Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture
Abstract
:1. Introduction
1.1. Fertilizer Shortcomings
1.2. Controlled Environment Agriculture and Wastewater
1.3. Modeling in Hydroponics
1.4. Objectives
2. Materials and Methods
2.1. Experimental Configuration
2.1.1. Controlled Environment Chambers
2.1.2. Vertical Nutrient Film Technique (NFT) Hydroponic Systems
2.1.3. Seedling Germination and Transplantation
2.1.4. Treatment Preparation
2.1.5. Controlling pH in Solution
2.1.6. Stabilizing Water Composition during Nutrient Uptake
2.2. Sample Preparation and Storage
2.3. Sample Analyses
2.4. Modeling Approach
2.4.1. Initiating Models
2.4.2. Average Nitrogen Concentration
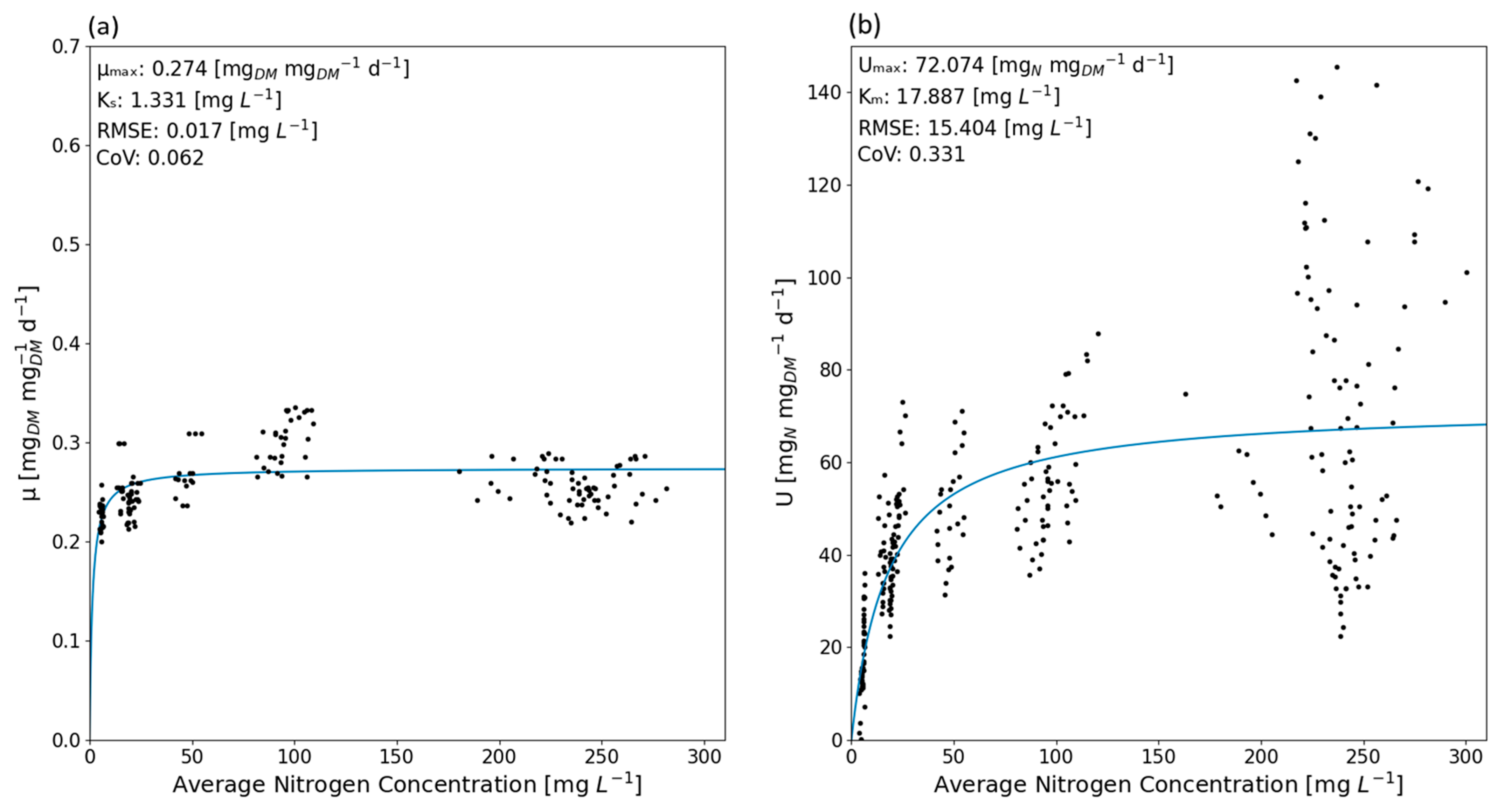
2.4.3. Monod Growth Model
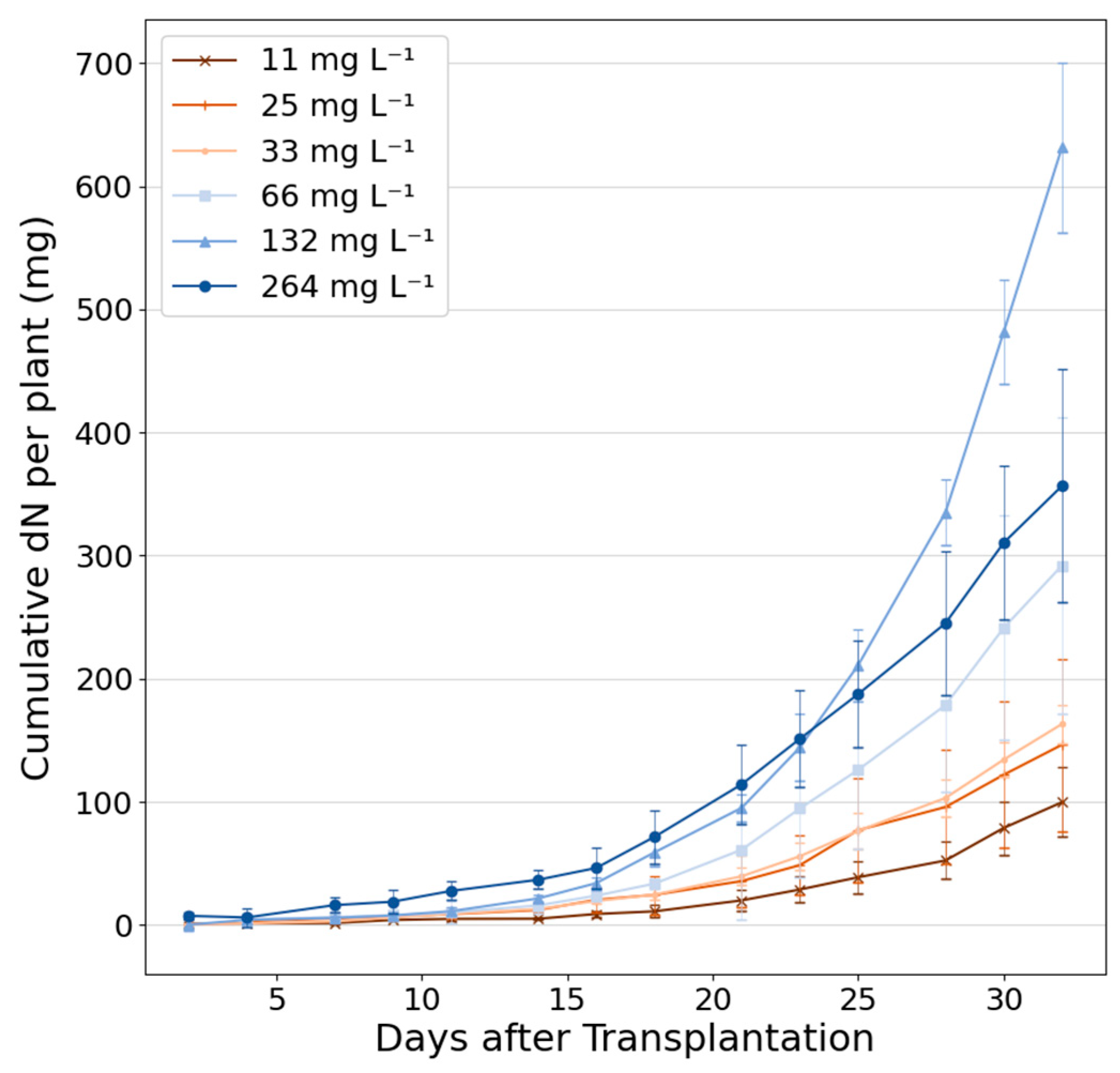
2.4.4. Nitrogen Uptake
2.4.5. Michaelis–Menten Uptake Model
2.4.6. Shoot-Specific Cell Yield
3. Results and Discussion
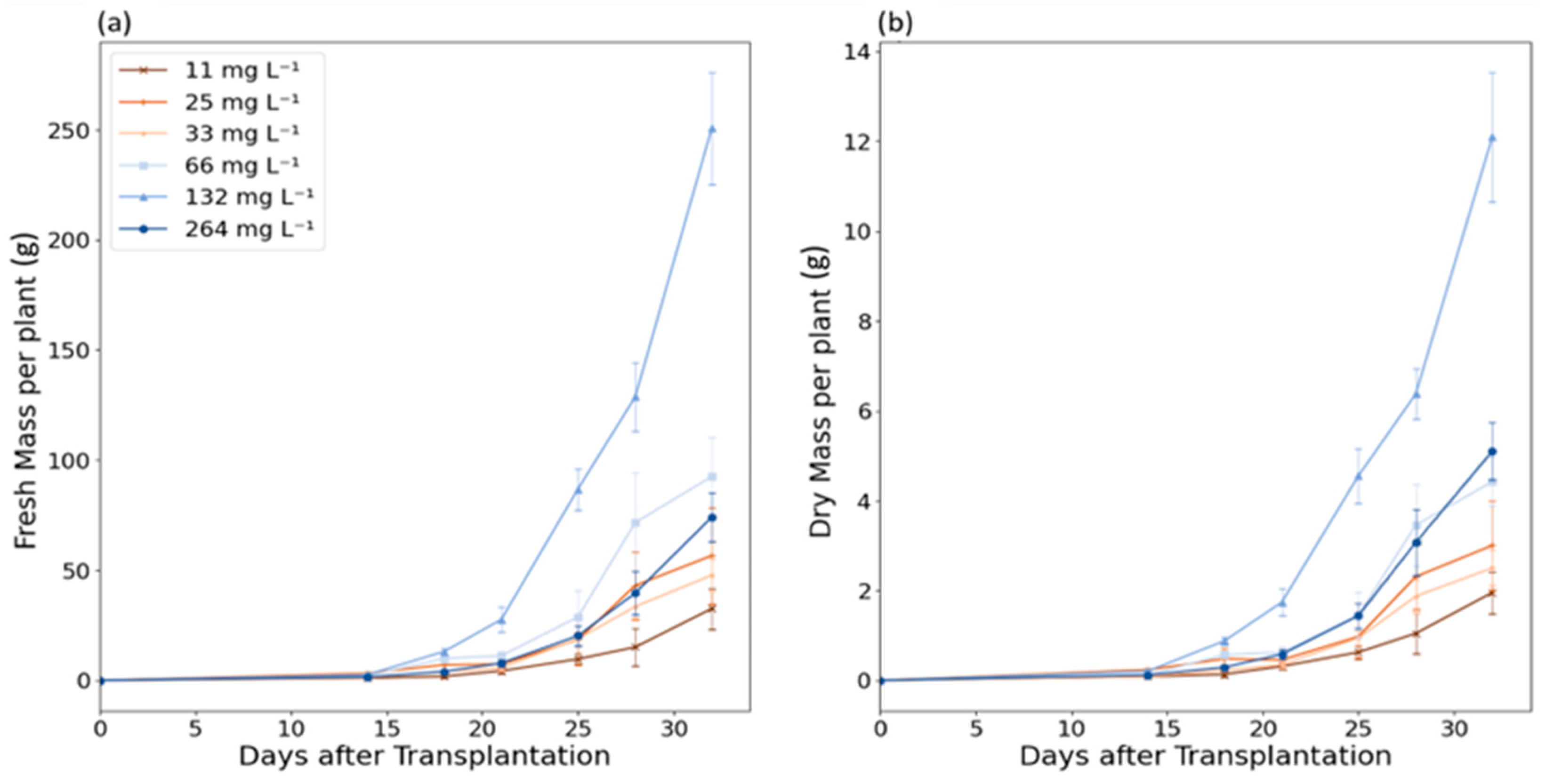
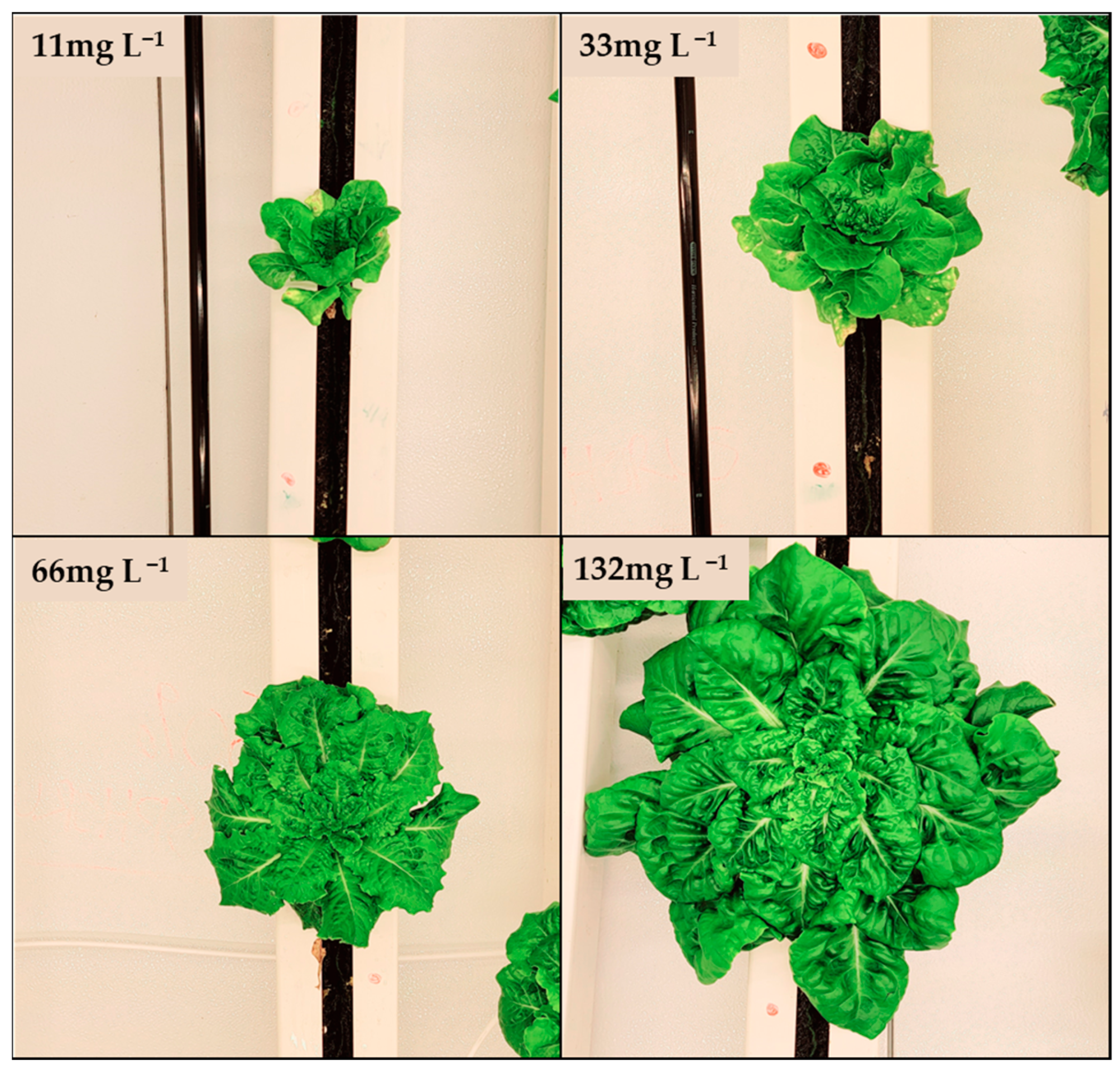
3.1. Biomass Growth and Nitrogen Depletion
3.2. Monod and Michaelis–Menten Modeling
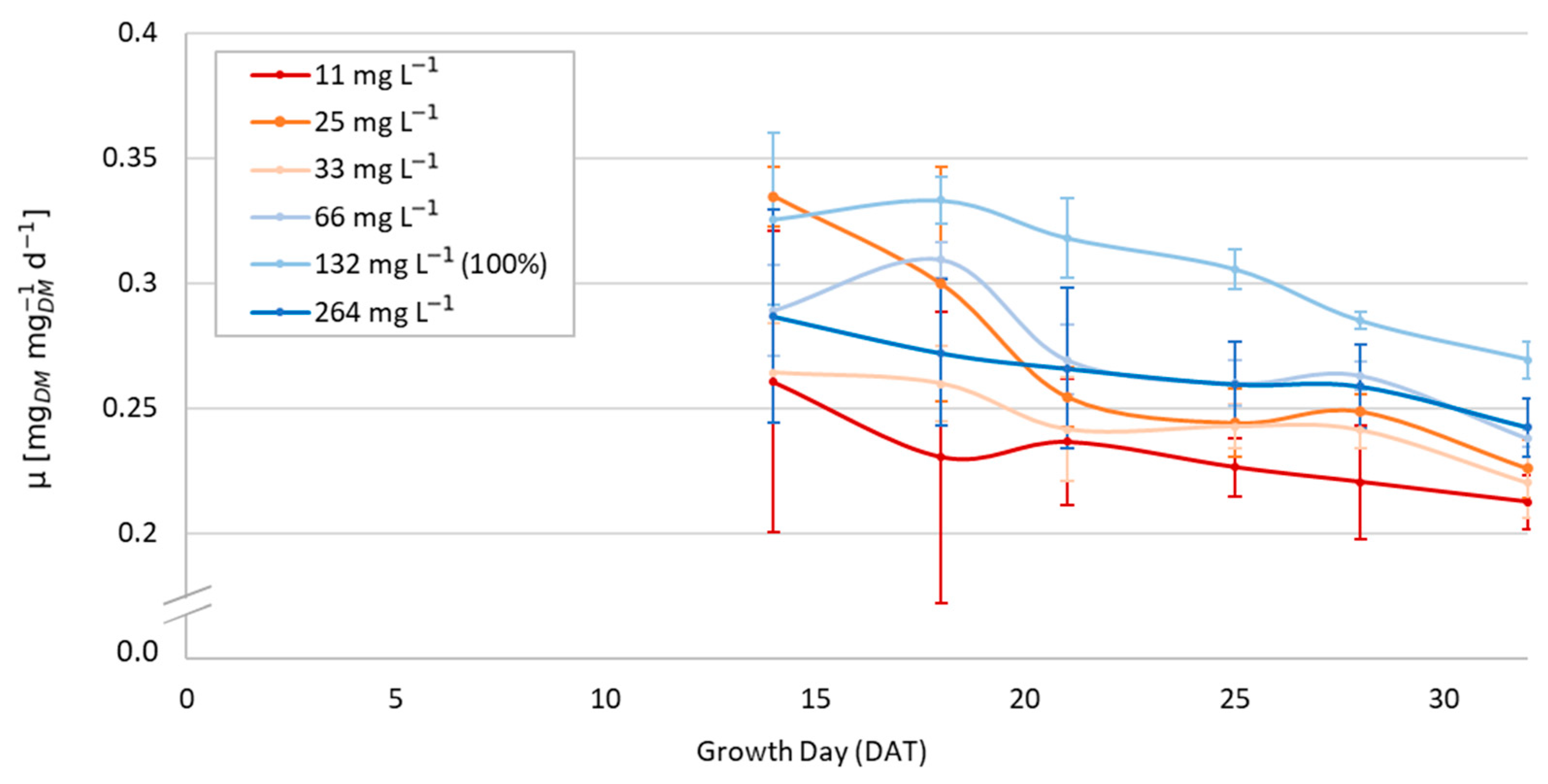
3.3. Dynamic Growth Rates
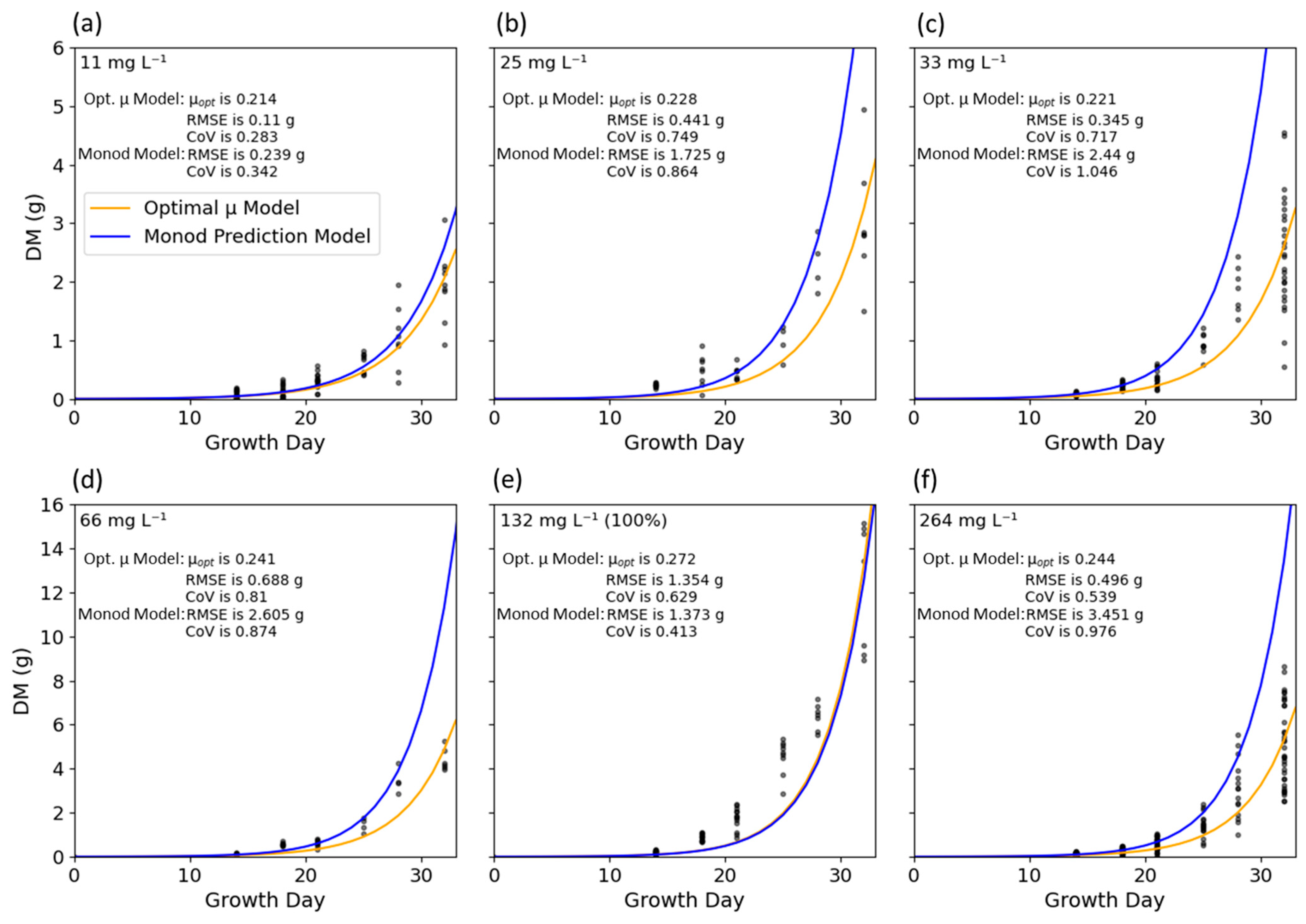
3.4. Visualizing Growth Models
3.5. Shoot-Specific Cell Yield
3.6. Final Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Halal, W.E.; Davies, O. State of the future, 19.0. World Futures Rev. 2018, 10, 95–98. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Environmental Engineering for the 21st Century: Addressing Grand Challenges; National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-47652-2. [Google Scholar]
- NAE Grand Challenges for Engineering: 14 Grand Challenges for Engineering in the 21st Century; National Academy of Engineering: Washington, DC, USA, 2006.
- Enriquez, J.P. Food self-sufficiency: Opportunities and challenges for the current food system. Biomed. J. Sci. Tech. Res. 2020, 31, 23984–23989. [Google Scholar] [CrossRef]
- Kaviti, G.; Asadu, C.; Wiseman, P. Russian war worsens fertilizer crunch, risking food supplies. Associated Press News, 12 April 2022. [Google Scholar]
- Zhang, W.; Ma, W.; Ji, Y.; Fan, M.; Oenema, O.; Zhang, F. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in china. Nutr. Cycl. Agroecosyst. 2008, 80, 131–144. [Google Scholar] [CrossRef]
