Influence of Growth Stages and Additives on the Fermentation Quality and Microbial Profiles of Whole-Plant Millet Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Silage Preparation
2.2. Silage Extract Preparation
2.3. Nutritional Component Analysis
2.4. Fermentation Quality Analysis
2.5. Aerobic Stability Analysis
2.6. Microbial Count
2.7. Bacterial Community Analysis
2.8. Data Processing
3. Results
3.1. Effect of Different Growth Stages on Nutritional Components of Whole-Plant Millet Silage
3.2. Effect of Different Additives on the Quality of Whole-Plant Millet Silage
3.3. Effect of Different Additives on the Aerobic Stability of Whole-Plant Millet Silage
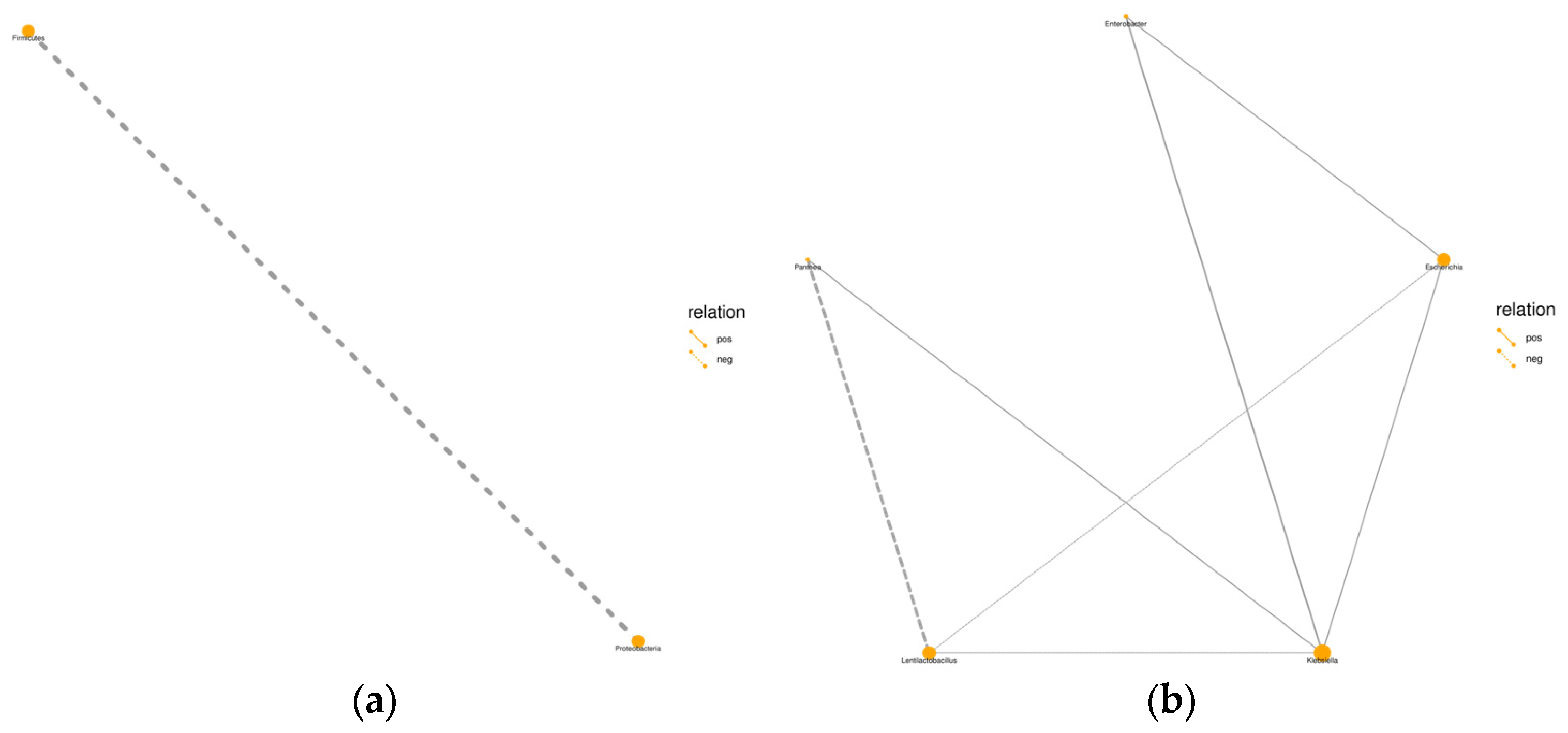
3.4. Effect of Different Additives on the Bacterial Communities of Whole-Plant Millet Silage
4. Discussion
4.1. Effect of Different Growth Stages on Nutritional Components of Whole-Plant Millet Silage
4.2. Effect of Different Additives on the Quality of Whole-Plant Millet Silage
4.3. Effect of Different Additives on the VFA Contents and Aerobic Stability of Whole-Plant Millet Silage
4.4. Effect of Different Additives on the Bacterial Communities of Whole-Plant Millet Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Zhang, J.; Liu, K.B.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X.; et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Hao, X.; Li, Y.; Zhang, Q.; Wang, C.; Han, H. Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri. Processes 2022, 10, 2369. [Google Scholar] [CrossRef]
- Costa, R.; Costa, K.; Souza, W.; Epifânio, P.; Santos, C.; Silva, J.; Oliveira, S. Production and quality of silages pearl millet and paiaguas palisadegrass in monocropping and intercropping in different forage systems. Biosci. J. 2018, 34, 957–967. [Google Scholar] [CrossRef]
- Amer, S.; Mustafa, A.F. Short communication: Effects of feeding pearl millet silage on milk production of lactating dairy cows. J. Dairy Sci. 2010, 93, 5921–5925. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.; Neves, A.L.A.; Pereira, L.G.R.; Sollenberger, L.E.; Muniz, E.N.; Souza, E.Y.B.; Sobral, A.J.S.; Costa, N.V.; Gonçalves, L.C. Performance, agronomic traits, ensilability and nutritive value of pearl millet cultivar harvested at different growth stages. J. Agric. Sci. 2020, 158, 225–232. [Google Scholar] [CrossRef]
- Hill, G.M.; Utley, P.R.; Gates, R.N.; Hanna, W.W.; Johnson, J.C., Jr. Pearl Millet Silage for Growing Beef Heifers and Steers. J. Prod. Agric. 1999, 12, 653–658. [Google Scholar] [CrossRef]
