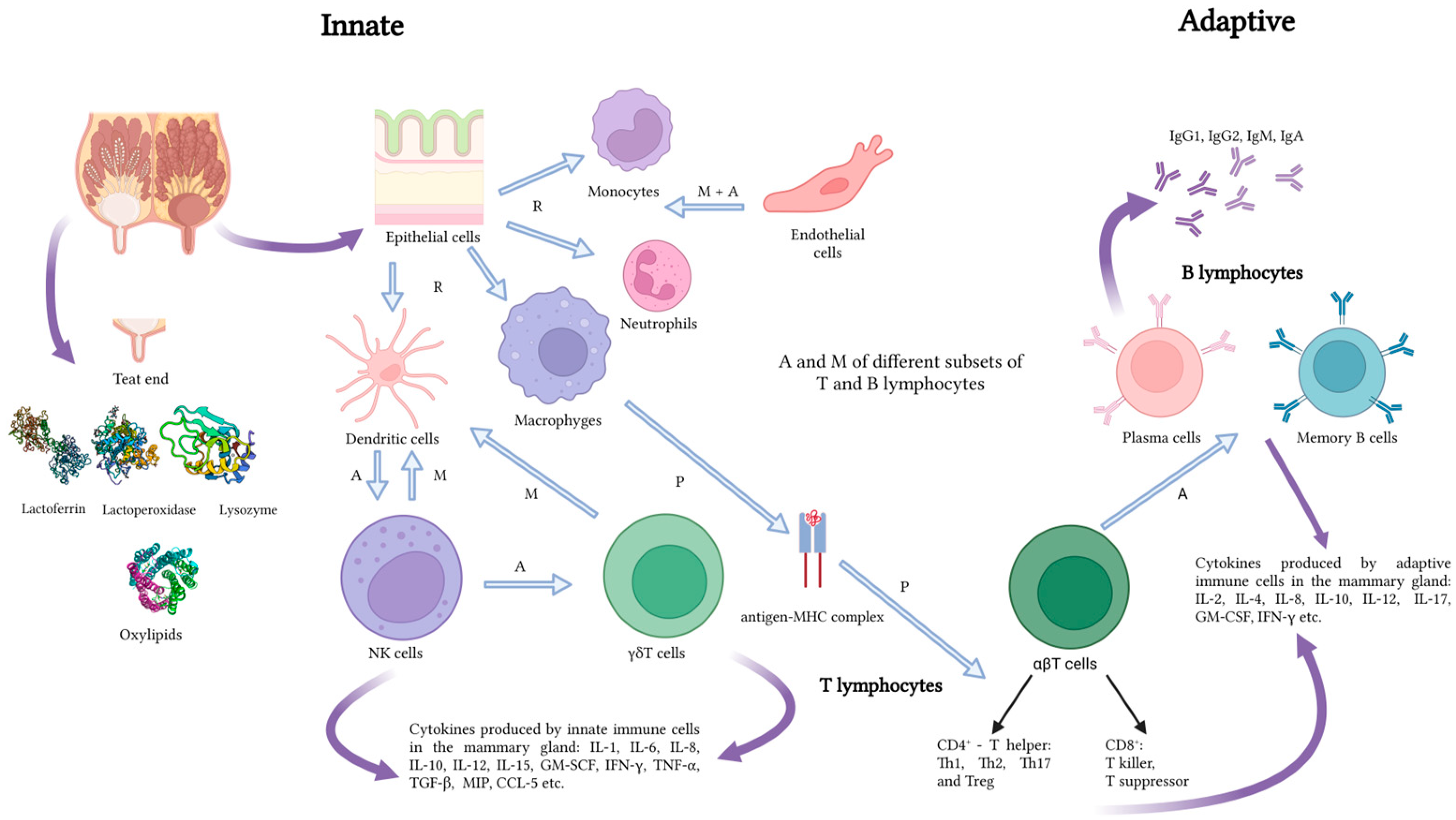

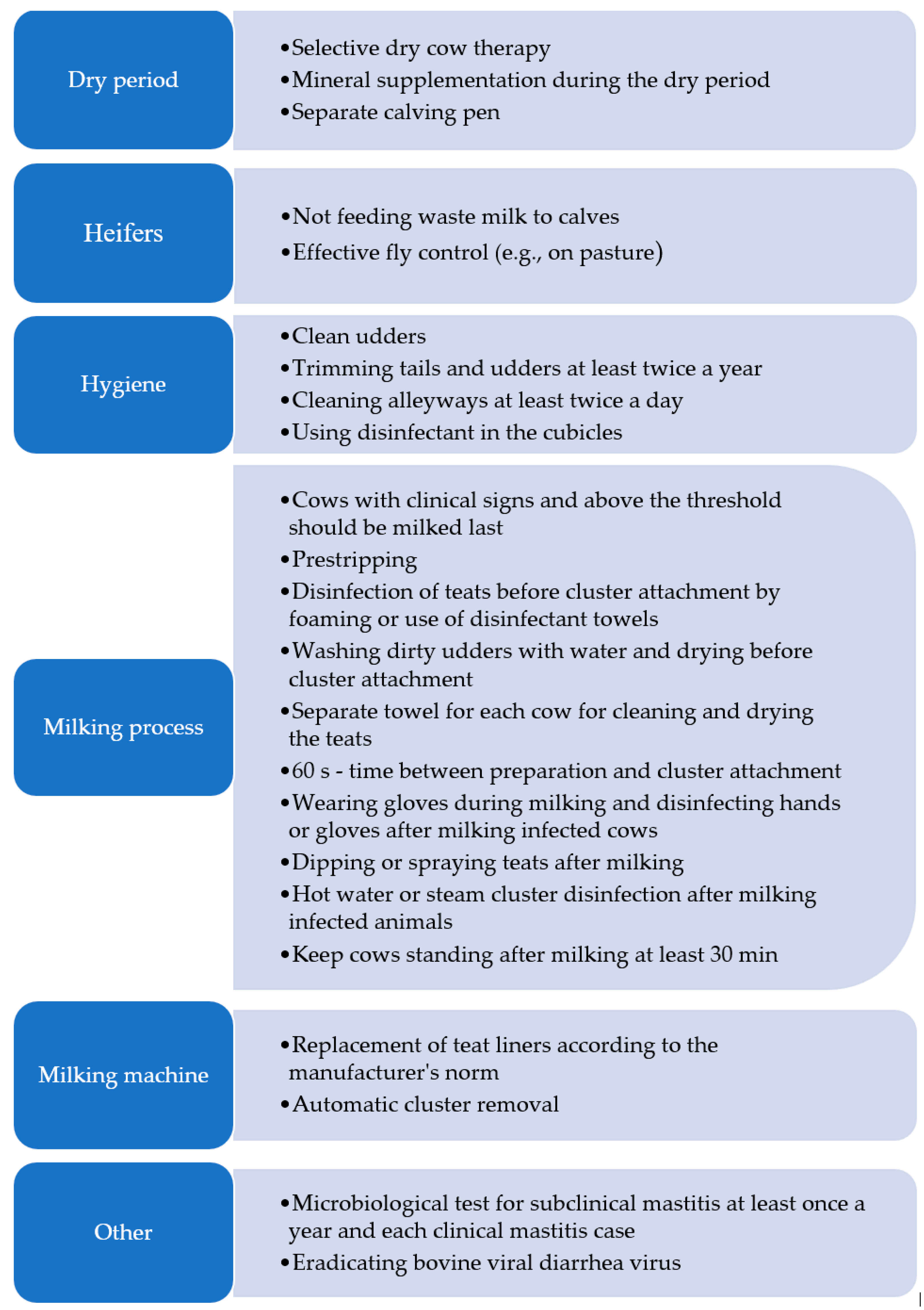
| Topic Area | Keywords |
|---|---|
| Pathogens involved and severity of clinical signs of mastitis | (“bovine” OR “cattle”) AND (“mastitis”) AND (“etiology” OR “pathogenesis”) |
| Losses associated with dairy cow mastitis | (“bovine” OR “cattle”) AND (“mastitis”) AND (“losses” OR “cost”) |
| Mastitis detection | (“bovine” OR “cattle”) AND (“mastitis”) AND (“diagnosis” OR “detection”) AND (“sensitivity” OR “specificity”) |
| Some management practices in mastitis prevention | (“bovine” OR “cattle”) AND (“mastitis”) AND (“prevention” OR “management”) |
| Method for Diagnosis | Optimal Cut-Off (Treshold) Value | Sensitivity | Specificity | Area under the ROC Curve (95% CI) | Positive Predicted Value | Negative Predicted Value | Authors |
|---|---|---|---|---|---|---|---|
| SCC | 100,000–200,000 cells/mL | 0.21–1.00 | 0.21–0.95 | 0.52–0.75 | 0.21–0.75 | 0.65–0.97 | [3,68,69,70,71,72,73,74] |
| CMT | Trace and above | 0.37–0.95 | 0.26–0.95 | 0.52–0.95 | 0.14–0.59 | 0.67–0.97 | [70,71,77,78,79,80] |
| Premilking and postmilking sampling | - | 0.25–1.00 | 0.83–1.00 | - | 0.63–1.00 | 0.45–1.00 | [81,82,83,84] |
| DSCC | 49.5–80.0% | 0.11–0.87 | 0.49–1.00 | 0.58–0.62 | 0.19–0.48 | 0.79–0.98 | [73,85,86,87] |
| SCC + DSCC | 100,000–200,000 cells/mL + 49.5–80.0% | 0.52–0.97 | 0.37–0.92 | 0.64 | 0.19–0.45 | 0.84–0.98 | [72,73] |
