Physiological Phenotyping and Biochemical Characterization of Mung Bean (Vigna radiata L.) Genotypes for Salt and Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample and Plant Acclimatization
2.2. Measurement of Growth and Plant Biomass
2.3. Measurement of RWC
2.4. Plant Height Stress Index
2.5. Chlorophyll Fluorescence
2.6. Loss of Plasma Membrane Integrity by Histochemical Analysis
3. Biochemical Analysis
3.1. Lipid Peroxidation
3.2. LOX (Lipo-Oxygenase Assay)
3.3. Hydrogen Peroxide
3.4. Proline Content
3.5. Extraction and Assessment of Antioxidant Enzymes
3.6. SOD (Superoxide Dismutase)
3.7. GPX (Guaiacol Peroxidase)
3.8. GR (Glutathione Reductase)
3.9. Ascorbate Peroxidase (APX)
3.10. MDHAR (Monodehydroascorbate Reductase)
3.11. DHAR (Dehydroascorbate Reductase)
3.12. CAT (Catalase)
3.13. Statistical Analysis
4. Results
4.1. Measurement of Plant Height
4.2. Fresh and Dry Biomass
4.3. Estimation of Relative Water Content
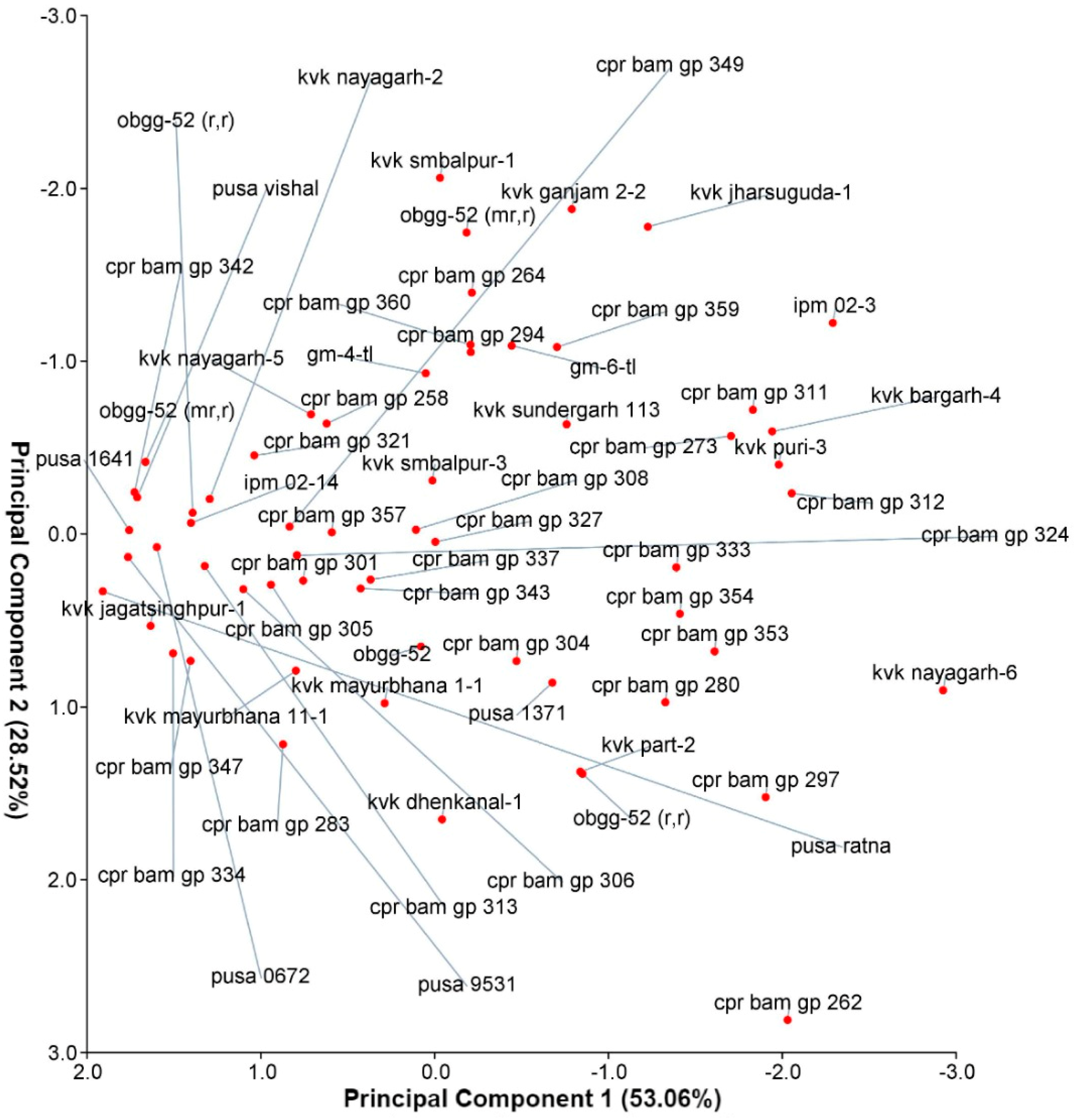
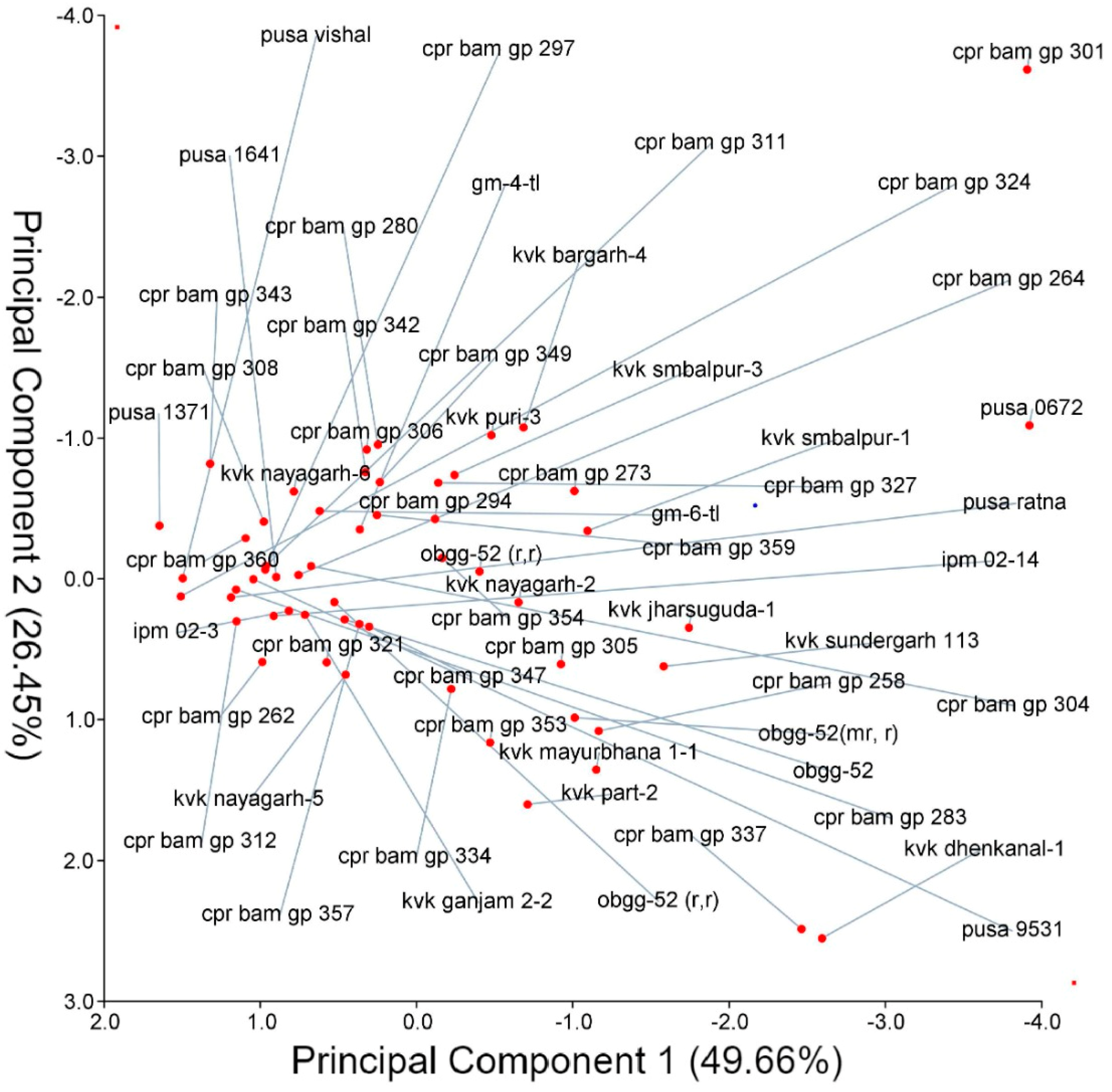
4.4. Principal Component Analysis (PCA)
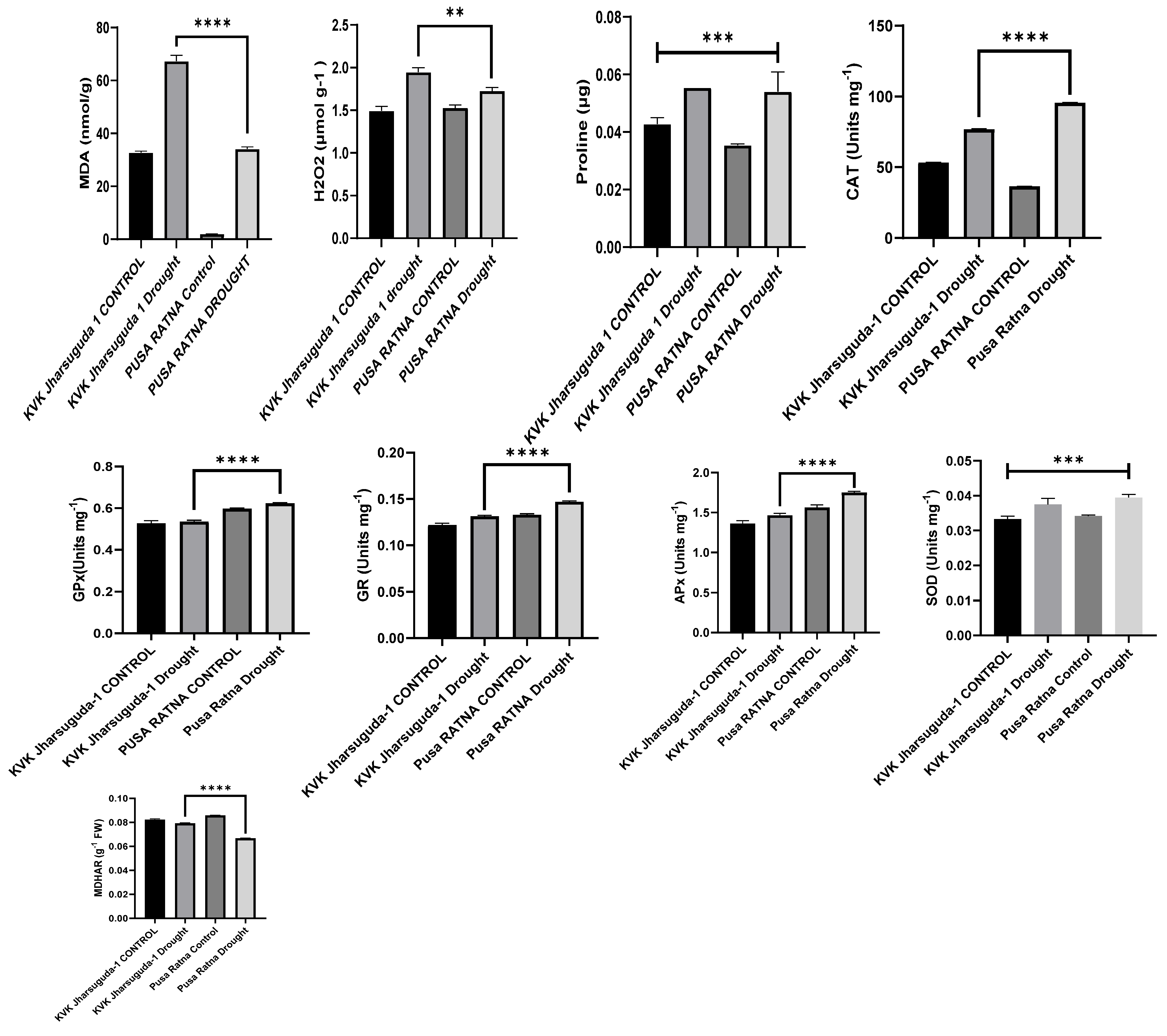
4.5. Biochemical Analysis
4.6. Histochemical Analysis
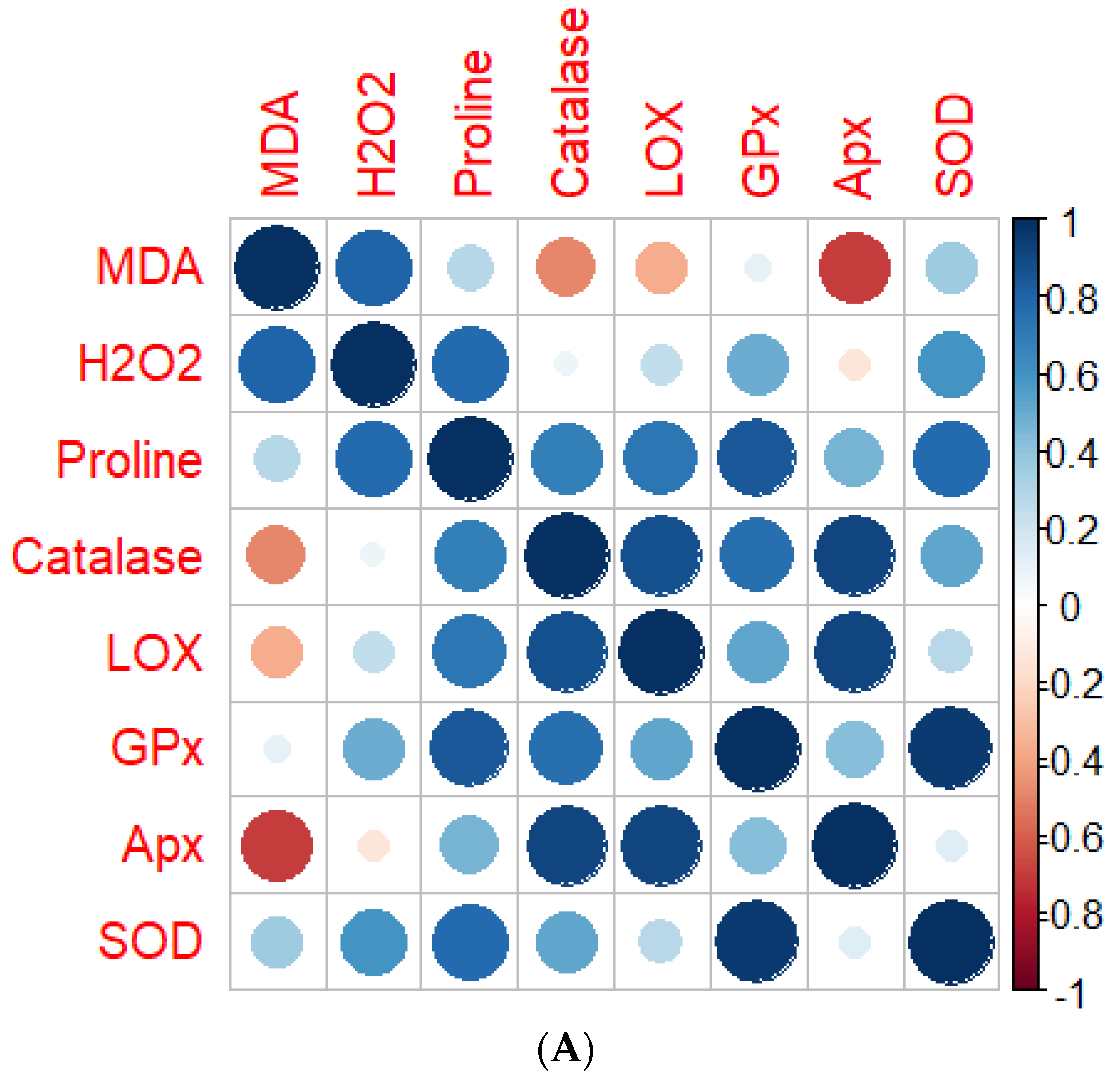
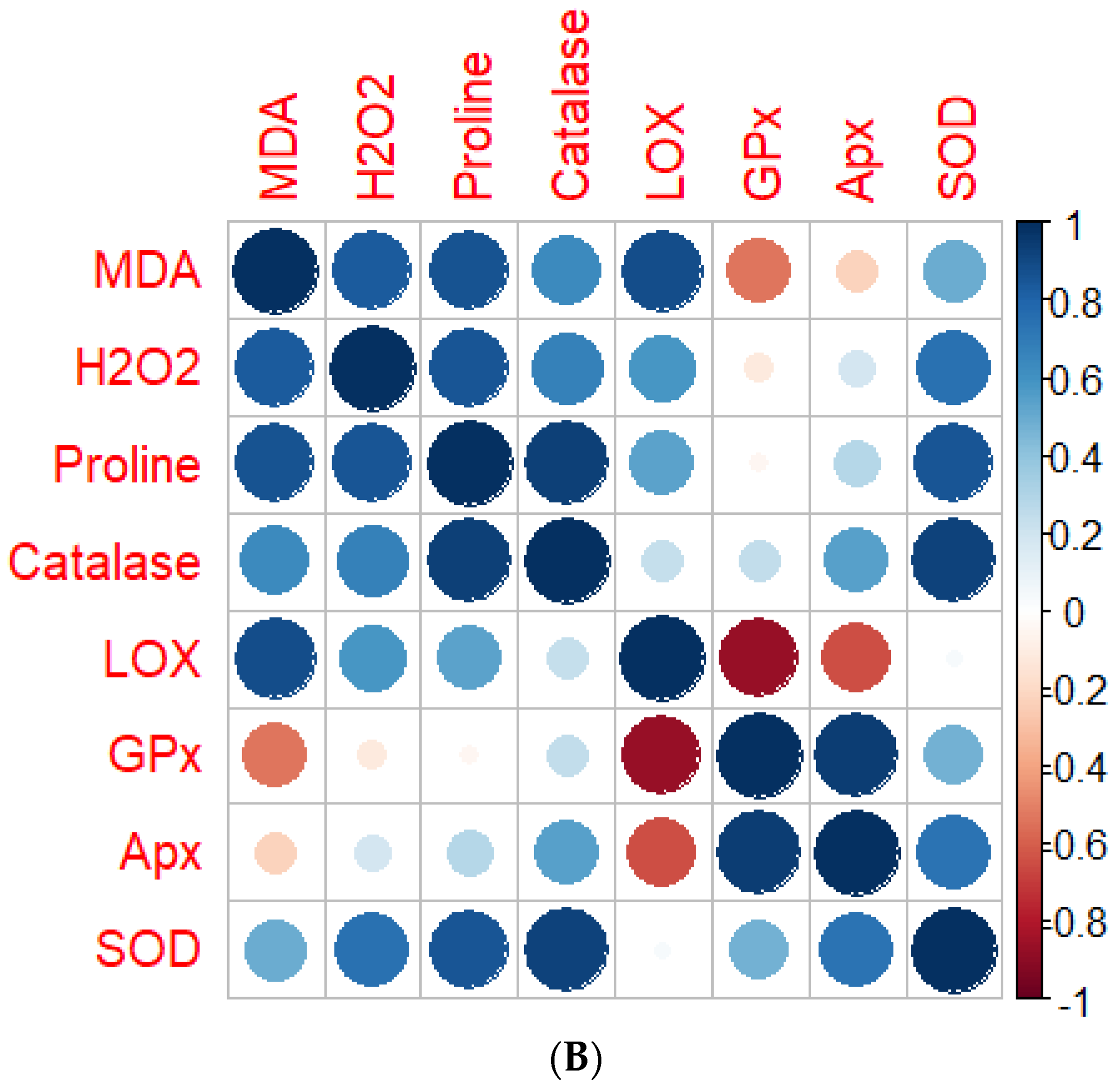
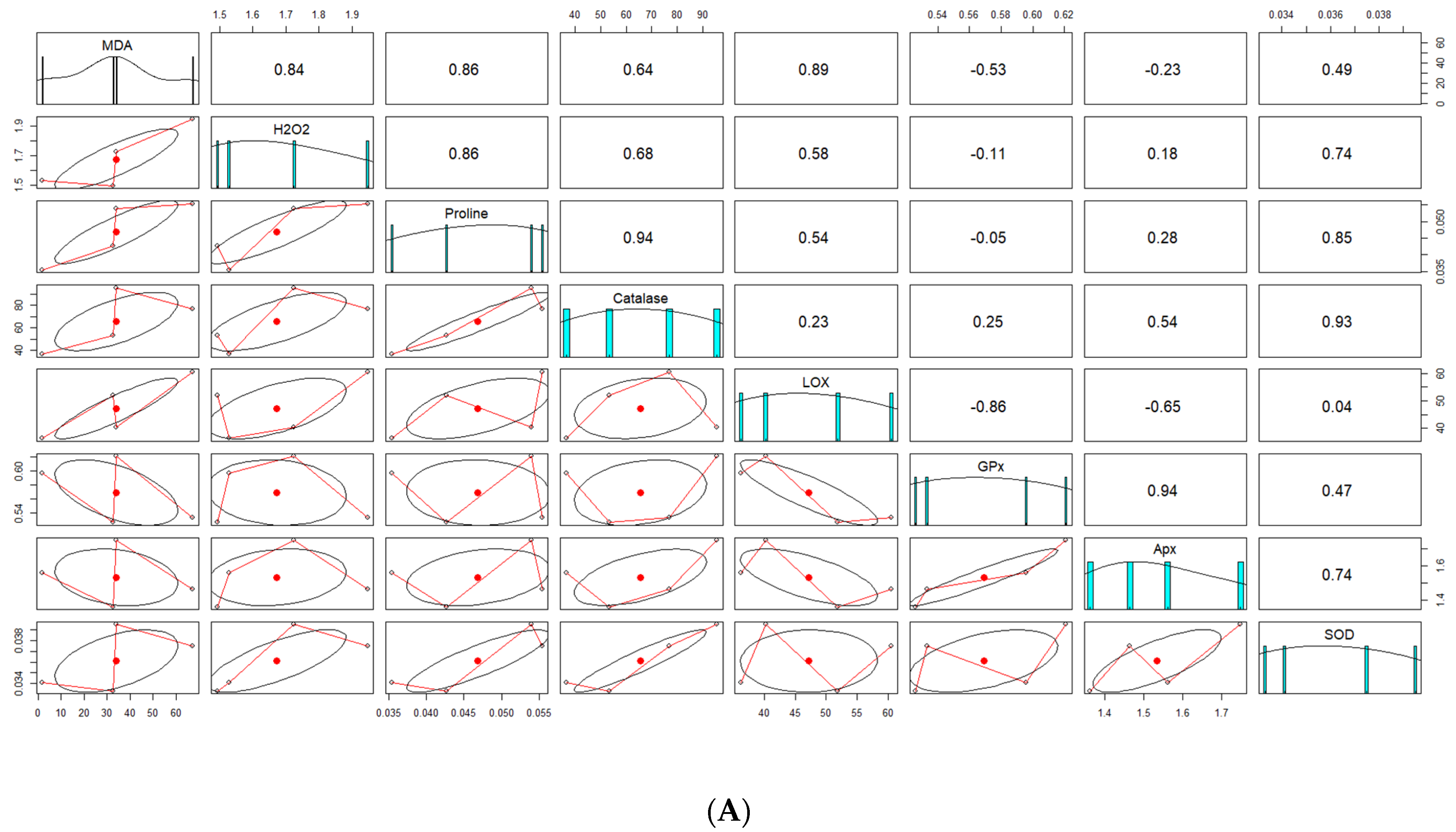
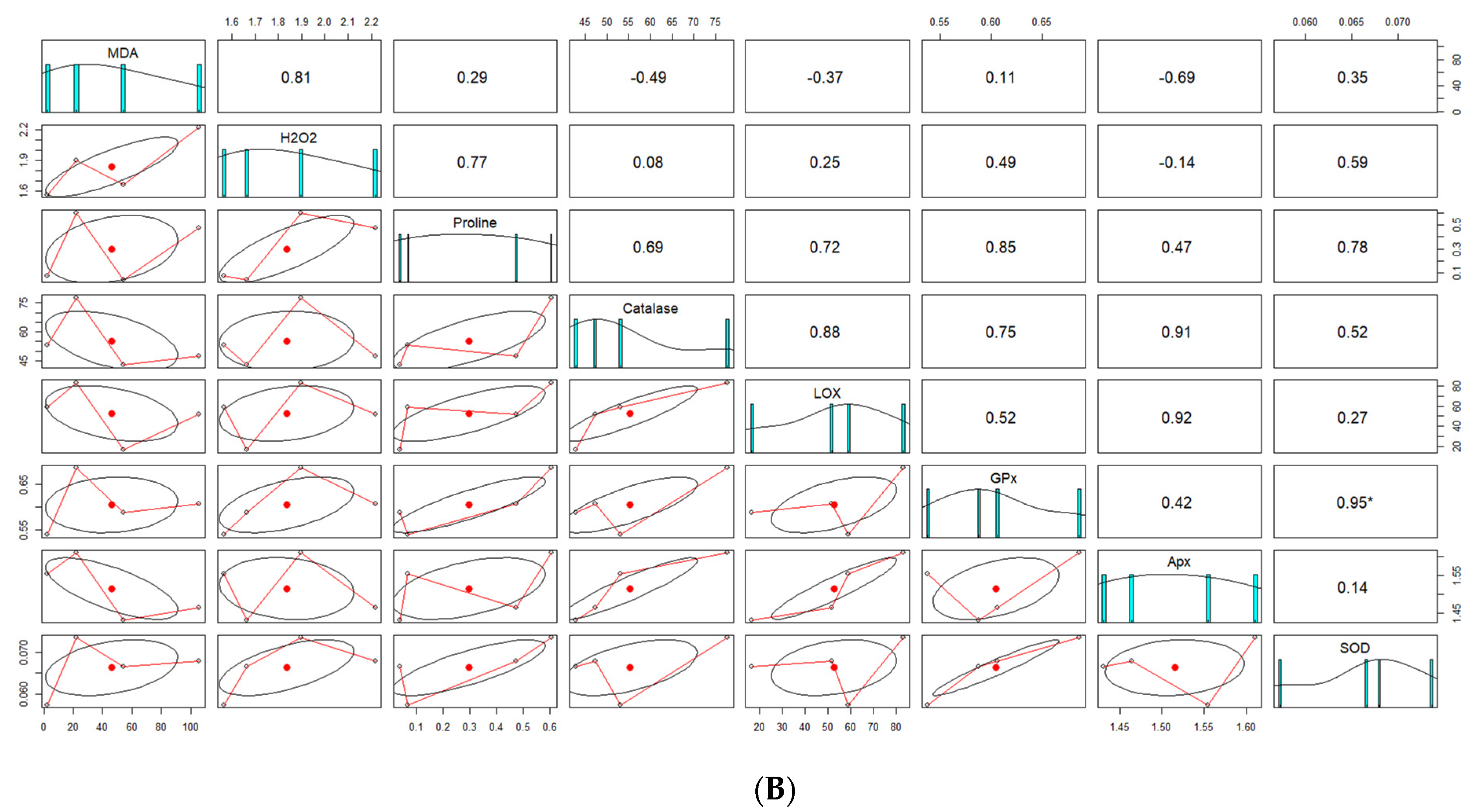
4.7. Correlation Analysis between Screened Genotypes
4.8. Stress Tolerant and Sensitive Genotypes
4.9. Plant Height Tolerance Index
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 22–53. [Google Scholar] [CrossRef]