- Nussio, L.G.; Park, R.S.; Stronge, M.D. Silage Production from Tropical Forages; Wageningen Academic: Wageningen, The Netherlands, 2005. [Google Scholar]
- Hassanat, F.; Mustafa, A.F.; Seguin, P. Effects of inoculation on ensiling characteristics, chemical composition and aerobic stability of regular and brown midrib millet silages. Anim. Feed. Sci. Technol. 2007, 139, 125–140. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Rinne, M.; Franco, M.; Tapio, I.; Stefanski, T.; Bayat, A.; Mäntysaari, P. Effects of Grass Silage Additive Type and Barley Grain Preservation Method on Rumen Fermentation, Microbial Community and Milk Production of Dairy Cows. Agriculture 2022, 12, 266. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xie, Y.; Zhang, Y.; Lin, Y.; Zheng, Y.; Yang, X.; Wang, N.; Ni, K.; Yang, F. Effect of Sucrose and Lactic Acid Bacteria Additives on Fermentation Quality, Chemical Composition and Protein Fractions of Two Typical Woody Forage Silages. Agriculture 2021, 11, 256. [Google Scholar] [CrossRef]
- Kasmaei, K.M.; Dicksved, J.; Spörndly, R.; Udén, P. Separating the effects of forage source and field microbiota on silage fermentation quality and aerobic stability. Grass Forage Sci. 2017, 72, 281–289. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Fang, D.; Dong, Z.; Wang, D.; Li, B.; Shi, P.; Yan, J.; Zhuang, D.; Shao, T.; Wang, W.; Gu, M. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J. Appl. Microbiol. 2022, 132, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.R.; O’Kiely, P. Preharvest Plant Factors Affecting Ensiling. In Silage Science and Technology; Agronomy Monographs; Springer: Cham, Switzerland, 2003; pp. 199–250. [Google Scholar]
- Khan, S.H.; Azim, A.; Sarwar, M.; Khan, A.G. Effect of maturity on comparative nutritive value and fermentation characteristics of maize, sorghum and millet silage. Pak. J. Bot. 2011, 43, 2967–2970. [Google Scholar]
- Allen, M.; Coors, J.G.; Roth, G. Silage Science and Technology. Agronomy Monograph 42. Corn Silage 2003, 42, 547–608. [Google Scholar]
- Oskey, M.; Velasquez, C.; Peña, O.M.; Andrae, J.; Bridges, W.; Ferreira, G.; Aguerre, M.J. Yield, nutritional composition, and digestibility of conventional and brown midrib (BMR) pearl millet as affected by planting and harvesting dates and interseeded cowpea. Animals 2023, 13, 260. [Google Scholar] [CrossRef]
- Kiliçalp, N. Cultivar and Harvest Stage Effects on Nutritive Value of Whole Crop Oat (Avena sativa L.) Silages. J. Agric. Nat. 2022, 26, 437–449. [Google Scholar] [CrossRef]