- Steen, I.; Agro, K. Phosphorus availability in the 21st century—Management of a non-renewable resource. Phosphorus Potassium 1998, 217, 25–31. [Google Scholar]
- Hosseinian, A.; Pettersson, A.; Ylä-Mella, J.; Pongrácz, E. Phosphorus recovery methods from secondary resources, assessment of overall benefits and barriers with focus on the nordic countries. J. Mater. Cycles Waste Manag. 2023, 25, 3104–3116. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Micro-Organisms, 14th ed.; Pearson: London, UK, 1992; ISBN 9780321897398. [Google Scholar]
- Gellings, C.W.; Parmenter, K.E. Energy efficiency in fertilizer production and use. In Efficient Use and Conservation of Energy: Encyclopedia of Life Support Systems; EOLSS Publishers: Oxford, UK, 2004. [Google Scholar]
- Kool, A.; Marinussen, M.; Blonk, H. LCI Data for the Calculation Tool Feedprint for Greenhouse Gas Emissions of Feed Production and Utilization: GHG Emissions of N, P and K Fertilizer Production; Blonk Consultants: Gouda, The Netherlands, 2012. [Google Scholar]
- FAO. “Energy-Smart” Food for People and Planet; FAO: Rome, Italy, 2011. [Google Scholar]
- Brown, B.D.; Engel, R.; Management, N.; Olson-Rutz, K.; Associate, R. Management to Minimize Nitrogen Fertilizer Volatilization; Montana State University: Bozeman, MT, USA, 2020. [Google Scholar]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Takaya, N.; Catalan-Sakairi, M.A.B.; Sakaguchi, Y.; Kato, I.; Zhou, Z.; Shoun, H. Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Appl. Environ. Microbiol. 2003, 69, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Soussana, J.F.; Allard, V.; Pilegaard, K.; Ambus, P.; Amman, C.; Campbell, C.; Ceschia, E.; Clifton-Brown, J.; Czobel, S.; Domingues, R.; et al. Full accounting of the greenhouse gas (CO2, N2O, CH4) budget of nine european grassland sites. Agric. Ecosyst. Environ. 2007, 121, 121–134. [Google Scholar] [CrossRef]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.B.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006, 21, 192–199. [Google Scholar] [CrossRef]
- Krauter, C.T.; Potter, C.; Klooster, S. Ammonia Emission Related to Nitrogen Fertilizer Application Practices. In Proceedings of the California Department of Food and Agriculture Fertilizer Education Program Conference, Tulare, CA, USA, 2001. [Google Scholar]