- Waseem, M.; Liu, P.; Aslam, M.M. Salinity and drought stress in plants: Understanding physiological, biochemical and molecular responses. Front. Plant Sci. 2023, 14, 1277859. [Google Scholar] [CrossRef]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Ahmed, M.A. Physiological effects of salt stress on plant growth. Tikrit J. Agric. Sci. 2022, 22, 93–97. [Google Scholar] [CrossRef]
- Rasheed, R.; Iqbal, M.; Anwar, S.; Shereen, A.; Khan, M.A. Assessment of rice (Oryza sativa L.) genotypes for drought stress tolerance using morpho-physiological indices as a screening technique. Pak. J. Bot. 2021, 53, 45–58. [Google Scholar]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.A. Drought stress in grain legumes: Effects, tolerance mechanisms and management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef]
- Nair, R.; Schreinemachers, P. Global status and economic importance of mungbean. In The Mungbean Genome; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–8. [Google Scholar]
- Mohan Naik, G.; Abhirami, P.; Venkatachalapathy, N. Mung bean. In Pulses: Processing and Product Development; Springer Cham: Cham, Switzerland, 2020; pp. 213–228. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Baza, M.; Shanka, D.; Bibiso, M. Agronomic and economic performance of mung bean (Vigna radiata L.) varieties in response to rates of blended NPS fertilizer in Kindo Koysha district, Southern Ethiopia. Open Life Sci. 2022, 17, 1053–1063. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Sharma, A.K.; Kumar, V.; Bhat, K.V. Salt stress and mungbean [Vigna radiata (L.) Wilczek]: Effects, physiological perspective and management practices for alleviating salinity. Arch. Agron. Soil Sci. 2019, 65, 1287–1301. [Google Scholar] [CrossRef]