- Hassanat, F.; Mustafa, A.F.; Seguin, P. Effect of the brown midrib trait and stage of development at harvest on cell wall composition and degradability of forage pearl millet leaves and stems. Can. J. Anim. Sci. 2007, 87, 421–429. [Google Scholar] [CrossRef]
- Morales, J.U.; Alatorre, J.A.H.; Nieto, C.A.R.; José, F.; Becerra, C. Forage production and nutritional content of silage from three varieties of pearl millet (Pennisetum glaucum) harvested at two maturity stages. J. Anim. Plant Sci. 2015, 27, 4161–4169. [Google Scholar]
- Kurniawan, W.; Napirah, A.; Isnaeni, P.; Bain, A. Nutrient qualities hydroponic corn green fodder (HCGF) of yellow-maize on different harvest time. IOP Conf. Ser. Earth Environ. Sci. 2024, 1341, 012062. [Google Scholar] [CrossRef]
- Ferraretto, L.; Shaver, R.; Luck, B. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Chu, P.F.; Yu, Z.W.; Wang, D.; Zhang, Y.L.; Xu, Z.Z. Effects of irrigation stage and amount on winter wheat fructan accumulation and translocation after anthesis and water use efficiency. Ying Yong Sheng Tai Xue Bao 2009, 20, 2691–2698. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of Soluble Sugars in Metabolism and Sensing Under Abiotic Stress. Plant Growth Regul. Signal. Under Stress Cond. 2021, 14, 305–334. [Google Scholar]
- Webster, J. The Biochemistry of Silage; McDonald, P., Henderson, A.R., Heron, S.J.E., Marlow, B., Eds.; Chalcombe Publications: Southampton, UK, 1991; p. 340. ISBN 0-948617-225. [Google Scholar]
- Kung, L. Silage fermentation and additives. Arch. Latinoam. Prod. Anim. 2018, 26, 61–66. [Google Scholar]
- Gerlach, K.; Daniel, J.L.P.; Jobim, C.C.; Nussio, L.G. A data analysis on the effect of acetic acid on dry matter intake in dairy cattle. Anim. Feed. Sci. Technol. 2021, 272, 114782. [Google Scholar] [CrossRef]
- Maмaeв, A.; Ocипян, Б.; Koзлoвa, B. Role of acetic acid in the sustainability of silage from cereal grasses to adverse micloflore at air access. Adapt. Fodd. Prod. 2019, 2019, 48–57. [Google Scholar] [CrossRef]
- Daniel, J.L.P.; Amaral, R.C.; Sá Neto, A.; Cabezas-Garcia, E.H.; Bispo, A.W.; Zopollatto, M.; Cardoso, T.L.; Spoto, M.H.F.; Santos, F.A.P.; Nussio, L.G. Performance of dairy cows fed high levels of acetic acid or ethanol. J. Dairy Sci. 2013, 96, 398–406. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Liu, Y.; Chen, T.; Li, M.; Zhou, H.; Tang, J. Effects of Sucrose, Glucose and Molasses on Fermentation Quality and Bacterial Community of Stylo Silage. Fermentation 2022, 8, 191. [Google Scholar] [CrossRef]