- Lammel, G.; Graßl, H. Greenhouse effect of NOX. Environ. Sci. Pollut. Res. 1995, 2, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Müller, R. The impact of the rise in atmospheric nitrous oxide on stratospheric ozone. Ambio 2021, 50, 35–39. [Google Scholar] [CrossRef]
- Shine, K.P. Radiative forcing of climate change. Space Sci. Rev. 2000, 94, 363–373. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492.e3. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—Could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Muñoz-Liesa, J.; Rieradevall, J.; Gabarrell, X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? J. Clean. Prod. 2020, 261, 121213. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Rittmann, B.E. Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Muhaidat, R.; Al-Qudah, K.; Al-Taani, A.A.; AlJammal, S. Assessment of nitrate and nitrite levels in treated wastewater, soil, and vegetable crops at the upper reach of Zarqa river in Jordan. Environ. Monit. Assess. 2019, 191, 153. [Google Scholar] [CrossRef]
- McClintock, S.A.; Sherrard, J.H.; Novak, J.T.; Randall, C.W. Nitrate versus oxygen respiration in the activated sludge process. J. Water Pollut. Control Fed. 1988, 60, 342–350. [Google Scholar]
- Tomei, M.C.; Carozza, N.A.; Mosca Angelucci, D. Post-aerobic digestion of waste sludge: Performance analysis and modelling of nitrogen fate under alternating aeration. Int. J. Environ. Sci. Technol. 2016, 13, 21–30. [Google Scholar] [CrossRef]
- Lee, E.; Rout, P.R.; Bae, J. The applicability of anaerobically treated domestic wastewater as a nutrient medium in hydroponic lettuce cultivation: Nitrogen toxicity and health risk assessment. Sci. Total Environ. 2021, 780, 146482. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. Biomed. Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, D.I. Uptake of Common Pharmaceutical Compounds in Hydroponically Grown Lactuca sativa. Master’s Thesis, University of Connecticut, Storrs, CT, USA, 2018. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I. Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2012; Volume 11, ISBN 978-94-007-5448-5. [Google Scholar]
