The Allelopathy of the Invasive Plant Species Ludwigia decurrens against Rice and Paddy Weeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Evaluation of the Allelopathic Activity of L. decurrens
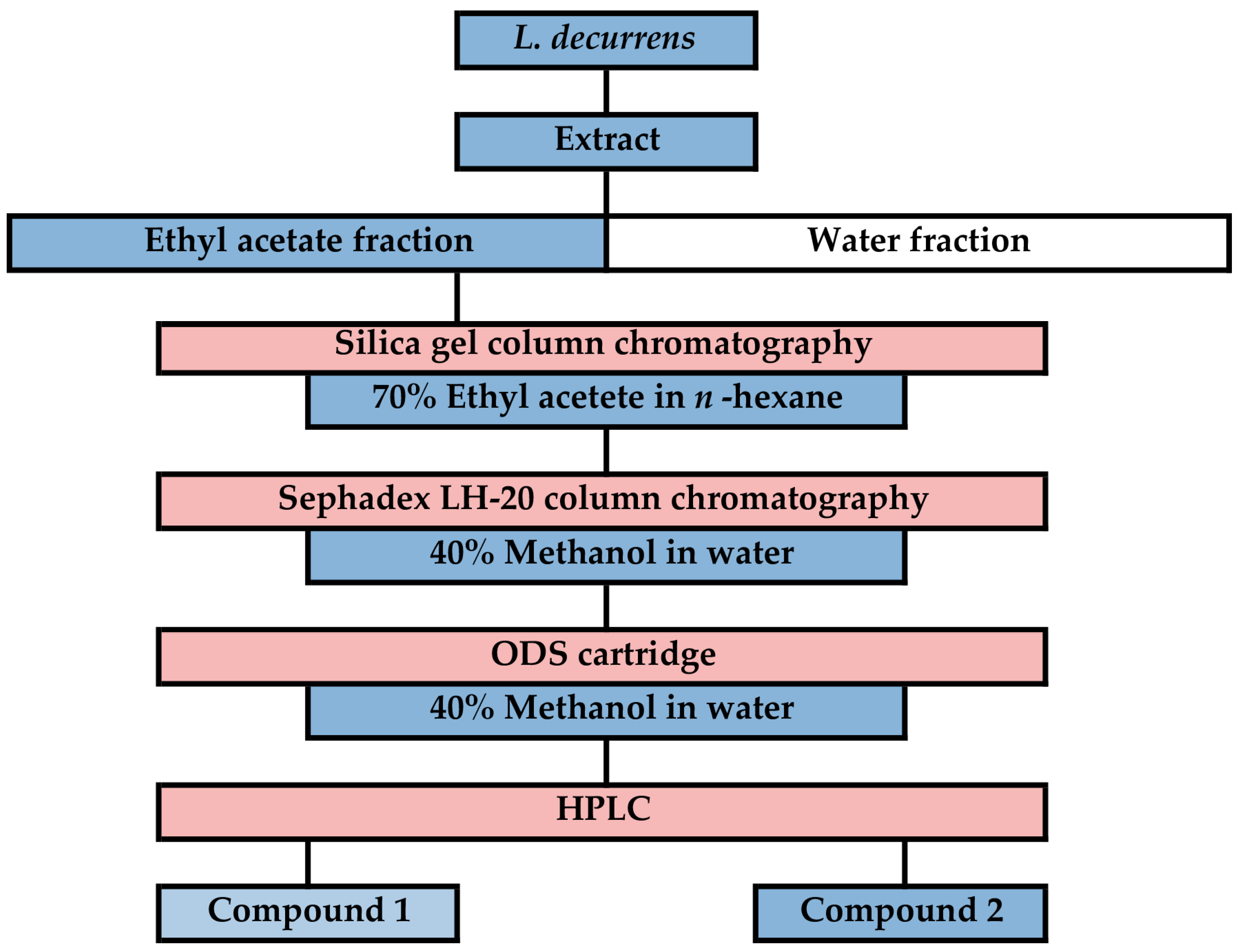
2.3. Isolation of Allelochemicals from L. decurrens
2.4. Allelopathic Activity of the Isolated Compounds
2.5. Statistics
