Solid-State Fermentation for Phenolic Compounds Recovery from Mexican Oregano (Lippia graveolens Kunth) Residual Leaves Applying a Lactic Acid Bacteria (Leuconostoc mesenteroides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. EOs Extraction Process
2.4. Solid-State Fermentation Process
2.5. Polyphenolic Compounds Evaluation in the Solid-State Fermentation Process (SSF)
2.5.1. Phenolic Compounds Determination
Total Polyphenolic Compounds (TPC)
Hydrolyzed Tannins (Folin–Ciocalteu)
Condensed Tannins (HCl–Butanol)
Total Flavonoid Compounds (TFC)
2.6. Antioxidant Activity in the Solid-State Fermentation Process (SSF)
2.6.1. DPPH• Radical Scavenging Assay
2.6.2. Ferric Reducing Capacity (FRAP)
2.7. Inhibitory Activity of Extracts against α-Amylase
2.8. Antimicrobial Activity via Agar Diffusion Assay (ADA)
2.9. RP-HPLC-ESI-MS Analysis of Extracts
2.10. Statistical Analysis
3. Results and Discussion
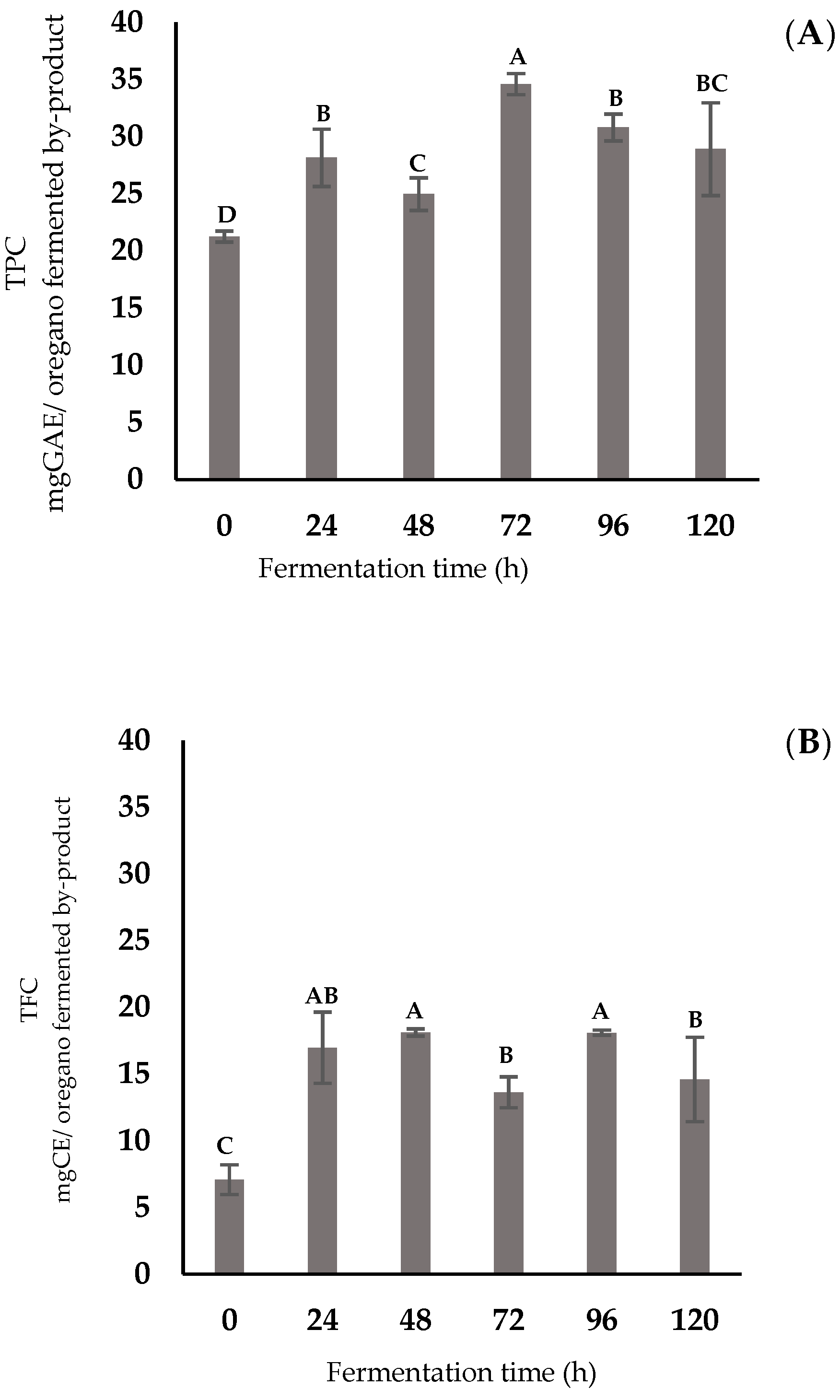
3.1. Phenolic Compounds
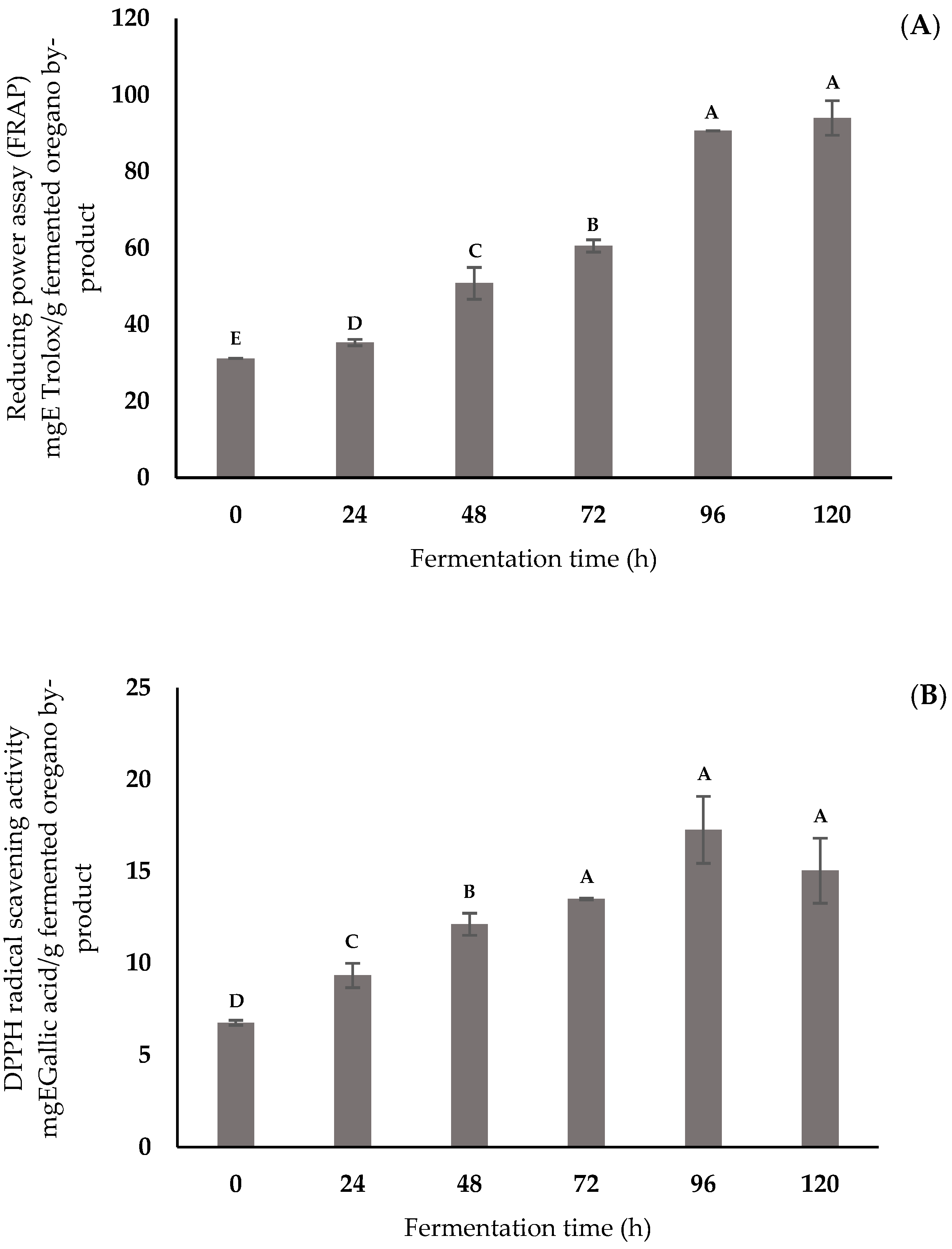
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
3.4. Potential α-Amylase Inhibition
3.5. RP-HPLC-ESI-MS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Hoof, B.; Solano, A.; Riano, J.; Mendez, C.; Medaglia, A. Decision-making for circular economy implementation in agri-food systems: A transdisciplinary case study of cacao in Colombia. J. Clean. Prod. 2024, 434, 140307. [Google Scholar] [CrossRef]
- Papamichael, I.; Voukkali, I.; Loizia, P.; Stylianou, M.; Economou, F.; Vardopoulos, I.; Klontza, E.E.; Lekkas, D.F.; Zorpas, A.A. Measuring Circularity: Tools for monitoring a smooth transition to Circular Economy. Sustain. Chem. Pharm. 2023, 36, 101330. [Google Scholar] [CrossRef]
- Galván Calamaco, Z.; Ramos Clamont Montfort, G.; Marszalek, J.E.; Vargas González, G. Revisión Sobre El orégano Mexicano Lippia graveolens HBK. (Sinonimia Lippia berlandieri Schauer) Y Su Aceite Esencial. Investig. Desarro. Cienc. Tecnol. Aliment. 2023, 8, 861–871. [Google Scholar] [CrossRef]
- Comisión Nacional Forestal. Paquete Tecnológico para la Producción de Orégano (Lippia spp.), 1st ed.; CONAFOR: Zapopan, Mexico, 2016. [Google Scholar]
- El Diario de Chihuahua. Available online: https://diario.mx/estado/2016/jan/23/produce-chihuahua-mil-toneladas-de-oregano-al-ano-586440.html (accessed on 29 July 2024).
- Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Available online: https://www.gob.mx/inifap/articulos/domesticacion-del-oregano-para-zonas-semideserticas-de-coahuila (accessed on 29 July 2024).