- Hoque, M.M.; Ajwa, H.A.; Smith, R. Nitrite and ammonium toxicity on lettuce grown under hydroponics. Commun. Soil. Sci. Plant Anal. 2007, 39, 207–216. [Google Scholar] [CrossRef]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- McCall, D.; Willumsen, J. Effects of nitrate, ammonium and chloride application on the yield and nitrate content of soil-grown lettuce. J. Hortic. Sci. Biotechnol. 1998, 73, 698–703. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Q. Effects of ammonium on the root architecture and nitrate uptake kinetics of two typical lettuce genotypes grown in hydroponic systems. J. Plant Nutr. 2012, 35, 1497–1508. [Google Scholar] [CrossRef]
- Zhang, K.; Burns, I.G.; Turner, M.K. Derivation of a dynamic model of the kinetics of nitrogen uptake throughout the growth of lettuce: Calibration and validation. J. Plant Nutr. 2008, 31, 1440–1460. [Google Scholar] [CrossRef]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, T.X.; Zhang, X.Z. Nutrient budget and soil nutrient status in greenhouse system. Agric. Sci. China 2010, 9, 871–879. [Google Scholar] [CrossRef]
- Epstein, E.; Hagen, C.E. A kinetic study of the absorption of alkali cations by barley roots. Plant Physiol. 1951, 27, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; York, L.M. Targeting root ion uptake kinetics to increase plant productivity and nutrient use efficiency. Plant Physiol. 2020, 182, 1854–1868. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.A.S. Soil Nutrient Bioavailability: A Mechanistic Approach, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1984. [Google Scholar]
- Wheeler, E.F.; Albright, L.D.; Spanswick, R.M.; Walker, L.P.; Langhans, R.W. Nitrate uptake kinetics in lettuce as influenced by light and nitrate nutrition. Trans. ASAE 1998, 41, 859–867. [Google Scholar] [CrossRef]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Strigul, N.; Dette, H.; Melas, V.B. A practical guide for optimal designs of experiments in the Monod model. Environ. Model. Softw. 2009, 24, 1019–1026. [Google Scholar] [CrossRef]
- Grosfils, A.; Vande Wouwer, A.; Bogaerts, P. On a general model structure for macroscopic biological reaction rates. J. Biotechnol. 2007, 130, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Seginer, I.; Buwalda, F.; van Straten, G. Nitrate concentration in greenhouse lettuce: A modeling study. Acta Hortic. 1998, 456, 189–197. [Google Scholar] [CrossRef]
- Seginer, I.; Van Straten, G.; Buwalda, F. Lettuce growth limited by nitrate supply. Acta Hortic. 1999, 507, 141–148. [Google Scholar] [CrossRef]