| Method for Diagnosis | Optimal Cut-Off (Treshold) Value | Sensitivity | Specificity | Area under the ROC Curve (95% CI) | Positive Predicted Value | Negative Predicted Value | Authors |
|---|---|---|---|---|---|---|---|
| EC | ≥5.1–≥7.0 mS/cm | 0.40–0.58 | 0.47–0.98 | 0.47–0.58 | 0.20–0.54 | 0.50–0.88 | [71] |
| EC | 25–30 mΩ/cm and below | 0.20–0.89 | 0.87–0.99 | 0.71–0.91 | - | - | [79] |
| RNNs in AMS | - | 0.68–0.90 | 0.84 | - | - | - | [109] |
| IRT | 32.6–35.1 °C | 0.42–0.83 | 0.72–0.98 | 0.70–0.90 | - | - | [111,112] |
| 3D acceleration | - | 0.76 | 0.76 | 0.80–0.88 | - | - | [113] |
| Method for Diagnosis | Optimal Cut-Off (Treshold) Value | Sensitivity | Specificity | Area under the ROC Curve (95% CI) | Positive Predicted Value | Negative Predicted Value | Authors |
|---|---|---|---|---|---|---|---|
| SAA in serum | 159.1 µg/mL | 0.90 | 0.72 | 0.84 | - | - | [137] |
| SAA in milk | 1.28–1.81 µg/mL | 0.65–0.77 | 0.76–0.83 | 0.88 | - | - | [134,135] |
| Hp in serum | 3.5 µg/mL | 0.74 | 0.69 | 0.75 | - | - | [125] |
| Hp in milk | 2.20–5.40 µg/mL | 0.82–0.96 | 0.92–0.99 | - | - | - | [134,136] |
| CRP in serum | 10.98 pg/mL | 0.98 | 1.00 | 0.99 | - | - | [140] |
| APP in milk (G+ vs G− differentiation DT model) | CRP < 9.5, LF ≥ 325, MAA < 16 μg/ml | 0.64 | 0.91 | 0.84 | - | - | [141] |