- Secretariat of Economy. Data Mexico. Available online: https://www.economia.gob.mx/datamexico/en/profile/product/essential-oils-perfumes-cosmetics-toiletries?tradeBalanceSelector=2013&timeNetTradeSelector=Year (accessed on 29 July 2024).
- Orona, I.; Salvador, A.J.; Espinoza, J.J.; Vázquez, C. Collection and marketing of oregano (Lippia spp.) in the Mexican semi-desert, a case study of the Sierra and Canyon Municipal Ecological Reserve of Jimulco, Mexico. Rev. Mex. Agronegocios 2017, 41, 684–695. [Google Scholar]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar-Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Dina, E.; Vontzalidou, A.; Cheilari, A.; Bagatzounis, P.; Agapidou, E.; Giannenas, I.; Grigoriadou, K.; Aligiannis, N. Sustainable Use of Greek Herbs By-Products, as an Alternative Source of Biologically Active Ingredients for Innovative Products. Front. Nutr. 2022, 9, 867666. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.; Ilina, A.; Torres-León, C.; Verma, D.K.; Chávez-González, M.L. Phenolic compounds and antioxidant activity of Lippia graveolens Kunth residual leaves fermented by two filamentous fungal strains in solid-state process. Food Bioprod. Process. 2022, 136, 24–35. [Google Scholar] [CrossRef]
- García-Pérez, E.; Castro-Álvarez, F.F.; Gutiérrez-Uribe, J.A.; García-Lara, S. Revisión de la producción, composición fitoquímica y propiedades nutracéuticas del orégano mexicano. Rev. Mex. Cienc. Agrícolas 2012, 3, 339–353. [Google Scholar]
- Hrncic, M.K.; Cor, D.; Simonovska, J.; Knez, Z.; Kavrakovski, Z.; Rafajlovska, V. Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species. Molecules 2020, 25, 4735. [Google Scholar] [CrossRef]
- Timothy, C.N.; Priya, V.V.; Gayathri, G.R. Phytochemical analysis and total phenolic content of Origanum vulgare (oregano). Drug Invent. Today 2018, 10, 10. [Google Scholar]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and antimicrobial activity of Mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Bourgine, J.; Thomsen, M. Closing the loop of cereal waste and residues with sustainable technologies: An overview of enzyme production via fungal solid-state fermentation. Sustain. Prod. Consum. 2021, 27, 845–857. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Manan, M.A.; Webb, C. Modern microbial solid state fermentation technology for future biorefineries for the production of added-value products. Biofuel J. 2017, 16, 730–740. [Google Scholar] [CrossRef]