- Mathieu, J.; Linker, R.; Levine, L.; Albright, L.; Both, A.J.; Spanswick, R.; Wheeler, R.; Wheeler, E.; de Villiers, D.; Langhans, R. Evaluation of the Nicolet model for simulation of short-term hydroponic lettuce growth and nitrate uptake. Biosyst. Eng. 2006, 95, 323–337. [Google Scholar] [CrossRef]
- Ioslovich, I.; Seginer, I.; Baskin, A. Fitting the Nicolet lettuce growth model to plant-spacing experimental data. Biosyst. Eng. 2002, 83, 361–371. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; De-Alba-Romenus, K.; Ramírez-Sosa, M.I.M.; Benavides-Mendoza, A.; Robledo-Torres, V. An experimental validation of NICOLET B3 mathematical model for lettuce growth in the southeast region of Coahuila Mexico by dynamic simulation. In Proceedings of the 2010 7th International Conference on Electrical Engineering Computing Science and Automatic Control CCE, Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 128–133. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J.; Ephrath, J.E. A model for nutrient and water flow and their uptake by plants grown in a soilless culture. Plant Soil. 2005, 271, 309–319. [Google Scholar] [CrossRef]
- Silberbush, M. Nutrients and toxic substances accumulation in the plant and their effect on uptake: Simulation study in hydroponics. Acta Hortic. 2002, 593, 235–242. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J. Hydroponic Lettuce Handbook; Cornell Controlled Environment Agriculture; Cornell University: Ithaca, NY, USA, 1996; Volume 834, pp. 504–509. [Google Scholar]
- Modarelli, G.C.; Paradiso, R.; Arena, C.; De Pascale, S.; Van Labeke, M.C. High light intensity from blue-red LEDs enhance photosynthetic performance, plant growth, and optical properties of red lettuce in controlled environment. Horticulturae 2022, 8, 114. [Google Scholar] [CrossRef]
- Mattson, N.S.; Peters, C. A recipe for hydroponic success. Inside Grower. 2014, Jan, 16–19. [Google Scholar]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.A.P.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Safety data sheet for pH up liquid. In General Hydroponics. 2017, Volume 5, pp. 1–10. Available online: https://generalhydroponics.com/resources/ph-up-liquid-safety-data-sheet/ (accessed on 20 June 2024).