- Kang, J.; Tang, S.; Zhong, R.; Tan, Z.; Wu, D. Alfalfa Silage Treated with Sucrose Has an Improved Feed Quality and More Beneficial Bacterial Communities. Front. Microbiol. 2021, 12, 670165. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Zhang, Z.X.; Shimojo, M.; Wang, T.; Masuda, Y. Comparison of Fermentation Characteristics of Italian Ryegrass (Lolium multiflorum Lam.) and Guineagrass (Panicum maximum Jacq.) during the Early Stage of Ensiling. Asian-Australas. J. Anim. Sci. 2005, 18, 1727–1734. [Google Scholar] [CrossRef]
- Rezaei, J.; Rouzbehan, Y.; Fazaeli, H. Nutritive value of fresh and ensiled amaranth (Amaranthus hypochondriacus) treated with different levels of molasses. Anim. Feed. Sci. Technol. 2009, 151, 153–160. [Google Scholar] [CrossRef]
- You, J.; Zhang, H.; Zhu, H.; Xue, Y.; Cai, Y.; Zhang, G. Microbial Community, Fermentation Quality, and In Vitro Degradability of Ensiling Caragana with Lactic Acid Bacteria and Rice Bran. Front. Microbiol. 2022, 13, 804429. [Google Scholar] [CrossRef]
- He, L.; Chen, N.; Lv, H.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef]
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed. Sci. Technol. 2008, 145, 122–135. [Google Scholar] [CrossRef]
- Bao, J.; Wang, L.; Yu, Z. Effects of Different Moisture Levels and Additives on the Ensiling Characteristics and In Vitro Digestibility of Stylosanthes Silage. Anim. Open Access J. 2022, 12, 1555. [Google Scholar] [CrossRef]
- Larsen, S.U.; Hjort-Gregersen, K.; Vazifehkhoran, A.H.; Triolo, J.M. Co-ensiling of straw with sugar beet leaves increases the methane yield from straw. Bioresour. Technol. 2017, 245 Pt A, 106–115. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, B.; Liu, Y.; Du, G.; Wei, X.; Imam, K.M.S.U.; Zhou, H.; Fan, S.; Wang, F.; Wang, Y.; et al. A reverse catalytic triad Asp containing loop shaping a wide substrate binding pocket of a feruloyl esterase from Lactobacillus plantarum. Int. J. Biol. Macromol. 2021, 184, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, W.; Shao, T.; Li, J.; Dong, Z.; Yuan, X. Effect of hexanoic acid, Lactobacillus plantarum and their combination on the aerobic stability of napier grass silage. J. Appl. Microbiol. 2020, 129, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Wang, J.; Guo, G.; Wen, A.Y.; Desta, S.T.; Shao, T. Effects of ethanol, molasses and Lactobacillus plantarum on fermentation characteristics and aerobic stability of total mixed ration silages. Grass Forage Sci. 2016, 71, 328–338. [Google Scholar] [CrossRef]