- Venil, C.K.; Yusof, N.Z.B.; Ahmad, W.A. Solid State Fermentation Utilizing Agro-Industrial Waste for Microbial Pigment Production. In Sustainable Agriculture towards Food Security; Dhanarajan, A., Ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid-state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Torres-león, C.; Ramirez-Guzman, N.; Ascacio-valdes, J.; Serna-Cock, L.; Santos, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT Food Sci. Technol. 2019, 112, 108236. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Macromolecules 2016, 86, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda, L.; Morlett-Cháve, J.; Meléndez-Renteria, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.; Aguilar, C.N. Solid-State Fermentation with Aspergillus niger GH1 to Enhance Polyphenolic Content and Antioxidative Activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Darvishi, N.; Fard, N.A.; Sadrnia, M. Genomic and proteomic comparisons of bacteriocins in probiotic species Lactobacillus and Bifidobacterium and inhibitory ability of Escherichia coli MG 1655. Biotechnol. Rep. 2021, 31, e00654. [Google Scholar] [CrossRef]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Saadoun, J.; Calani, L.; Cirlini, M.; Bernini, V.; Neviani, E.; del Rio, D.; Galaverna, G.; Lazzi, C. Effect of fermentation with single and co-culture of lactic acid bacteria on okara: Evaluation of bioactive compounds and volatile profiles. Food Funct. 2021, 12, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Roasa, J.; de Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- Khan, S.A.; Liu, L.; Lai, T.; Zhang, R.; Wei, Z.; Xiao, J.; Deng, Y.; Zhang, M. Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. J. Food Sci. Technol. 2018, 55, 4782–4791. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, K.-T.; Paik, H.-D. Improved Antioxidant, Anti-inflammatory, and Anti-adipogenic Properties of Hydroponic Ginseng Fermented by Leuconostoc mesenteroides KCCM 12010P. Molecules 2019, 24, 3359. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; AmIot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Sepúlveda-Torre, L.; Ascacio-Vadlés, J.A.; Chavez-González, M.L. Comparative extraction study of grape pomace bioactive compounds by submerged and solid-state fermentation. J. Chem. Technol. Biotechnol. 2021, 97, 1494–1505. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, E. The phenolic constituents of Prunus domestica. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina-Campos, O.N.; Ávila-Nava, A.; González-Reyes, S.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Weng, Y.M.; Yu, Z.R.; Koo, M.; Wang, B.J. Extraction temperature affects the activities of antioxidation, carbohydrate-digestion enzyme and antiogestin-converting enzyme of Pleurotus citrinopileatus extract. J. Food Drug Anal. 