3. Results
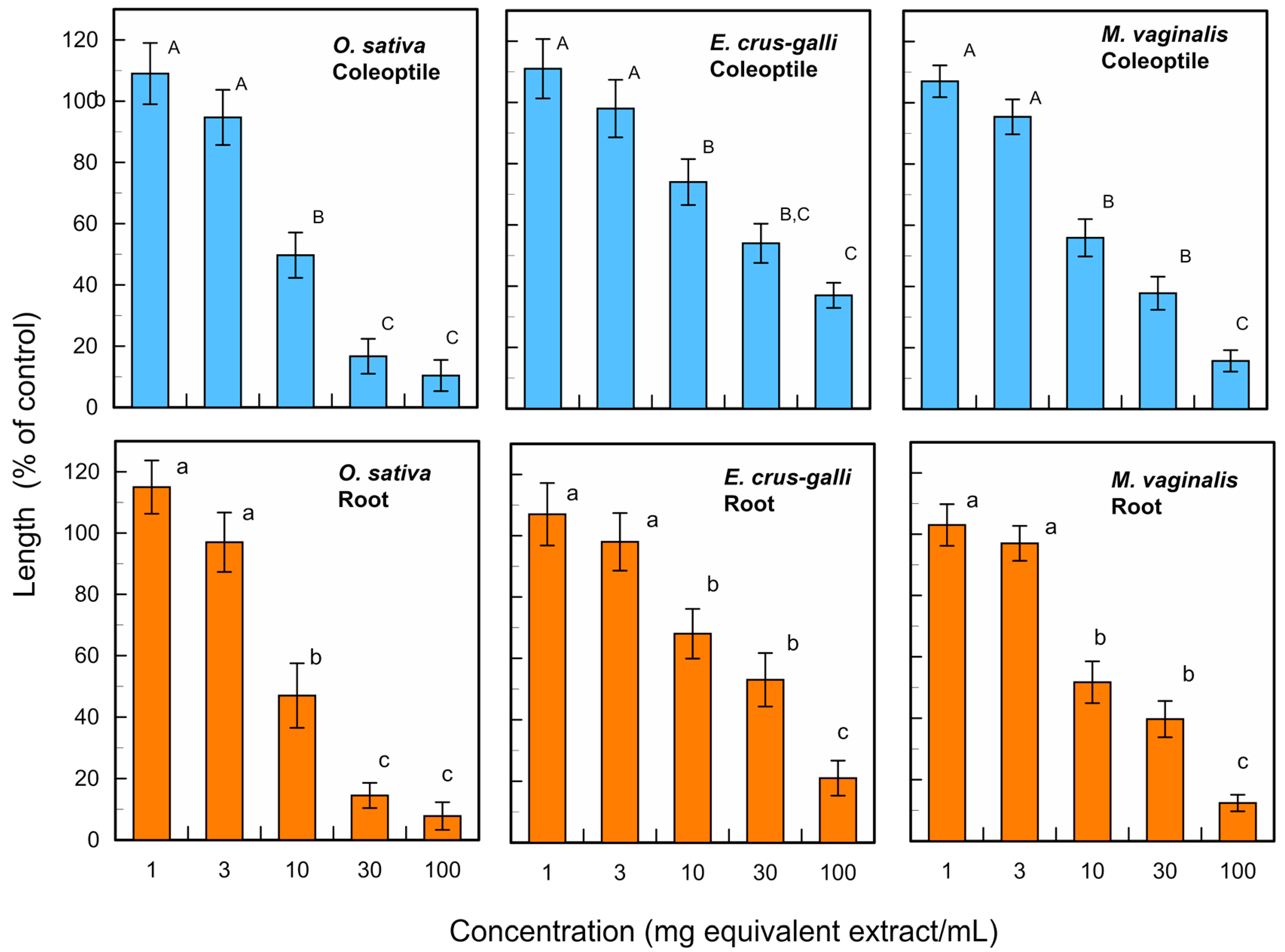
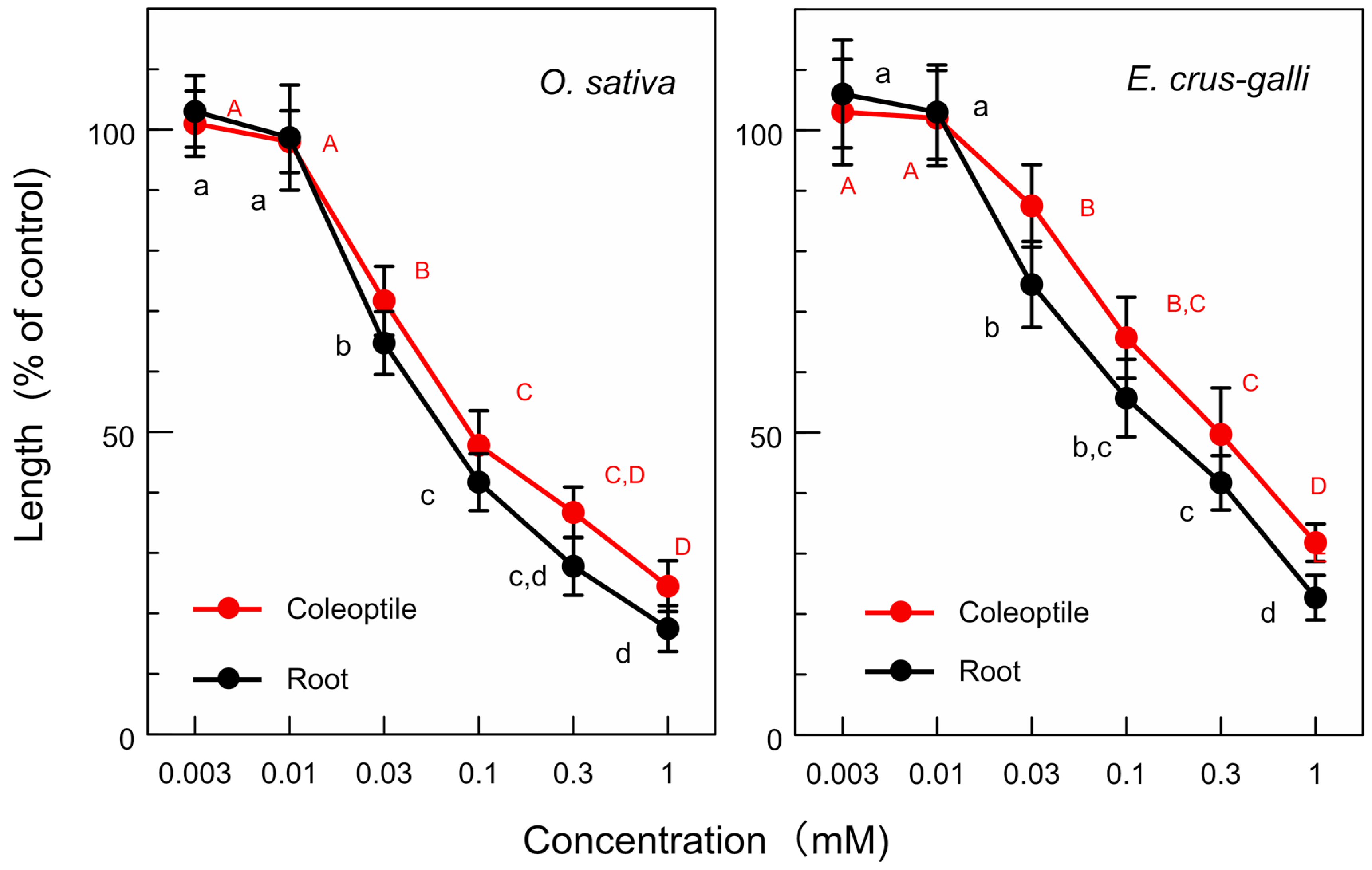
3.1. Allelopathic Activity of L. decurrens
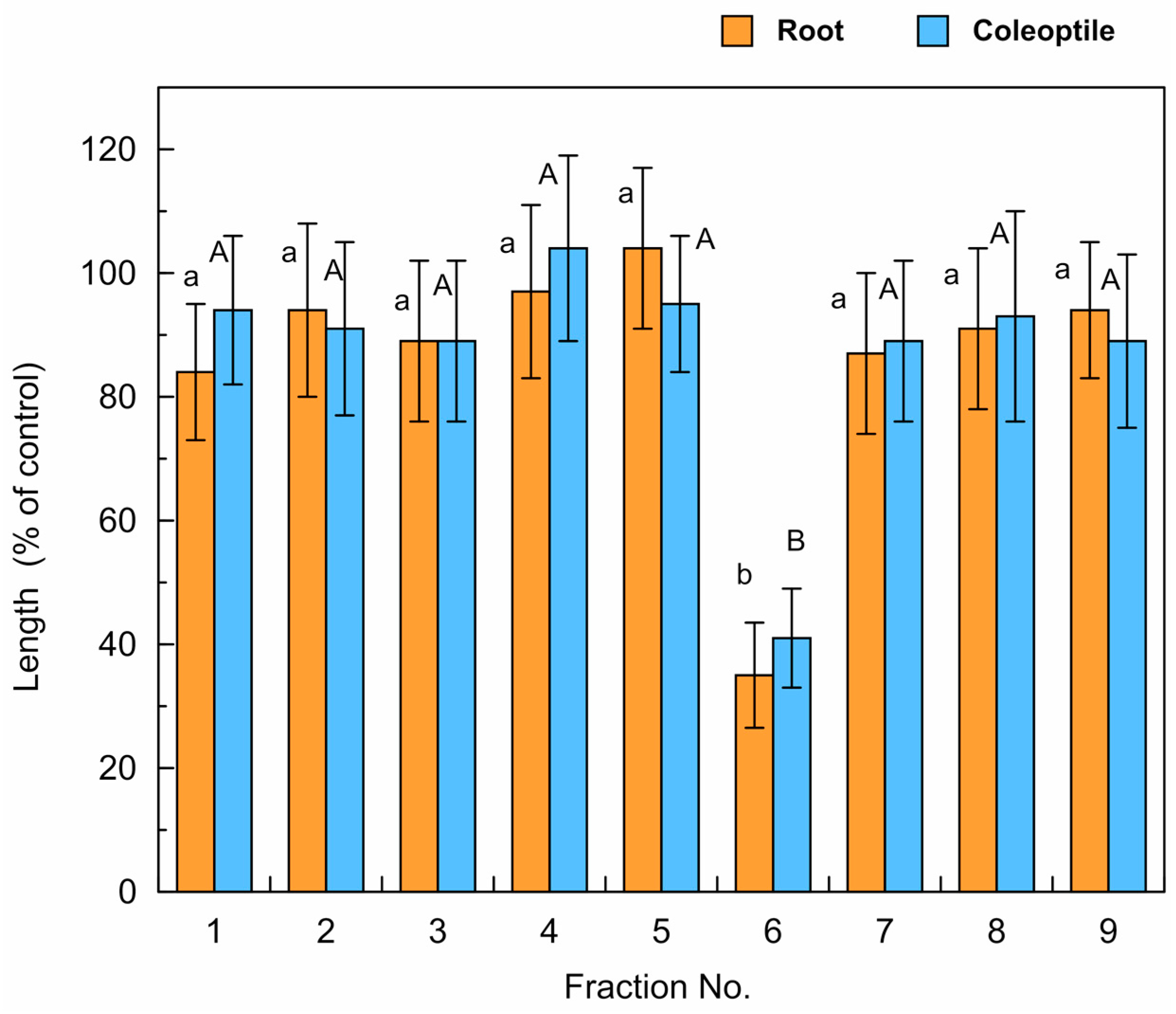
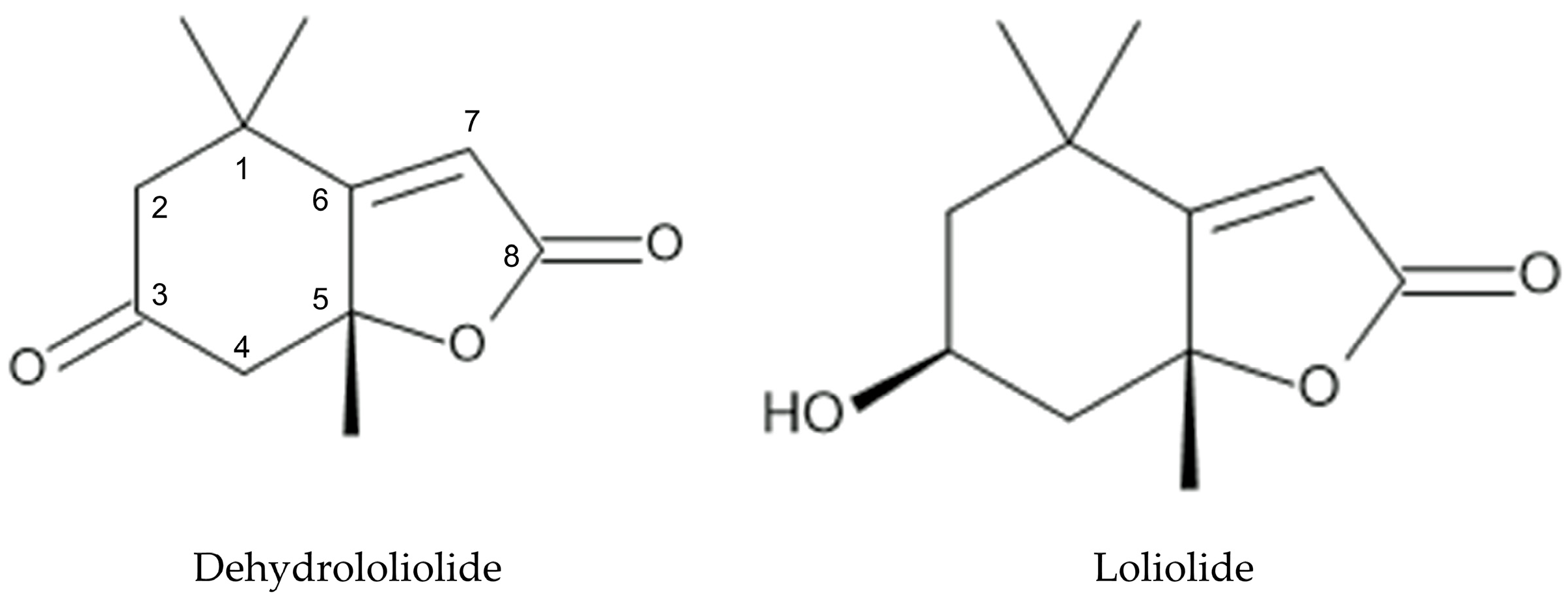
3.2. Isolation and Identification of Allelochemicals in L. decurrens
3.3. Allelopathic Activity of the Isolated Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard. I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128. [Google Scholar] [CrossRef]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.M.; Crawley, M.L. Exotic plant invasions and the enemy release hypothesis. Trend. Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nature 2003, 421, 625–627. [Google Scholar] [CrossRef]
- Cappuccino, N.; Carpenter, D. Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol. Lett. 2005, 1, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.G.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.S. Allelopathy in weed management, In Allelopathy Update. Vol. 2. Basic and Applied Aspects; Narwal, S.S., Ed.; Science Publishers Inc.: Enfield, NH, USA, 1999; pp. 203–254. [Google Scholar]
- Chandrasena, J.P.N.R. Ludwigia decurrens Walt. A new rice-field weed in Sri Lanka. J. Natil. Sci. Counc. Sri Lanka 1988, 16, 97–103. [Google Scholar] [CrossRef]
- Ludwigia decurrens Walter. Available online: https://warcapps.usgs.gov/PlantID/Species/Details/1659 (accessed on 2 April 2024).