- Safety data sheet for pH down liquid. In General Hydroponics. 2017, Volume 5, pp. 1–10. Available online: https://generalhydroponics.com/resources/ph-down-liquid-safety-data-sheet/ (accessed on 20 June 2024).
- Geilfus, C.M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef]
- Nissim, W.G.; Masi, E.; Pandolfi, C.; Mancuso, S.; Atzori, G. The response of halophyte (Tetragonia tetragonioides (pallas) kuntz.) and glycophyte (Lactuca sativa L.) crops to diluted seawater and NaCl solutions: A comparison between two salinity stress types. Appl. Sci. 2021, 11, 6336. [Google Scholar] [CrossRef]
- Fernandez, D. Phosphorous toxicity and concentration in higher plants. Sci. Hydroponics 2017. Available online: https://scienceinhydroponics.com/2017/05/phosphorous-toxicity-concentration-higher-plants.html (accessed on 20 June 2024).
- Kim, J.-G.; Kim, M.S. Effects of phosphorus and iron on the phytotoxicity of lettuce (Lactuca sativa L.) in arsenic-contaminated soil. Ecol. Resilient Infrastruct. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Uchida, R.; Silva, J.A. Essential nutrients for plant growth: Nutrient functions and deficiency symptoms. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; College of Tropical Agriculture and Human Resources: Honolulu, Hawaii, 2000; pp. 31–55. ISBN 978-1-929325-08-5. [Google Scholar]
- Kirsten, W.J. Automatic methods for the simultaneous determination of carbon, hydrogen, nitrogen, and sulfur, and for sulfur alone in organic and inorganic materials. Anal. Chem. 1979, 51, 1173–1179. [Google Scholar] [CrossRef]
- USEPA. Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; Test methods evaluation solid waste; US Environmental Protection Agency: Washington, DC, USA, 1996.
- Creed, J.T.; Brockhoff, C.A.; Martin, T.D. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-mass Spectrometry; Revisiom 5.4 EMMC Version; Environmental Monitoring System Laboratory Office Research Development, US Environmental Protection Agency: Washington, DC, USA, 1994; pp. 1–57.
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Mueller, A.; Grisel, O.; Niculae, V.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; et al. API design for machine learning software: Experiences from the Scikit-Learn project. arXiv 2013. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine learning in python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Karlowsky, S.; Gläser, M.; Henschel, K.; Schwarz, D. Seasonal nitrous oxide emissions from hydroponic tomato and cucumber cultivation in a commercial greenhouse company. Front. Sustain. Food Syst. 2021, 5, 626053. [Google Scholar] [CrossRef]