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Avaiyarasi, N.D.; Ravindran, A.D.; Venkatesh, P.; Arul, V. In vitro selection, characterization, and cytotoxic effect of bacteriocin of Lactobacillus sakei GM3 isolated from goat milk. Food Control 2016, 69, 124–133. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Sawangwan, T.; Porncharoennop, C.; Nimrasksa, H. Antioxidant compounds from rice bran fermentation by lactic acid bacteria. Agric. Food 2021, 6, 578–587. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Chun, L.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Herreros, M.A.; Fresno, J.M.; González Prieto, M.J.; Tornadijo, M.E. Technological characterization of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese). Int. Dairy J. 2003, 13, 469–479. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Kim, Y.M.; Moon, J.H. Change in Flavonoid Composition and Antioxidative Activity during Fermentation of Onion (Allium cepa L.) by Leuconostoc mesenteroides with Different Salt Concentrations. J. Food Sci. 2016, 81, C1385–C1393. [Google Scholar] [CrossRef]
- Bertrand, A.; Morel, S.; Lefoulon, F.; Rolland, Y.; Monsan, P.; Remaud-Simeon, M. Leuconostoc mesenteroides glucansucrase synthesis of flavonoid glucosides by acceptor reactions in aqueous-organic solvents. Carbohydr. Res. 2006, 341, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Kim, H.; Gil, C.; Park, C.; Wook, S.; Kim, D. Enzyme and Microbial Technology Biochemical characterization of synthesized fisetin glucoside by dextransucrase from Leuconostoc mesenteroides NRRL B-1299CB4 with enhanced water solubility. Enzym. Microb. Technol. 2022, 161, 110111. [Google Scholar] [CrossRef]
- Miyagusuku-Cruzado, G.; García-Cano, I.; Rocha-Mendoza, D.; Jiménez-Flores, R.; Giusti, M.M. Monitoring Hydroxycinnamic Acid Decarboxylation by Lactic Acid Bacteria Using High-Throughput UV-Vis Spectroscopy. Molecules 2020, 25, 3142. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Niu, K.M.; Lee, J.H.; Cho, S.; Han, S.G.; Kim, S.K. Characteristics of Chinese chives (Allium tuberosum) fermented by Leuconostoc mesenteroides. Appl. Biol. Chem. 2016, 59, 349–357. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Moon, J.H. Isolation and antioxidative activity of amino acid derivatives produced by Leuconostoc mesenteroides. Food Sci. Biotechnol. 2016, 25, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Sun, Q.; Zhang, S.M.; Sun, X.Y.; Li, C.Y.; Zheng, M.X.; Xiang, W.L.; Tang, J. Characterization and Antioxidant Activity of Released Exopolysaccharide from Potential Probiotic Leuconostoc mesenteroides LM187. J. Microbiol. Biotechnol. 2021, 31, 1144–1153. [Google Scholar] [CrossRef]