| Method for Diagnosis | Optimal Cut-Off (Treshold) Value | Sensitivity | Specificity | Area under the ROC Curve (95% CI) | Positive Predicted Value | Negative Predicted Value | Authors |
|---|---|---|---|---|---|---|---|
| LDH | ln 0.47 | 0.61–0.80 | 0.56–0.85 | 0.61–0.88 | 0.46 | 0.77 | [74,162] |
| LDH | 99.5–103.5 U/L | 0.80–0.81 | 0.86–0.87 | - | - | - | [136] |
| SCC + LDH | - | 0.96–1.00 | 0.92–0.99 | 0.98–0.99 | - | - | [74] |
| NAGase | ln 1.77 | 0.63 | 0.58 | 0.62 | 0.44 | 0.75 | [162] |
| CTHL in in milk | 0.00–0.05 | 0.76–0.98 | 0.86–0.99 | 0.78 | - | - | [62,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, P.; Żółkiewski, P.; Januś, E. A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives. Agriculture 2024, 14, 1292. https://doi.org/10.3390/agriculture14081292
Stanek P, Żółkiewski P, Januś E. A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives. Agriculture. 2024; 14(8):1292. https://doi.org/10.3390/agriculture14081292
Chicago/Turabian StyleStanek, Piotr, Paweł Żółkiewski, and Ewa Januś. 2024. "A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives" Agriculture 14, no. 8: 1292. https://doi.org/10.3390/agriculture14081292
APA StyleStanek, P., Żółkiewski, P., & Januś, E. (2024). A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives. Agriculture, 14(8), 1292. https://doi.org/10.3390/agriculture14081292