- Dong, Z.; Li, J.; Wang, S.; Dong, D.; Shao, T. Time of Day for Harvest Affects the Fermentation Parameters, Bacterial Community, and Metabolic Characteristics of Sorghum-Sudangrass Hybrid Silage. Msphere 2022, 7, e00168-22. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, E.; Shapiro, O.H.; Siboni, N.; Kushmaro, A. Advantage of Using Inosine at the 3′ Termini of 16S rRNA Gene Universal Primers for the Study of Microbial Diversity. Appl. Environ. Microbiol. 2006, 72, 6902–6906. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Niu, D.; Zhao, Y. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation. Bioprocess Biosyst. Eng. 2015, 38, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vitorino, M.V.; Godinho, C.P.; Bourbon-Melo, N.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Yeast adaptive response to acetic acid stress involves structural alterations and increased stiffness of the cell wall. Sci. Rep. 2021, 11, 12652. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of acetic acid bacteria and lactic acid bacteria in multispecies solid-state fermentation of traditional Chinese cereal vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, C.; Zhong, Y.; Bu, X.; Huang, S.; Tahir, M.; Du, Z.; Liu, W.; Yang, W.; Li, J.; et al. Effect of storage time on the silage quality and microbial community of mixed maize and faba bean in the Qinghai-Tibet Plateau. Front. Microbiol. 2022, 13, 1090401. [Google Scholar] [CrossRef]
- Wayne Polley, H.; Wilsey, B.J.; Derner, J.D. Dominant species constrain effects of species diversity on temporal variability in biomass production of tallgrass prairie. Oikos 2007, 116, 2044–2052. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.-Y.; Chen, X.-Y.; Zhang, Q. Effects of Wilting and Lactobacillus plantarum Addition on the Fermentation Quality and Microbial Community of Moringa oleifera Leaf Silage. Front. Microbiol. 2018, 9, 1817. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.-A.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Dong, Z.; Shao, T. The reconstitution mechanism of napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 2020, 297, 122391. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of Gallic Acid on Fermentation Parameters, Protein Fraction, and Bacterial Community of Whole Plant Soybean Silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.-J.; Yu, Z. Effects of sucrose, formic acid and lactic acid bacteria inoculant on quality, in vitro rumen digestibility and fermentability of drooping wild ryegrass (Elymus nutans Griseb.) silage. J. Anim. Feed. Sci. 2017, 26, 26–32. [Google Scholar] [CrossRef]