- Baharin, N.H.; Khairil Mokhtar, N.F.; Mohd Desa, M.N.; Gopalsamy, B.; Mohd Zaki, N.N.; Yuswan, M.H.; Muthanna, A.; Dzaraly, N.D.; Abbasiliasi, S.; Mohd Hashim, A.; et al. The characteristics and roles of antimicrobial peptides as potential treatment for antibiotic-resistant pathogens: A review. PeerJ 2021, 9, e12193. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, Y.H.; Chen, J.H.; Hsu, P.S.; Wu, T.H.; Lin, C.F.; Peng, C.C.; Wu, M.C. A potential probiotic Leuconostoc mesenteroides TBE-8 for honey bee. Sci. Rep. 2021, 11, 18466. [Google Scholar] [CrossRef]
- Molet-Rodríguez, A.; Turmo-Ibarz, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Incorporation of antimicrobial nanoemulsions into complex foods: A case study in an apple juice-based beverage. Lwt 2021, 141, 110926. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Zaynullin, R.A.; Kunakova, R.V.; Khusnutdinova, E.K.; Yalaev, B.; Segura-Ceniceros, E.P.; Chavez-Gonzalez, M.L.; Martínez-Hernández, J.L.; Gernet, M.V.; Batashov, E.S.; Ilyina, A. Dihydroquercetin: Known antioxidant—New inhibitor of alpha-amylase activity. Med. Chem. Res. 2018, 27, 966–971. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, A.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metab. Clin. Exp. 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Tolmie, M.; Bester, M.J.; Apostolides, Z. Inhibition of α-glucosidase and α-amylase by herbal compounds for the treatment of type 2 diabetes: A validation of in silico reverse docking with in vitro enzyme assays. J. Diabetes 2021, 13, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, H.; Hwang, I.S.; Kim, A.R.; Woo, E.R.; Lee, D.G. Synergistic antibacterial and antibiofilm effect between (+)-medioresinol and antibiotics in vitro. Appl. Biochem. Biotechnol. 2013, 170, 1934–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, X.; Gao, C.L.; Ruan, W.; Zhao, S.; Kai, G.; Li, F.; Pang, T. Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol. Res. 2021, 169, 105640. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An Insight on Its Pharmacological Activities, Mechanisms of Action and Toxicity. Front. Pharmacol. 2021, 11, 553404. [Google Scholar]
- Cabrera, J.; Saavedra, E.; del Rosario, H.; Perdomo, J.; Loro, J.F.; Cifuente, D.A.; Tonn, C.E.; García, C.; Quintana, J.; Estévez, F. Gardenin B-induced cell death in human leukemia cells involves multiple caspases but is independent of the generation of reactive oxygen species. Chem. Biol. Interact. 2016, 256, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Al-Karmalawy, A.A.; Farid, M.M.; Mostafa, A.; Ragheb, A.Y.; Mahmoud, S.H.; Shehata, M.; Abo Shama, N.M.; Gaballah, M.; Mostafa-Hedeab, G.; Marzouk, M.M. Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS-CoV-2. Molecules 2021, 26, 6559. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.F.; Lima, K.S.B.; Sousa, T.S.; Marinho-Filho, J.D.B.; Pessoa, C.; Silveira, E.R.; Pessoa, O.D.L.; Costa-Lotufo, L.V.; Moraes, M.O.; Araújo, A.J. In vitro antitumor effect of a lignan isolated from Combretum fruticosum, trachelogenin, in HCT-116 human colon cancer cells. Toxicol. Vitr. 2018, 47, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.J.; Jin, Y.S.; Chen, H.S.; Xu, Q.Q.; Ren, H.; Zhu, S.Y.; Tang, H.L.; Wang, Y.; Zhao, P.; Qi, Z.T.; et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016, 97, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Siddiqui, M.J.A.; Ang, L.F.; Sadikun, A.; Chan, S.H.; Tan, S.C.; Asmawi, M.Z.; Yam, M.F. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement. Altern. Med. 2012, 12, 176. [Google Scholar] [CrossRef]
- Timalsina, D.; Bhusal, D.; Devkota, H.P.; Pokhrel, K.P.; Sharma, K.R. α-Amylase Inhibitory Activity of Catunaregam spinosa (Thunb.) Tirveng.: In Vitro and in Silico Studies. BioMed Res. Int. 2021, 2021, 4133876. [Google Scholar] [CrossRef] [PubMed]
- Berton, S.B.R.; Cabral, M.R.P.; de Jesus, G.A.M.; Sarragiotto, M.H.; Pilau, E.J.; Martins, A.F.; Bonafé, E.G.; Matsushita, M. Ultra-high-performance liquid chromatography supports a new reaction mechanism between free radicals and ferulic acid with antimicrobial and antioxidant activities. Ind. Crops Prod. 2020, 154, 112701. [Google Scholar] [CrossRef]
- Solís-Salas, L.M.; Sierra-Rivera, C.A.; Cobos-Puc, L.E.; Ascacio-Valdés, J.A.; Silva-Belmares, S.Y. Antibacterial potential by rupture membrane and antioxidant capacity of purified phenolic fractions of Persea americana leaf extract. Antibiotics 2021, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Deng, X.; Tan, M.; Yu, C.; Zhang, M.; Sun, Y.; Jiang, N. In vitro antibiofilm activity of resveratrol against avian pathogenic Escherichia coli. BMC Vet. Res. 2021, 17, 249. [Google Scholar] [CrossRef]
- Skroza, D.; Simat, V.; Mozina, S.S.; Katalinic, V.; Boban, N.; Mekinic, I.G. Interactions of resveratrol with other phenolics and activity against food-borne pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef]

| Fermentation Time (h) | Bioactivities | |
|---|---|---|
| Antimicrobial Activity (E. coli) Inhibition mm | α-Amylase Inhibition (%) | |
| 0 | 3.3 ± 0.56 C | 3.24 ± 1.28 C |
| 24 | 4.73 ± 1.3 C | 2.17 ± 1.28 C |
| 48 | 2.96 ± 1.72 C | 4.22 ± 1.03 C |
| 72 | 4.03 ± 0.45 C | 4.61 ± 1.27 C |
| 96 | 12.06 ± 2.66 B | 11.32 ± 3.7 B |
| 120 | 2.8 ± 0.1 C | 14.10 ± 0.45 B |
| Tetracycline (1 mg/mL) | 22.36 ± 0.55 A | ___ |
| Acarbose (1 mg/mL) | ____ | 94.07 ± 2.6 A |