- Karlowsky, S.; Buchen-Tschiskale, C.; Odasso, L.; Schwarz, D.; Well, R. Sources of nitrous oxide emissions from hydroponic tomato cultivation: Evidence from stable isotope analyses. Front. Microbiol. 2023, 13, 1080847. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.W.; Yang, D.S.; Kays, S.J.; Kim, J.H.; Woo, J.H.; Park, K.W. Effects of nutrient solution electrical conductivity and sulfur, magnesium, and phosphorus concentration on sesquiterpene lactones in hydroponically grown lettuce (Lactuca sativa L.). Sci. Hortic. 2009, 122, 369–374. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Commonwealth Agricultural Bureaux: Wallingford, UK, 1965. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347. [Google Scholar]
- Bollard, E.G.; Butler, G.W. Mineral nutrition of plants. Annu. Rev. Plant Physiol. 1966, 17, 77–112. [Google Scholar] [CrossRef]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the Sixth International Congress on Soilless Culture, ISOSC, Lunteren, The Netherlands, 29 April–5 May 1984; pp. 633–649. [Google Scholar]
- Smith, G.S.; Johnston, C.M.; Cornforth, I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983, 94, 537–548. [Google Scholar] [CrossRef]


| Nitrogen Treatment % | ||||||
|---|---|---|---|---|---|---|
| 200% | 100% | 50% | 25% | 19% | 8% | |
| Macronutrient Concentrations (mg/L) | ||||||
| N | 264.47 | 132.24 | 66.12 | 33.06 | 25.30 | 10.58 |
| P | * | 30.97 | * | 109.86 | 93.94 | 154.05 |
| K | 218.42 | 210.01 | * | * | * | * |
| Ca | * | 82.49 | * | * | * | * |
| S | * | 32.22 | * | * | * | * |
| Mg | * | 24.25 | * | * | * | * |
| Fe | * | 1.26 | * | * | * | * |
| Micronutrient Concentrations (µg/L) | ||||||
| B | * | 162.50 | * | * | * | * |
| Mn | * | 249.04 | * | * | * | * |
| Zn | * | 130.39 | * | * | * | * |
| Cu | * | 23.54 | * | * | * | * |
| Mo | * | 24.09 | * | * | * | * |
| DAT | 0 | 2 | 4 | 7 | 9 | 11 | 14 | 16 | 18 | 21 | 23 | 25 | 28 | 30 | 32 |
| n | 0 | 1 | 2 | 3 * | 4 | 5 | 6 * | 7 | 8 * | 9 * | 10 * | 11 * | 12 * | 13 * | 14 |