- Lv, H.; Pian, R.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of citric acid on fermentation characteristics and bacterial diversity of Amomum villosum silage. Bioresour. Technol. 2020, 307, 123290. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Wang, X.; Zeng, Z.; Hu, Y.; Cui, Z.-J. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage. World J. Microbiol. Biotechnol. 2009, 25, 965–971. [Google Scholar] [CrossRef]
- Jiang, F.-G.; Cheng, H.-J.; Liu, D.; Wei, C.; An, W.-J.; Wang, Y.-F.; Sun, H.-T.; Song, E.-L. Treatment of Whole-Plant Corn Silage with Lactic Acid Bacteria and Organic Acid Enhances Quality by Elevating Acid Content, Reducing pH, and Inhibiting Undesirable Microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. In Silage Science and Technology; American Society of Agronomy: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Cheng, Q.; Chen, L.; Chen, Y.; Li, P.; Chen, C. Effects of LAB Inoculants on the Fermentation Quality, Chemical Composition, and Bacterial Community of Oat Silage on the Qinghai-Tibetan Plateau. Microorganisms 2022, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Pech Cervantes, A.A.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Wang, Z.; Bao, J.; Zhao, M.; Si, Q.; Sun, P.; Ge, G.; Jia, Y. Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure. Microorganisms 2023, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, Z.; Sun, Y.; Kong, X.; Dong, P.; Zhang, J. A reused method for molasses-processed wastewater: Effect on silage quality and anaerobic digestion performance of Pennisetum purpereum. Bioresour. Technol. 2017, 241, 1003–1011. [Google Scholar] [CrossRef]
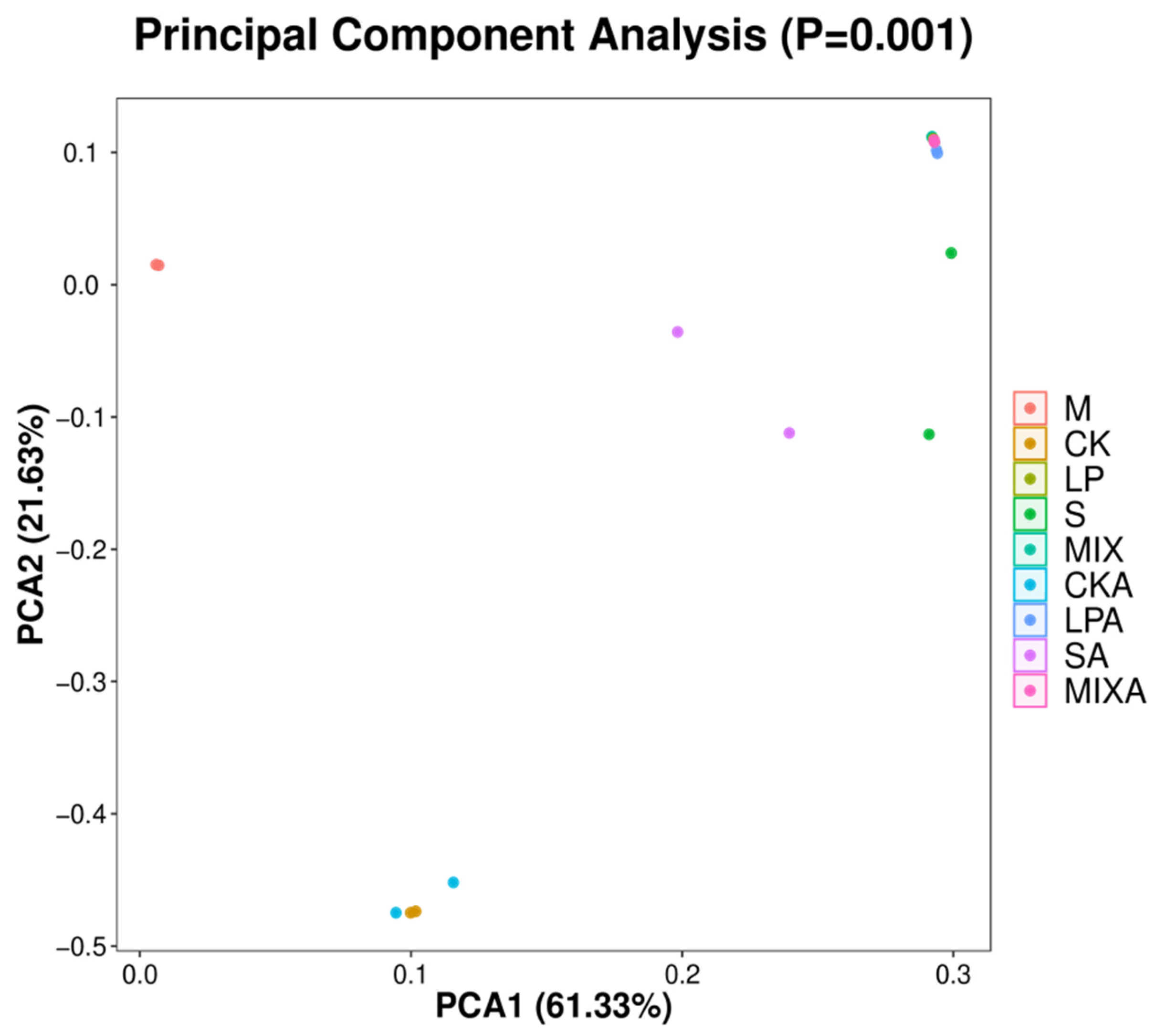

| Items | Growth Stages | |||||
|---|---|---|---|---|---|---|
| Heading Stage | Sizing Stage | Milk Stage | Dough Stage | Maturity Stage | ||
| pre-ensiling | DM (g/kg DM) | 255 ± 2.25 c | 270 ± 1.32 c | 296 ± 1.70 b | 329 ± 1.65 b | 371 ± 1.05 a |
| CP (g/kg DM) | 78.7 ± 6.45 a | 54.7 ± 0.95 b | 55.5 ± 1.59 b | 49.8 ± 2.45 b | 52.4 ± 1.45 b | |
| WSC (g/kg DM) | 111 ± 6.65 ab | 125 ± 3.35 a | 68.1 ± 4.50 d | 92.7 ± 5.20 bc | 73.6 ± 7.85 cd | |
| NDF (g/kg DM) | 651 ± 5.40 a | 661 ± 9.10 a | 672 ± 17.9 a | 659 ± 5.75 a | 599 ± 7.70 b | |
| ADF (g/kg DM) | 359 ± 17.2 | 364 ± 8.60 | 358 ± 6.20 | 365 ± 12.65 | 337 ± 2.44 | |
| post-ensiling | DM (g/kg DM) | 271 ± 1.71 e | 285 ± 1.11 d | 300 ± 0.65 c | 355 ± 1.98 b | 402 ± 2.90 a |
| CP (g/kg DM) | 77.8 ± 0.41 a | 51.8 ± 0.36 c | 52.0 ± 3.00 c | 66.8 ± 2.03 b | 77.1 ± 0.10 a | |
| WSC (g/kg DM) | 19.2 ± 0.81 c | 36.0 ± 0.60 a | 24.0 ± 0.91 b | 33.7 ± 0.91 a | 16.5 ± 0.50 d | |
| NDF (g/kg DM) | 631 ± 3.06 a | 591 ± 3.06 b | 622 ± 1.92 a | 629 ± 3.11 a | 542 ± 5.20 c | |