- Islam, M.R.; Sarker, U.; Azam, M.G.; Hossain, J.; Alam, M.A.; Ullah, R.; Bari, A.; Hossain, N.; El Sabagh, A.; Islam, M.S. Potassium augments growth, yield, nutrient content, and drought tolerance in mung bean (Vigna radiata L. Wilczek.). Sci. Rep. 2024, 14, 9378. [Google Scholar] [CrossRef]
- Sachdev, S.; Ahmad, S. Role of nanomaterials in regulating oxidative stress in plants. In Nanobiotechnology: Mitigation of Abiotic Stress in Plants; Springer Cham: Cham, Switzerland, 2021; pp. 305–326. [Google Scholar]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PloS One 2017, 12, e0176357. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N. Selenium (Se) regulates seedling growth in wheat under drought stress. Adv. Chem. 2014, 143567. [Google Scholar] [CrossRef]
- Saha, B.; Swain, D.; Borgohain, P.; Rout, G.R.; Koyama, H.; Panda, S.K. Enhanced exudation of malate in the rhizosphere due to AtALMT1 overexpression in blackgram (Vigna mungo L.) confers increased aluminium tolerance. Plant Biol. 2020, 22, 701–708. [Google Scholar]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001, 127, 887. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Reprint of: Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 2022, 726, 109248. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. [53] Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: Oxygen oxidoreductase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 71, pp. 441–451. [Google Scholar]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730. [Google Scholar] [CrossRef]
- Forlani, G.; Funck, D. A specific and sensitive enzymatic assay for the quantitation of L-proline. Front. Plant Sci. 2020, 11, 582026. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Amako, K.; Chen, G.X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Poli, Y.; Nallamothu, V.; Balakrishnan, D.; Ramesh, P.; Desiraju, S.; Mangrauthia, S.K.; Voleti, S.R.; Neelamraju, S. Increased catalase activity and maintenance of photosystem II distinguishes high-yield mutants from low-yield mutants of rice var. Nagina22 under low-phosphorus stress. Front. Plant Sci. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.84. 2017. 2020. Available online: https://github.com/taiyun/corrplot (accessed on 6 August 2024).