| h | 0 | - | - | - | - | - | 1 | - | 2 | 3 | - | 4 | 5 | - | 6 |
| DAT | 11 mg L−1 | 25 mg L−1 | 33 mg L−1 | 66 mg L−1 | 132 mg L−1 | 264 mg L−1 |
|---|---|---|---|---|---|---|
| 14 | 0.96 ± 0.33 | 3.20 ± 0.46 | 1.13 ± 0.16 | 2.00 ± 0.51 | 2.61 ± 0.48 | 1.53 ± 0.33 |
| 0.08 ± 0.03 (8.3%) | 0.24 ± 0.03 (7.5%) | 0.09 ± 0.01 (8.0%) | 0.13 ± 0.02 (6.5%) | 0.21 ± 0.04 (8.0%) | 0.12 ± 0.02 (7.8%) | |
| 18 | 1.85 ± 0.77 | 7.04 ± 3.80 | 4.13 ± 0.67 | 9.94 ± 1.56 | 13.07 ± 1.24 | 4.00 ± 0.73 |
| 0.14 ± 0.06 (7.6%) | 0.48 ± 0.22 (6.8%) | 0.23 ± 0.03 (5.6%) | 0.57 ± 0.07 (5.7%) | 0.88 ± 0.09 (6.7%) | 0.29 ± 0.05 (7.3%) | |
| 21 | 4.29 ± 1.05 | 7.37 ± 2.32 | 5.61 ± 1.28 | 11.20 ± 1.81 | 27.63 ± 5.67 | 7.84 ± 1.70 |
| 0.31 ± 0.07 (7.2%) | 0.46 ± 0.11 (6.2%) | 0.35 ± 0.08 (6.2%) | 0.62 ± 0.14 (5.5%) | 1.74 ± 0.30 (6.3%) | 0.58 ± 0.11 (7.4%) | |
| 25 | 9.68 ± 2.39 | 18.46 ± 11.40 | 18.79 ± 3.18 | 28.74 ± 12.30 | 86.81 ± 9.44 | 20.23 ± 4.68 |
| 0.63 ± 0.14 (6.5%) | 0.98 ± 0.47 (5.3%) | 0.94 ± 0.16 (5.0%) | 1.45 ± 0.51 (5.0%) | 4.55 ± 0.61 (5.2%) | 1.44 ± 0.28 (7.1%) | |
| 28 | 15.1 ± 8.48 | 43.07 ± 15.09 | 33.51 ± 6.40 | 71.63 ± 22.52 | 128.71 ± 15.45 | 39.64 ± 9.82 |
| 1.05 ± 0.45 (7.0%) | 2.31 ± 0.73 (5.4%) | 1.87 ± 0.36 (5.6%) | 3.46 ± 0.92 (4.8%) | 6.38 ± 0.56 (5.0%) | 3.07 ± 0.73 (7.7%) | |
| 32 | 32.57 ± 9.22 | 56.62 ± 21.95 | 47.73 ± 7.35 | 92.53 ± 17.97 | 250.73 ± 25.41 | 74.11 ± 10.90 |
| 1.96 ± 0.47 (6.0%) | 3.00 ± 0.99 (5.3%) | 2.50 ± 0.39 (5.2%) | 4.42 ± 0.54 (4.8%) | 12.10 ± 1.44 (4.8%) | 5.10 ± 0.64 (6.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharkey, A.; Altman, A.; Cohen, A.R.; Groh, T.; Igou, T.K.S.; Ferrarezi, R.S.; Chen, Y. Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture. Agriculture 2024, 14, 1358. https://doi.org/10.3390/agriculture14081358
Sharkey A, Altman A, Cohen AR, Groh T, Igou TKS, Ferrarezi RS, Chen Y. Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture. Agriculture. 2024; 14(8):1358. https://doi.org/10.3390/agriculture14081358
Chicago/Turabian StyleSharkey, Andrew, Asher Altman, Abigail R. Cohen, Teagan Groh, Thomas K. S. Igou, Rhuanito Soranz Ferrarezi, and Yongsheng Chen. 2024. "Modeling Bibb Lettuce Nitrogen Uptake and Biomass Productivity in Vertical Hydroponic Agriculture" Agriculture 14, no. 8: 1358. https://doi.org/10.3390/agriculture14081358