| ADF (g/kg DM) | 352 ± 13.0 | 360 ± 6.65 | 356 ± 3.75 | 355 ± 7.65 | 333 ± 11.20 | |
| Items | Growth Stages | ||||
|---|---|---|---|---|---|
| Heading Stage | Sizing Stage | Milk Stage | Dough Stage | Maturity Stage | |
| LAB (log CFU/g) | 4.40 ± 0.05 b | 4.26 ± 0.07 b | 3.48 ± 0.14 c | 4.23 ± 0.05 b | 6.80 ± 0.23 a |
| Yeasts (log CFU/g) | 5.32 ± 0.10 b | 6.06 ± 0.20 a | 3.31 ± 0.08 d | 5.26 ± 0.07 b | 4.36 ± 0.16 c |
| General bacteria (log CFU/g) | 7.11 ± 0.29 b | 7.48 ± 0.05 b | 8.26 ± 0.08 a | 6.20 ± 0.03 c | 6.18 ± 0.08 c |
| Items | Growth Stages | ||||
|---|---|---|---|---|---|
| Heading Stage | Sizing Stage | Milk Stage | Dough Stage | Maturity Stage | |
| pH | 4.13 ± 0.01 b | 4.08 ± 0.00 c | 4.03 ± 0.01 d | 4.10 ± 0.02 c | 4.20 ± 0.01 a |
| LA (g/kg DM) | 120 ± 0.61 a | 108 ± 0.46 c | 114 ± 0.58 b | 119 ± 2.87 a | 120 ± 1.81 a |
| AA (g/kg DM) | 11.5 ± 0.26 d | 11.3 ± 0.11 d | 22.6 ± 0.87 c | 30.3 ± 0.88 b | 34.1 ± 0.72 a |
| LA/AA | 10.4 ± 0.18 a | 9.53 ± 0.05 b | 5.06 ± 0.18 c | 3.95 ± 0.19 d | 3.52 ± 0.09 d |
| Items | Additives | g/kg DM | NDF (g/kg DM) | ADF g/kg DM) | CP (g/kg DM) | WSC (g/kg DM) |
|---|---|---|---|---|---|---|
| Pre-ensiling | M | 388.9 ± 9.75 | 621 ± 12.8 | 516 ± 9.20 | 68.8 ± 1.11 | 21.3 ± 1.98 |
| At silo opening | CK | 373 ± 0.06 b** | 586 ± 6.35 | 503 ± 4.89 a | 70.2 ± 1.07 * | 33.8 ± 8.65 b |
| LP | 376 ± 0.70 ab | 576 ± 2.73 | 496 ± 5.25 ab | 70.0 ± 0.70 | 46.3 ± 2.34 b** | |
| S | 385 ± 0.01 ** | 536 ± 19.4 | 458 ± 15.3 b | 69.8 ± 1.28 | 43.6 ± 3.88 b* | |
| MIX | 383 ± 0.80 ab** | 550 ± 25.6 | 480 ± 20.8 ab | 69.4 ± 0.58 | 69.4 ± 5.80 a** | |
| After 7 d aerobic stability test | CK | 442 ± 0.10 | 582 ± 8.20 A | 497 ± 9.20 A | 66.4 ± 0.47 | 25.0 ± 4.07 B |
| LP | 439 ± 0.22 | 531 ± 21.9 AB | 456 ± 18.8 AB | 68.4 ± 0.49 | 25.8 ± 2.68 B | |
| S | 446 ± 0.08 | 524 ± 24.1 B | 453 ± 19.3 AB | 66.9 ± 2.18 | 27.5 ± 1.41 B | |
| MIX | 449 ± 0.01 | 513 ± 5.15 B | 435 ± 10.3 B | 61.5 ± 4.38 | 37.1 ± 1.31 A |
| Items | Additives | LAB (log CFU/g) | Yeasts (log CFU/g) | General Bacteria (log CFU/g) |
|---|---|---|---|---|
| At silo opening | CK | 7.19 ± 0.18 b | 7.18 ± 0.24 b | 7.37 ± 0.9 a** |
| LP | 8.02 ± 0.10 a | 7.51 ± 0.08 ab* | 6.15 ± 0.08 c** | |
| S | 7.98 ± 0.20 a | 7.69 ± 0.28 ab | 5.94 ± 0.10 c* | |
| MIX | 8.19 ± 0.13 a | 7.92 ± 0.11 a* | 6.63 ± 0.15 b | |
| After 7 d aerobic stability test | CK | 6.98 ± 0.18 B | 6.93 ± 0.18 C | 6.77 ± 0.12 |
| LP | 8.14 ± 0.05 A | 8.33 ± 0.02 AB | ND | |
| S | 7.90 ± 0.14 A | 8.04 ± 0.14 B | 7.58 ± 0.50 | |