- Bedoya, A.M.; Madriñán, S. Evolution of the aquatic habit in Ludwigia (Onagraceae): Morpho-anatomical adaptive strategies in the Neotropics. Aquat. Bot. 2015, 120 Pt B, 352–362. [Google Scholar] [CrossRef]
- USDA; NRCS. Plant Guide; Willow Primrose. Available online: https://plants.sc.egov.usda.gov/home/plantProfile?symbol=LUDE4 (accessed on 2 April 2024).
- Flora of North America. Ludwigia decurrens. Available online: http://floranorthamerica.org/Ludwigia_decurrens (accessed on 2 April 2024).
- Species Profile: Ludwigia decurrens. Available online: https://static1.squarespace.com/static/58740d57579fb3b4fa5ce66f/t/65c559d889a2ab029feadf15/1707432409855/Species+Profile+Ludwigia+decurrens.pdf (accessed on 2 April 2024).
- Invasive Species Factsheet: Water Primrose. Available online: https://storymaps.arcgis.com/stories/d8fcab60eb6744738c5ff061447d50c3 (accessed on 2 April 2024).
- Barua, I.C. The genus Ludwigia (Onagraceae) in India. Rhedea 2010, 20, 59–70. [Google Scholar]
- Oyedeji, O.; Oziegbe, M.; Taiwo, F.O. Antibacterial, antifungal and phytochemical analysis of crude extracts from the leaves of Ludwigia abyssinica A. Rich. and Ludwigia decurrens Walter. J. Med. Plants Res. 2011, 5, 1192–1199. [Google Scholar]
- Kong, L.P.; Peng, Y.F.; You, K.; Peng, H.H.; Wang, G.H. Ludwigia decurrens Walt., a naturalized hydrophyte in mainland China. J. Trop. Subtrop. Bot. 2019, 27, 338–342. [Google Scholar]
- Kurniadie, D.; Widianto, R.; Widayat, D.; Umiyati, U.; Nasahi, C.; Kato-Noguchi, H. Herbicide-resistant invasive plant species Ludwigia decurrens Walter. Plants 2021, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Cronk, Q.C.B.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Earthscan Publications: London, UK, 2001; pp. 1–241. [Google Scholar]
- Oziegbe, M.; Faluyi, J.O. Reproductive biology of Ludwigia leptocarpa and L. adscendens subsp. diffusa in Ile Ife, Nigeria. Turk. J. Bot. 2012, 36, 167–173. [Google Scholar] [CrossRef]
- Thouvenot, L.; Puech, C.; Martinez, L.; Haury, J.; Thiébaut, G. Strategies of the invasive macrophyte Ludwigia grandiflora in its introduced range: Competition, facilitation or coexistence with native and exotic species? Aquat. Bot. 2013, 107, 8–16. [Google Scholar] [CrossRef]
- Reddy, A.M.; Pratt, P.; Grewell, B.J.; Harms, N.E.; Walsh, G.C.; Hernández, M.C.; Faltlhauser, A.; Cibils-Stewart, X. Biological control of invasive water primroses, Ludwigia spp., in the United States: A feasibility assessment. J. Aquat. Plant Manag. 2021, 59, 67–77. [Google Scholar]
- Grewell, B.J.; Netherland, M.D.; Thomason, M.J.S. Establishing Research and Management Oriorities for Invasive Water Primroses (Ludwigia spp.); U.S. Army Crops of Engineers: Washington, DC, USA, 2016; pp. 1–42. [Google Scholar]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef]
- Dharmaratne, P.G.; Ranamukaarachchi, S.L. Sensitivity of rice to Ludwigia decurrens (L.). Trop. Agric. Res. 1991, 3, 180–194. [Google Scholar]
- Kim, S.C.; Heu, H.; Park, P.K. Study on weed control on paddy field. Three studies on competition between major annual weeds and rice in transplanted rice paddy field. Res. Rep. Rural Dev. 1977, 19, 133–144. [Google Scholar]
- Dandelot, S.; Robles, C.; Pech, N.; Cazaubon, A.; Verlaque, R. Allelopathic potential of two invasive alien Ludwigia spp. Aquat. Bot. 2008, 88, 311–316. [Google Scholar] [CrossRef]
- Roy, N.; Basu, M.; Chattopadhyay, C.; Barik, A. Allelopathic effects of rice field weed, Ludwigia adscendens (L.) on germination and seedling growth of three economic crop plants. Int. J. Horti. Crop Sci. Res. 2011, 1, 15–20. [Google Scholar]