| ID | Tentative Assignment | [M − H]− (m/z) | Class | Fermentation Time (h) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | ||||
| 1 | Caffeic acid 4-O-glucoside | 341.1 | Hydroxycinnamic acids | X | X | X | X | ||
| 2 | 5-nonadecylresorcino | 375.1 | Alkylphenols | X | X | X | |||
| 3 | Medioresinol | 387.2 | Lignans | X | X | X | X | X | X |
| 4 | Sinensetin | 371.1 | Methoxyflavones | X | X | X | X | X | X |
| 5 | Phlorin | 287.1 | Other polyphenols | X | |||||
| 6 | Petunidin 3-O-(6″-acetyl-glucoside) | 520.4 | Anthocyanins | X | |||||
| 7 | Kaempferol 3-O-(2″-rhamnosyl-6″-acetyl-galactoside) 7-O-rhamnoside | 783.2 | Flavonols | X | |||||
| 8 | Resveratrol 3-O-glucoside | 389.1 | Stilbenes | X | X | X | X | X | |
| 9 | Gardenin B | 357.3 | Methoxyflavones | X | |||||
| 10 | 3,4-DHPEA-EA | 377.1 | Tyrosols | X | |||||
| 11 | Trachelogenin | 387.2 | Lignans | X | |||||
| 12 | Quercetin 3-O-glucuronide | 447.1 | Flavonols | X | |||||
| 13 | Phloretin | 273 | Dihydrochalcones | X | X | ||||
| 14 | 5-O-galloylquinic acid | 343.1 | Hydroxybenzoic acids | X | |||||
| 15 | p-coumaroyl tyrosine | 327.2 | Hydroxycinnamic acids | X | |||||
| 16 | Ferulic acid | 193.1 | Methoxycinnamic acids | X | |||||
| 17 | Hydroxycaffeic acid | 195.1 | Hydroxycinnamic acids | X | |||||
| 18 | Feruloyl glucose | 355.1 | Methoxycinnamic acids | X | |||||
| 19 | Tangeretin | 1075 | Methoxyflavones | X | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Hernández, I.; Gómez-García, R.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Chávez-González, M.L. Solid-State Fermentation for Phenolic Compounds Recovery from Mexican Oregano (Lippia graveolens Kunth) Residual Leaves Applying a Lactic Acid Bacteria (Leuconostoc mesenteroides). Agriculture 2024, 14, 1342. https://doi.org/10.3390/agriculture14081342
Bautista-Hernández I, Gómez-García R, Aguilar CN, Martínez-Ávila GCG, Torres-León C, Chávez-González ML. Solid-State Fermentation for Phenolic Compounds Recovery from Mexican Oregano (Lippia graveolens Kunth) Residual Leaves Applying a Lactic Acid Bacteria (Leuconostoc mesenteroides). Agriculture. 2024; 14(8):1342. https://doi.org/10.3390/agriculture14081342
Chicago/Turabian StyleBautista-Hernández, Israel, Ricardo Gómez-García, Cristóbal N. Aguilar, Guillermo C. G. Martínez-Ávila, Cristian Torres-León, and Mónica L. Chávez-González. 2024. "Solid-State Fermentation for Phenolic Compounds Recovery from Mexican Oregano (Lippia graveolens Kunth) Residual Leaves Applying a Lactic Acid Bacteria (Leuconostoc mesenteroides)" Agriculture 14, no. 8: 1342. https://doi.org/10.3390/agriculture14081342