- Hossen, M.S.; Karim, M.F.; Fujita, M.; Bhuyan, M.B.; Nahar, K.; Masud, A.A.C.; Mahmud, J.A.; Hasanuzzaman, M. Comparative physiology of Indica and Japonica rice under salinity and drought stress: An intrinsic study on osmotic adjustment, oxidative stress, antioxidant defense and methylglyoxal detoxification. Stresses 2022, 2, 156–178. [Google Scholar] [CrossRef]
- Mehraban, A.; Tobe, A.; Gholipouri, A.; Amiri, E.; Ghafari, A.; Rostaii, M. The effects of drought stress on yield, yield components, and yield stability at different growth stages in bread wheat cultivar (Triticum aestivum L.). Pol. J. Environ. Stud. 2019, 28. [Google Scholar]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 1–14. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Lee, J.G. Monitoring of salinity, temperature, and drought stress in grafted watermelon seedlings using chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 786309. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Adhinarayanreddy, V.; Vemanna, R.S.; Sreeman, S.; Makarla, U. Quantification of membrane damage/cell death using Evan’s blue staining technique. Bio-Protocol 2017, 7, e2519. [Google Scholar]
- Omisun, T.; Sahoo, S.; Saha, B.; Panda, S.K. Relative salinity tolerance of rice cultivars native to North East India: A physiological, biochemical and molecular perspective. Protoplasma 2018, 255, 193–202. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of osmotic stress in Brassica napus, B. campestris, and B. juncea by ascorbic acid application. Biol. Plant. 2014, 58, 697–708. [Google Scholar]
- Naderi, R.; Valizadeh, M.; Toorchi, M.; Shakiba, M.R. Antioxidant enzyme changes in response to osmotic stress in wheat (Triticum aestivum L.) seedling. Acta Biol. Szeged. 2014, 58, 95–101. [Google Scholar]
- Lopez-Delacalle, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization and interaction of proline, ascorbate and oxidative stress pathways under abiotic stress combination in tomato plants. BioRxiv 2020. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Gupta, D.; Saini, A.; Kumari, A.; Priya, R.; Panda, S.K. Physiological Phenotyping and Biochemical Characterization of Mung Bean (Vigna radiata L.) Genotypes for Salt and Drought Stress. Agriculture 2024, 14, 1337. https://doi.org/10.3390/agriculture14081337
Patel M, Gupta D, Saini A, Kumari A, Priya R, Panda SK. Physiological Phenotyping and Biochemical Characterization of Mung Bean (Vigna radiata L.) Genotypes for Salt and Drought Stress. Agriculture. 2024; 14(8):1337. https://doi.org/10.3390/agriculture14081337
Chicago/Turabian StylePatel, Mayur, Divya Gupta, Amita Saini, Asha Kumari, Rishi Priya, and Sanjib Kumar Panda. 2024. "Physiological Phenotyping and Biochemical Characterization of Mung Bean (Vigna radiata L.) Genotypes for Salt and Drought Stress" Agriculture 14, no. 8: 1337. https://doi.org/10.3390/agriculture14081337
APA StylePatel, M., Gupta, D., Saini, A., Kumari, A., Priya, R., & Panda, S. K. (2024). Physiological Phenotyping and Biochemical Characterization of Mung Bean (Vigna radiata L.) Genotypes for Salt and Drought Stress. Agriculture, 14(8), 1337. https://doi.org/10.3390/agriculture14081337