- Mukherjee, A.; Barik, A. Potential allelopathic effects of Ludwigia adscendens L. on the seed germination and seedling growth of rice. Indian J. Agric. Res. 2013, 47, 1–15. [Google Scholar]
- Thiébaut, G.; Thouvenot, L.; Rodríguez-Pérez, H. Allelopathic effect of the invasive Ludwigia hexapetala on growth of three macrophyte species. Front. Plant Sci. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Le Rouzic, B.; Thiébaut, G. Seasonal dependence and functional implications of macrophyte-phytoplankton allelopathic interactions. Freshw. Biol. 2018, 63, 1161–1172. [Google Scholar] [CrossRef]
- Sim, D.Z.; Mowe, M.A.; Mitrovic, S.M.; Tulsian, N.K.; Anand, G.S.; Yeo, D.C. Nutrient conditions influence allelopathic capabilities of Ludwigia adscendens and other tropical macrophytes against Microcystis aeruginosa. Freshw. Biol. 2024, 69, 538–555. [Google Scholar] [CrossRef]
- Pelella, E.; Questino, B.; Ceschin, S. Impact of the alien aquatic plant Ludwigia hexapetala on the native Utricularia australis: Evidence from an indoor experiment. Plants 2023, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Sakpere, A.M.; Oziegbe, M.; Obisesan, I.A. Allelopathic effects of Ludwigia decurrens and L. adscendens subsp. diffusa on germination, seedling growth and yield of Corchorus olitorious L. Not. Sci. Biol. 2010, 2, 75–80. [Google Scholar] [CrossRef][Green Version]
- Wang, G.; Kusanagi, T.; Itoh, K. Environmental factors relating to dormancy breaking, germination and emergence of seeds in Monochoria korsakowii Regel et Maack and M. vaginalis (Burm. f.) Kunth. Weed Res. 1996, 41, 247–254. [Google Scholar]
- Ogorek, L.P.; Striker, G.G.; Mollard, F.P.O. Echinochloa crus-galli seed physiological dormancy and germination responses to hypoxic floodwaters. Plant Biol. 2019, 21, 1159–1166. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, Y.; Kojima, M.; Kumagai, S.; Iwasaki, A.; Suenaga, K. Allelopathic substances of Osmanthus spp. for developing sustainable agriculture. Plants 2023, 12, 376. [Google Scholar] [CrossRef]
- Ravi, B.N.; Murphy, P.T.; Lidgard, R.O.; Warren, R.G.; Wells, R.J. C18 terpenoid metabolites of the brown alga Cystophora moniliformis. Aust. J. Chem. 1982, 35, 171–182. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undariapinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, S.K.; Kim, C.S.; Kim, K.S.; Moon, D.C. Phytochemical constituents of Carpesium macrocephalum FR-et SAV. Arch. Pharmacal Res. 2004, 27, 1029–1033. [Google Scholar] [CrossRef]
- Hodges, R.; Porte, A.L. The structure of loliolide: A terpene from Lolium perenne. Tetrahedron 1964, 20, 1463–1467. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Potaniec, B.; Anioł, M. Loliolide-the most ubiquitous lactone. Acta Univ. Lodziensis. Folia Biol. Oecol. 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Lu, H.; Xie, H.; Gong, Y.; Wang, Q.; Yang, Y. Secondary metabolites from the sea weed Gracilaria lemaneiformis and their allelopathic effects on Skeletonemacostatum. Biochem. Syst. Ecol. 2011, 39, 397–400. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Moriyasu, M.; Ohno, O.; Suenaga, K. Growth limiting effects on various terrestrial plant species by an allelopathic substance, loliolide, from water hyacinth. Aquat. Bot. 2014, 117, 56–61. [Google Scholar] [CrossRef]
- Global Invasive Species Database, Species Profile: Eichhornia crassipes. Available online: https://www.iucngisd.org/gisd/speciesname/Eichhornia+crassipes (accessed on 4 April 2024).