| MIX | 8.18 ± 0.16 A | 8.47 ± 0.11 A | 7.16 ± 0.26 |
| Items | Additives | pH | LA(g/kg DM) | AA(g/kg DM) | LA/AA |
|---|---|---|---|---|---|
| At silo opening | CK | 4.50 ± 0.03 a | 37.3 ± 1.81 b* | 47.3 ± 4.96 c | 0.80 ± 0.04 c |
| LP | 4.07 ± 0.03 b* | 58.8 ± 2.86 a** | 66.7 ± 2.07 a** | 0.88 ± 0.03 bc | |
| S | 4.03 ± 0.01 bc | 46.4 ± 4.92 b | 48.9 ± 4.63 bc | 0.95 ± 0.03 b | |
| MIX | 3.96 ± 0.04 c | 65.9 ± 2.94 a | 60.9 ± 1.84 ab** | 1.08 ± 0.70 a | |
| After 7 d aerobic stability test | CK | 4.55 ± 0.03 A | 28.0 ± 1.01 B | 32.1 ± 3.18 B | 0.89 ± 0.05 |
| LP | 4.15 ± 0.01 BC | 30.9 ± 5.45 B | 27.5 ± 1.27 B | 0.90 ± 0.03 | |
| S | 4.27 ± 0.16 B | 56.6 ± 0.62 A | 43.9 ± 1.26 A | 1.15 ± 0.12 | |
| MIX | 4.00 ± 0.02 C | 55.6 ± 3.87 A | 49.1 ± 0.71 A | 1.14 ± 0.09 |
| Items | Additives | Shannon | Simpson | Chao1 | Goods_Coverage |
|---|---|---|---|---|---|
| Raw material | M | 3.89 ± 0.05 a | 0.88 ± 0.01 a | 65.0 ± 6.35 | 1.00 |
| At silo opening | CK | 3.19 ± 0.03 b | 0.82 ± 0.01 a | 47.0 ± 0.71 | 1.00 |
| LP | 0.97 ± 0.02 d* | 0.23 ± 0.01 c* | 51.0 ± 1.42 | 1.00 | |
| S | 2.44 ± 0.24 c | 0.65 ± 0.07 b | 52.5 ± 1.77 | 1.00 | |
| MIX | 0.73 ± 0.06 d | 0.17 ± 0.01 c | 34.0 ± 11.3 | 1.00 | |
| After 7 d aerobic stability test | CK | 2.97 ± 0.10 A | 0.79 ± 0.02 A | 46.0 ± 10.6 | 1.00 |
| LP | 1.21 ± 0.01 B | 0.31 ± 0.01 B | 48.0 ± 1.41 | 1.00 | |
| S | 3.08 ± 0.08 A | 0.80 ± 0.02 A | 57.0 ± 0.71 | 1.00 | |
| MIX | 0.97 ± 0.01 B | 0.25 ± 0.01 B | 43.0 ± 5.65 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Hao, X.; Yin, M.; Li, C.; Wang, C.; Han, H. Influence of Growth Stages and Additives on the Fermentation Quality and Microbial Profiles of Whole-Plant Millet Silage. Agriculture 2024, 14, 1323. https://doi.org/10.3390/agriculture14081323
Zhao N, Hao X, Yin M, Li C, Wang C, Han H. Influence of Growth Stages and Additives on the Fermentation Quality and Microbial Profiles of Whole-Plant Millet Silage. Agriculture. 2024; 14(8):1323. https://doi.org/10.3390/agriculture14081323
Chicago/Turabian StyleZhao, Na, Xia Hao, Maozhe Yin, Changqing Li, Chao Wang, and Hongyan Han. 2024. "Influence of Growth Stages and Additives on the Fermentation Quality and Microbial Profiles of Whole-Plant Millet Silage" Agriculture 14, no. 8: 1323. https://doi.org/10.3390/agriculture14081323
APA StyleZhao, N., Hao, X., Yin, M., Li, C., Wang, C., & Han, H. (2024). Influence of Growth Stages and Additives on the Fermentation Quality and Microbial Profiles of Whole-Plant Millet Silage. Agriculture, 14(8), 1323. https://doi.org/10.3390/agriculture14081323