- Invasive Species Compendium, Eichhornia crassipes. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.20544 (accessed on 4 April 2024).
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Uegaki, R.; Fujimori, T.; Kaneko, H.; Kato, K.; Noguchi, M. Isolation of dehydrololiolide and 3-oxo-actinidol from Nicotiana tabacum. Agric. Biol. Chem. 1979, 43, 1149–1150. [Google Scholar] [CrossRef][Green Version]
- Wang, J.H.; Dai, C.D.; Long, R.; Yao, J.H.; Chen, X.; Yi, J.L.; Nan, X.Y.; Zhou, X.M. Phenolic and sesquiterpene derivatives from Fissistigma retusum. Biochem. Syst. Ecol. 2022, 102, 104417. [Google Scholar] [CrossRef]
- Oyawaluja, A.A.; Oyawaluja, B.O.; Aydogan, F.; Ali, Z.; Khan, I.A.; Zhao, J.; Odukoya, O.A.; Samuel, T.A.; Coker, H.A.B. Chemical constituents from the leaves of Nephrolepis exaltata. Biochem. Syst. Ecol. 2024, 112, 104772. [Google Scholar] [CrossRef]
- Hossen, K.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Identification of four allelopathic compounds including a novel compound from Elaeocarpus floribundus Blume and determination of their allelopathic activity. J. Environ. Manag. 2023, 326, 116728. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Wang, Y.; Xu, J.; He, X. Potential in vitro anti-neuroinflammatory sterols from mango fruits (Mangifera indica L.). J. Funct. Foods 2021, 84, 104576. [Google Scholar] [CrossRef]
- Chen, J.; Shao, J.; Zhao, C.; Shen, J.; Dong, Z.; Liu, W.; Zhao, M.; Fan, J. Chemical constituents from Viburnum fordiae Hance and their anti-inflammatory and antioxidant activities. Arch Pharm. Res. 2018, 41, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ju, B.; Lan, X.; Ying, X.; Stien, D. Seven compounds from Portulaca oleracea L. and their anticholinesterase activities. Nat. Prod. Res. 2022, 36, 4703–4707. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Kato, M. The Allelopathy of the Invasive Plant Species Ludwigia decurrens against Rice and Paddy Weeds. Agriculture 2024, 14, 1297. https://doi.org/10.3390/agriculture14081297
Kato-Noguchi H, Kato M. The Allelopathy of the Invasive Plant Species Ludwigia decurrens against Rice and Paddy Weeds. Agriculture. 2024; 14(8):1297. https://doi.org/10.3390/agriculture14081297
Chicago/Turabian StyleKato-Noguchi, Hisashi, and Midori Kato. 2024. "The Allelopathy of the Invasive Plant Species Ludwigia decurrens against Rice and Paddy Weeds" Agriculture 14, no. 8: 1297. https://doi.org/10.3390/agriculture14081297
APA StyleKato-Noguchi, H., & Kato, M. (2024). The Allelopathy of the Invasive Plant Species Ludwigia decurrens against Rice and Paddy Weeds. Agriculture, 14(8), 1297. https://doi.org/10.3390/agriculture14081297






